Abstract
Background
To evaluate contrast sensitivity function (CSF) in convalescent Vogt-Koyanagi-Harada (VKH) disease and investigate the relationship