Abstract
A charge neutral complex (CNC) was formed in aqueous solution by combining an orange light emitting anionic conjugated polyelectrolyte and a saturated cationic polyelectrolyte at a 1:1 ratio (per repeat unit). Photoluminescence (PL) from the CNC can be quenched by both the negatively charged dinitrophenol (DNP) derivative, (DNP-BS−), and positively charged methyl viologen (MV2+). Use of the CNC minimizes nonspecific interactions (which modify the PL) between conjugated polyelectrolytes and biopolymers. Quenching of the PL from the CNC by the DNP derivative and specific unquenching on addition of anti-DNP antibody (anti-DNP IgG) were observed. Thus, biosensing of the anti-DNP IgG was demonstrated.
Conjugated polymers are a versatile class of organic materials that promise utility in a variety of applications ranging from antistatic coatings, electrodes, and transistors, to light-emitting diodes, large area displays, photodetectors, photovoltaic cells, and lasers (1–3). The electrical, optical, and electrochemical properties of conjugated polymers can be modified by chemical synthesis and are strongly affected by relatively small perturbations, including changes in temperature, solvent, or chemical environment. As a result of this sensitivity, conjugated polymers are promising as sensory materials (4, 5); sensing may be accomplished by transducing and/or amplifying physical or chemical changes into electrical, optical, or electrochemical signals. Conjugated polymers have been used to detect chemical species (chemosensors) (6), such as ions (7–11), gases (for example, trinitrotoluene) (6, 12–14), and other chemicals (15), or biomolecules such as proteins, antibodies (16–27), and DNA (28–31), using electrical (13, 15), chromic (7, 8, 16–19), electrochemical (7–9, 20–25, 28–31), photoluminescent (11, 26), chemoluminescent (27), or gravimetric (14) responses.
Contemporary biosensor and bioassay developments have focused on mimicking natural host–receptor (“lock-and-key”) interactions. “Lock-and-key” molecular recognition can be between enzyme and substrate, ligand and receptor, antibody and antigen, or between two strands of nucleic acids with complementary sequences. Although antibody-based ELISAs are widely used for detection of biological species with high sensitivity, these assays are relatively labor-intensive and time-consuming (hours to days) and require two different antibodies of defined specificities to adequately detect the target molecule (potentially making them cumbersome to perform) (32). Additionally, the detection of small molecules using ELISA can seldom be accomplished because of the recognition of one epitope by both capture and detection antibodies. Therefore, competition assays have to be performed that are both less accurate and more time consuming.
Biosensors based on conjugated polymers as sensory materials exhibit real-time response [electrochemical (20–25) or optical (16–19, 26)] to the ligand-receptor recognition event. The coupling of a recognition event to photoinduced electron transfer or a change in the electronic structure of the conjugated polymer produces changes in the luminescence, UV-visible absorption, or redox potential of the polymer (4, 5). Extensive research has been carried out by using conjugated polymers (derivatives of polydiacetylene, electrochemically polymerized polypyrrole, or polythiophene) as chromic (16–19) or electrochemical (20–25, 28–31) biosensors. However, the relatively low sensitivity of UV-visible absorption measurements, the complex electrochemical instrumentation required, and the nonspecific interactions between biomolecules and conjugated polymers have prevented practical and general use.
Water-soluble conjugated polymers (conjugated polyelectrolytes) show potential for use as a new class of high-sensitivity rapid-response chemical and biological sensors (26, 33, 34). The fluorescence of these polymers can be quenched by very small amounts of charged molecules (quenchers) that quench the excited state by energy transfer or electron transfer (26, 35, 36). This quenching can be adapted to biosensing by coupling a quencher to a biological ligand. In aqueous solution, the photoluminescence (PL) from the polymer is quenched when the quencher–ligand conjugate associates with the polyelectrolyte to form a relatively weak conjugate–polymer complex, as a consequence of electrostatic and hydrophobic interactions. Exposure of the conjugate–polymer complex to a biological receptor results in formation of a biospecific receptor–conjugate complex and release of the polymer with concomitant unquenching of the polymer fluorescence.
In this paper, we report an investigation of the PL from a conjugated polyelectrolyte complex in aqueous solution. We show that the anionic conjugated polymer alone is subject to nonspecific effects on the PL (luminescence enhancement), which complicates the use of conjugated polyelectrolytes in biosensing applications. We find, however, that when a cationic polymer is added in the solution to associate with the anionic conjugated polyelectrolyte and form a charge neutral complex (CNC) [formed in aqueous solution by an anionic conjugated polyelectrolyte and a saturated cationic polyelectrolyte at a 1:1 ratio (per repeat unit)], the CNC shows little evidence of nonspecific interactions with biopolymers that occur with the anionic conjugated polymer alone. Moreover, we demonstrate that PL from the CNC can be quenched by both cationic and anionic quenchers. We also demonstrate full PL recovery on the specific recognition between negatively charged dinitrophenol (DNP) derivative, (DNP-BS−) and anti-DNP IgG, indicating a conjugated polymer-based biosensor with moderate sensitivity and rapid response.
Experimental Methods
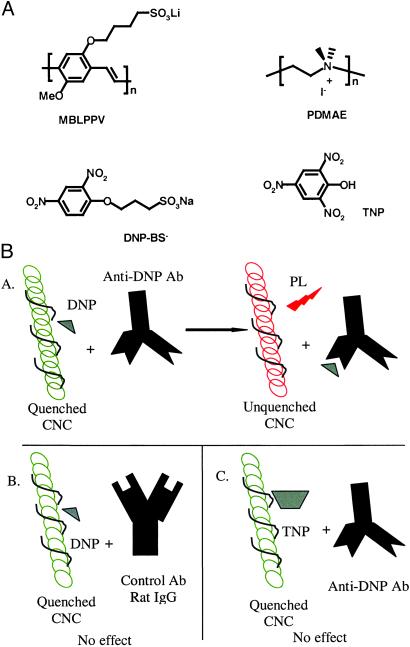
The anionic conjugated polyelectrolyte, poly[lithium 5-methoxy-2-(4-sulfobutoxy)-1, 4-phenylenevinylene] (MBL-PPV), cationic polyelectrolyte, poly(N,N-dimethylammonio-ethylene iodide), and anionic quencher, 1-sulfobutoxy-2, 4-dinitrobenzene (DNP-BS−), were synthesized at the University of California, Santa Barbara (37). The proteins used in this study, sheep anti-mouse F(ab′)2, mouse IgG, and rat IgG, were purchased from Sigma. Fab′ (fragment of antigen-binding: univalent antigen-binding fragment of an antibody) fragments were generated from sheep anti-mouse F(ab′)2 by 2-mercaptoethanolamine reduction by using standard techniques and covalently conjugated to MV2+ to generate Fab′-Q [Fab′ with covalently linked a methyl-viologen quencher (Q)] (37) The monoclonal hamster anti-DNP antibody (anti-DNP IgG) was isolated from the hybridoma CRL 1968 obtained from American Type Culture Collection by using standard techniques (38). The molecular structures of the polymers and conjugates are shown in Fig. 1.
Figure 1.
(A) Chemical structures. (B) Schematic of the DNP-BS− sensor. (Ba) PL from CNC is quenched by DNP-BS− and recovered on the specific binding between DNP-BS− and anti-DNP antibody. (Bb) PL from CNC is quenched by DNP-BS− and not recovered on addition of nonspecific antibody–rat IgG. (Bc) PL from CNC is quenched by TNP and not recovered on addition of nonspecific anti-DNP antibody.
PL spectra of the mixtures of conjugated polyelectrolyte and proteins were collected on a PSI (Photon Technology International, London, Ontario, Canada) Fluorescence System in 1×PBS buffer (2 mM NaH2PO4/8 mM Na2HPO4/150 mM NaCl, pH = 7.0). Emission spectra were measured “in situ,” and linear concentration corrections were made for the very dilute polyelectrolyte solutions (35, 39). In the PL measurements, a small slit width was used to assure that there was no photodegradation. On the basis of repeated measurements of the integrated intensity of the emission spectrum, we estimate the experimental error of the integrated PL intensity to be less than 1%.
Nonspecific Interactions.
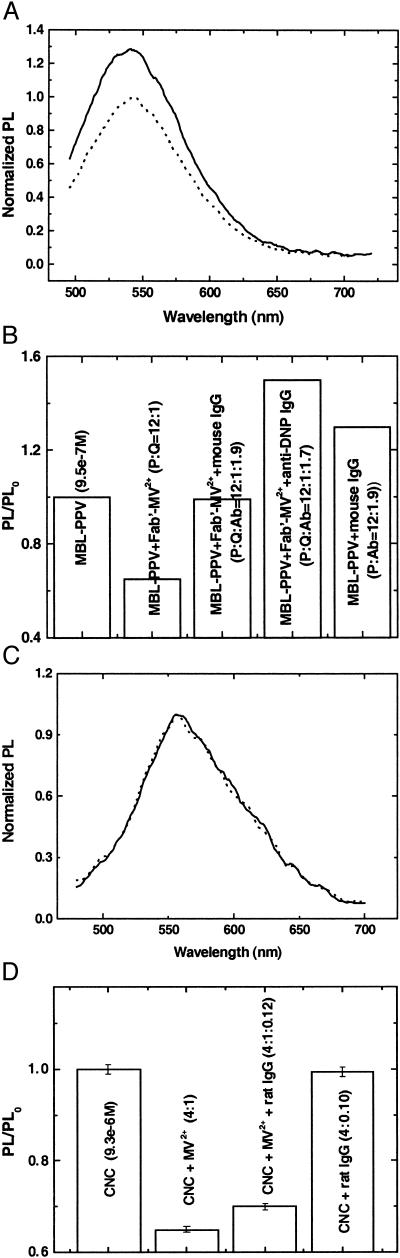
As shown elsewhere, the fluorescence of conjugated polyelectrolytes in aqueous solution is easily perturbed by the addition of various reagents (37, 40, 41). In the present investigation, we found that when proteins were added to the polyelectrolyte solutions, the PL from the conjugated polymers was altered. For example, the integrated PL intensity from MBL-PPV in 9.5 × 10−7 M solution was enhanced by 29.8% when the protein, 150 nM mouse IgG, was added, as shown by the dotted and solid lines in Fig. 2A. The observation of such effects at relatively low concentrations of both conjugated polymer and biopolymer was initially surprising, because both are anionic. That the polymer responds to these species implies there must be an interaction even at very low concentrations of both macromolecules, most likely association because of a combination of hydrophobic effects and interactions with cations from the buffer (42, 43).
Figure 2.
(A) PL changes caused by interaction with biopolymers. Antibody enhances PL of MBL-PPV. PL from 9.5e−7 M MBL-PPV in 1×PBS (dashed line) and PL when 150 nM mouse IgG was added (solid line). (B) PL from 9.5 × 10−7 M MBL-PPV in 1×PBS is quenched by anti-mouse Fab′-Q (8 × 10−8 M) and “recovered” by the addition of specific antibody, mouse IgG (1.5 × 10−7 M) and nonspecific antibody, anti-DNP (1.3 × 10−7 M), respectively. PL from 9.5e−7 M MBL-PPV in 1×PBS is enhanced by the addition of mouse IgG (1.5 × 10−7 M). (C) Formation of CNC minimizes the nonspecific changes in PL by biopolymers. PL from 9.3e−6 M CNC in 1×PBS (solid line) and PL after adding 240 nM rat IgG (dashed line). (D) The quenched PL from CNC (by methyl viologen and DNP-BS−) is not affected by addition of rat IgG. PL from 9.3 × 10−6 M CNC was quenched by 35% when 2.3 × 10−6 M of MV2+ was added. There is less than 5% change in PL by the addition of 2.8 × 10−7 M rat IgG. The PL from 9.3 × 10−6 M CNC is not affected by the addition of 2.3 × 10−7 rat IgG alone.
Association between charged macromolecules is a very general phenomenon. Unfortunately, the fact that this association influences the PL of conjugated polyelectrolytes complicates their use in biosensing applications that are based on the quench–unquench approach described above. The fluorescence of MBL-PPV is indeed quenched by 39% by the sheep Fab′-Q, a methyl-viologen quencher (Q) that was covalently linked to a sheep Fab′ antibody fragment, specific for mouse IgG. However, addition not only of the specific receptor for the conjugate, mouse IgG, but also of other nonspecific antibodies that do not bind with the Fab′-Q, such as hamster anti-DNP IgG, causes enhancement (“unquenching”) of the polymer fluorescence (37). As shown in Fig. 2B, PL from 9.5 × 10−7 M MBL-PPV in 1×PBS is quenched by anti-mouse Fab′-Q (8 × 10−8 M) and fully “recovered” by the addition of specific antibody, mouse IgG (1.5 × 10−7 M), but the PL is overrecovered (150%) by nonspecific anti-DNP antibody (1.3 × 10−7 M). Fig. 2B also shows that the integrated PL (from Fig. 2A) intensity from MBL-PPV in 9.5 × 10−7 M solution was enhanced by 29.8% by the addition of mouse IgG (1.5 × 10−7 M). Thus, the detected unquenching was not antigen-specific and therefore not of practical value as a biosensor.
Elimination of Nonspecific Interactions with the CNC.
In an attempt to minimize the nonspecific interactions, we formed a CNC comprising the anionic luminescent polymer (MBL-PPV) and a cationic saturated polymer (PDMAE); the structures of the two polymers are shown in Fig. 1A. Using the CNC, there was no change in the PL emission when nonspecific polyclonal IgG was added (Fig. 2 C and D). The reason for the major reduction in nonspecific interactions of charged biopolymers with the CNC is not completely clear. Although it is possible that the neutralization of charge attenuates the tendency of the polymer complex to associate with biopolymers, it is also possible that the CNC is less sensitive to effects (such as conformational changes) that modify the PL than are the individual polymers.
The 1:1 CNC formed between cationic polyelectrolyte and anionic conjugated polyelectrolyte should be overall neutral. Thus, it is instructive to evaluate its PL behavior when exposed to small charged molecules as well as to biopolymers.
To further study the stabilization of the PL by “charge-tuning” through formation of the CNC, the effect of proteins on the quenched PL was evaluated. As shown in Fig. 2D, PL from 9.3 × 10−6 M CNC was quenched by 35% when 2.3 × 10−6 M MV2+ was added. Only a small change in PL (less than 5%) was found when 2.8 × 10−7 M rat IgG was added, compared with ≈30% increase of the PL for the pure MBL-PPV in solution (Fig. 2 A and B). It is also shown in Fig. 2D that the addition of IgG does not change the PL of CNC (Integrated from Fig. 2C).
As noted above, the PL from anionic MBL-PPV can be quenched only by positive quenchers (via static quenching at very low quencher concentration regimes) (35, 39) but not by the anionic quencher DNP-BS−. As a result of the neutralization of the charge on the polyelectrolyte side groups (“charge-tuning”), the PL from the CNC can be “universally” quenched by both positively charged, e.g., MV2+, and negatively charged quenchers, e.g., DNP-BS−. Using MV2+, the Stern–Volmer constant (Ksv) was Ksv = 6.6 × 105 M−1 in 1×PBS buffer, compared with 1.9 × 107 M−1 for pure MBL-PPV quenched by MV2+ in water (39). The relatively low Ksv results from a combination of ionic screening in buffer solution (35) and the absence of static quenching for the CNC because it is overall neutral. The PL from the MBL-PPV component of the CNC can also be quenched by anionic DNP-BS− with Ksv = 2 × 105 M−1 in 1×PBS buffer.
Biosensing.
We sought to use the quenching of the PL from the MBL-PPV component of the CNC to design an antibody-based biosensor capable of detecting DNP-BS−. We reasoned that the addition of an anti-DNP antibody would bind to the DNP-BS−, removing it from the vicinity of CNC and thus restoring the PL (see ref. 26). We further reasoned that if the antibody antigen interaction were biospecific, then recovery of PL would occur only in the presence of the anti-DNP antibody and not in the presence of a control antibody with differing specificity (as shown in Fig. 1B).
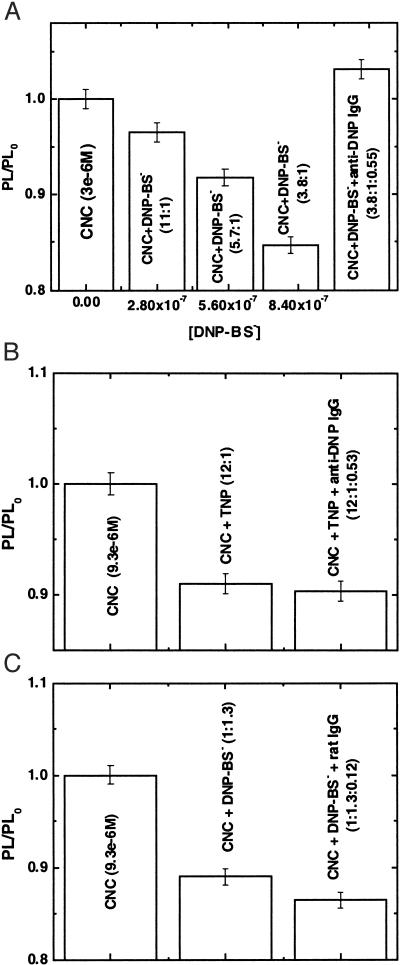
Fig. 3A shows the PL quenching/unquenching of the CNC by DNP-BS−/anti-DNP IgG. The PL from the complex (MBL-PPV 3 × 10−6 M) was quenched by successively higher concentrations of DNP-BS− (15% quenching at 7.9 × 10−7 M). The quenched PL (15%) was recovered when anti-DNP antibody specific for DNP was added, with the ratio of DNP-BS−/anti-DNP IgG roughly 2:1 (every anti-DNP IgG has two antigenic sites). Because the changes in PL efficiency caused by nonspecific interactions have been eliminated (see Fig. 2B), the recovery of the PL is attributed to the specific binding between DNP-BS− and anti-DNP antibody.
Figure 3.
(A) CNC quenching/unquenching by DNP-BS−/anti-DNP IgG. PL from the complex (MBL-PPV 3 × 10−6 M) was quenched on addition of successively greater concentrations of DNP-BS−. The quenched PL was recovered when antibody was added, with the ratio of DNP-BS−/anti-DNP IgG roughly 2:1. (B) Biological specificity of antigen control experiment: quenched PL from the CNC (by TNP) was not recovered on addition of anti-DNP IgG. PL from CNC (9.3 × 10−6 M) was quenched by about 9% by TNP (7.8 × 10−7 M), and no PL recovery on addition of 4.1 × 10−7 M anti-DNP IgG was observed. (C) Biological specificity of antibody control experiment: PL from 9.3 × 10−6 M CNC was quenched 11% when 1.2 × 10−5 M DNP-BS− was added. No PL enhancement was found by the addition of 1.1 × 10−6 M rat IgG.
Control experiments were carried out to demonstrate that the recovery of the PL from the CNC was indeed because of the specific binding between the DNP-BS− and anti-DNP IgG. Both antibody and antigen control experiments were tested. Fig. 3B shows that the PL from the CNC (9.3 × 10−6 M) was quenched by about 9% by 2, 4,6-trinitrophenol (TNP) (7.8 × 10−7 M), a DNP analog, but the PL is not recovered on the addition of 4.1 × 10−7 M anti-DNP IgG. Additionally, as shown in Fig. 3C, PL from 9.3 × 10−6 M CNC was quenched 11% by the addition of 1.2 × 10−5 M DNP-BS−, but no PL enhancement was detected when 1.1 × 10−6 M rat IgG was added. The control experiments confirmed that the PL recovery in Fig. 3A is because of the biologically specific recognition between DNP-BS− and anti-DNP IgG.
Although attractive as a homogeneous assay in aqueous solution, the biosensor demonstrated by the experiments reported here shows a modest detectable sensitivity of ≈300 nM analyte (Fig. 3A). Nevertheless, the data provide a clear demonstration of the validity of the method. If we are able to improve the Stern-Volmer constant to values obtained with charged photoluminescent polymers as suggested by the work of Jones et al. (44), one can anticipate sensitivities in the subnanomolar range.
Conclusion
In summary, the conjugated polyelectrolyte, MBL-PPV, was found to associate with negatively charged biopolymers (proteins). As a consequence of this nonspecific interaction, the PL efficiency of the anionic polyelectrolyte is enhanced, which complicates the use of luminescent conjugated polyelectrolytes in biosensor applications. When a cationic polyelectrolyte was associated with the anionic conjugated polyelectrolyte, the formation of the CNC enables tuning of the properties of the polyelectrolyte. The PL emission from the CNC can be quenched by both cationic and anionic quenchers. Moreover, the PL from the CNC is insensitive to the addition of nonspecific biopolymers that affect strongly the PL of the individual conjugated polyelectrolytes. The attenuation of the nonspecific effects on the PL allows the biospecific sensing of anti-DNP IgG through the antibody/antigen pair, DNP-BS− and anti-DNP IgG. Biospecific sensing was realized and demonstrated with detectable sensitivity of ≈300 nM of analyte. Because this approach could be applied to any antibody/antigen pair, the results demonstrate the potential of this approach for high-sensitivity real-time detection of biologically relevant molecules with a wide array of possible applications.
Acknowledgments
We are grateful to Dr. Guihua Hou of Shandong University, People's Republic of China, for initial work in generating monoclonal anti-DNP IgG. We thank Drs. David G. Whitten and Duncan W. McBranch of QTL Biosystems, LLC, Santa Fe, NM, for useful discussions. Research at University of California, Santa Barbara, was supported by the National Science Foundation (DMR-0099843).
Abbreviations
- CNC
charge neutral complex
- PL
photoluminescence
- MBL-PPV
poly[lithium 5-methoxy-2-(4-sulfobutoxy)-1, 4-phenylenevinylene]
- DNP
2, 4-dinitrophenol
- DNP-BS−,1-sulfobutoxy-2
4-dinitrobenzene
- TNP
2, 4,6-trinitrophenol
- MV2+
(methyl viologen) N, N′-dimethyl-4, 4′-bipyridinium
References
- 1.Skotheim T A, Reynolds J, Elsenbaumer R. Handbook of Conducting Polymers. 2nd Ed. New York: Dekker; 1998. [Google Scholar]
- 2.Friend R H, Gymer R W, Holmes A B, Burroughes J H, Marks R N, Taliani C, Bradley D D C, Dos Santos D A, Bredas J L, Logdlund M, et al. Nature (London) 1999;397:121–128. [Google Scholar]
- 3.McGehee M D, Miller E K, Moses D, Heeger A J. In: Twenty Years of Conducting Polymers: From Fundamental Science to Applications. Bernier P, editor. Amsterdam: Elsevier; 2000. [Google Scholar]
- 4.Leclerc M. Adv Mater. 1999;11:1491–1498. [Google Scholar]
- 5.McQuade D T, Pullen A E, Swager T M. Chem Rev. 2000;100:2537–2574. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- 6.Swager T M. Acc Chem Res. 1998;31:201–207. [Google Scholar]
- 7.Zhu S S, Carroll P J, Swager T M. J Am Chem Soc. 1996;118:8713–8714. [Google Scholar]
- 8.Zhu S S, Swager T M. J Am Chem Soc. 1997;119:12568–12577. [Google Scholar]
- 9.Korri-Youssoufi H, Godillot P, Srivastava P, Kassmi A E, Garnier F. Synth Metals. 1997;84:169–170. [Google Scholar]
- 10.Wang B, Wasielewski M R. J Am Chem Soc. 1997;119:12–21. [Google Scholar]
- 11.Rimmel G, Bauerle B. Synth Metals. 1999;102:1323–1324. [Google Scholar]
- 12.Yang J-S, Swager T M. J Am Chem Soc. 1998;120:11864–11873. [Google Scholar]
- 13.Schottland S, Bouguettaya M, Chevrot C. Synth Metals. 1999;102:1325. [Google Scholar]
- 14.Syritski V, Reut J, Opik A, Idla K. Synth Metals. 1999;102:1326–1327. [Google Scholar]
- 15.Stella R, Barisci J N, Serra G, Wallace G G, Rossi D D. Sens Actuators. 2000;B63:1–9. [Google Scholar]
- 16.Fäid K, Leclerc M. J Am Chem Soc. 1998;120:5274–5278. [Google Scholar]
- 17.Daves J, Glidle A, Cass A E G, Zhang J, Cooper J M. J Am Chem Soc. 1999;121:4302–4303. [Google Scholar]
- 18.Englebienne P. J Mater Chem. 1999;9:1043–1054. [Google Scholar]
- 19.Okada S, Peng S, Spevak W, Charych D. Acc Chem Res. 1998;31:229–239. [Google Scholar]
- 20.Lu W, Zhao H, Wallace G G. Anal Chem Acta. 1995;315:27–32. [Google Scholar]
- 21.Barisci J N, Hughes D, Minett A, Wallace G G. Anal Chem. 1998;371:39–48. [Google Scholar]
- 22.Torres-Rodriguez L M, Billion M, Roget A, Biden G. Anal Chem. 1999;102:1328–1329. [Google Scholar]
- 23.Biden G, Billion M, Livahe T, Mathis G, Roget A, Torres-Rodriguez L M. Synth Metals. 1999;102:1363–1365. [Google Scholar]
- 24.Cosnier S, Stoytcheva M, Senillou A, Perrot H, Furriel R P M, Leone F A. Anal Chem. 1999;71:3692–3697. doi: 10.1021/ac9901788. [DOI] [PubMed] [Google Scholar]
- 25.Cosnier S, Gondran C, Senillou A. Synth Metals. 1999;102:1366–1367. [Google Scholar]
- 26.Chen L, McBranch D W, Wang H L, Helgeson R, Wudl F, Whitten D G. Proc Natl Acad Sci USA. 1999;96:12287–12292. doi: 10.1073/pnas.96.22.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pande R, Kamtekar S, Ayyagari M S, Kamath M, Marx K A, Kumar J, Tripathy S K, Kaplan D L. Bioconj Chem. 1996;7:159–164. doi: 10.1021/bc950086z. [DOI] [PubMed] [Google Scholar]
- 28.Livache T, Roget A, Dejean E, Barthet C, Biden G, Teoule R. Synth Metals. 1995;71:2143–2146. [Google Scholar]
- 29.Emge A, Bauerle P. Synth Metals. 1999;102:1370–1373. [Google Scholar]
- 30.Garnier F, Korri-Youssoufi H, Srivastava P, Mandrand B, Delair T. Synth Metals. 1999;100:89–94. [Google Scholar]
- 31.Wang J, Jiang M, Fortes A, Mukherjee B. Anal Chem Acta. 1999;402:7–12. [Google Scholar]
- 32.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current Protocols in Immunology. New York: Wiley; 1998. [Google Scholar]
- 33.Heeger P S, Heeger A J. Proc Natl Acad Sci USA. 1999;96:12219–12221. doi: 10.1073/pnas.96.22.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Q, Swager T M. J Am Chem Soc. 1995;117:7017–7018. [Google Scholar]
- 35.Wang J, Wang D, Miller E K, Moses D, Bazan G C, Heeger A J. Macromolecules. 2000;33:5153–5158. [Google Scholar]
- 36.Lakowicz J R. Principles of Fluorescence Spectroscopy. 2nd Ed. New York: Kluwer/Plenum; 1999. [Google Scholar]
- 37.Wang D. Ph.D. thesis. Santa Barbara: Univ. of California; 2001. [Google Scholar]
- 38.Benichou G, Valujskikh A, Heeger P S. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 39.Wang D, Wang J, Moses D, Bazan G C, Heeger A J. Langmuir. 2001;17:1262–1266. [Google Scholar]
- 40.Chen L, Xu S, McBranch D W, Whitten D G. J Am Chem Soc. 2000;122:9302–9303. [Google Scholar]
- 41.Chen L, McBranch D W, Wang R, Whitten D G. Chem Phys Lett. 2000;330:27–33. [Google Scholar]
- 42.Wang D, Lal J, Moses D, Bazan G C, Heeger A J. Chem Phys Lett. 2001;348:411–415. [Google Scholar]
- 43.Wang, D., Moses, D., Bazan, G. C., Heeger, A. J. & Lal, J. (2001) J. Macromol. Sci., in press.
- 44.Jones R M, Bergstedt T S, McBranch D W, Whitten D G. J Am Chem Soc. 2001;123:6726–6727. doi: 10.1021/ja0157797. [DOI] [PubMed] [Google Scholar]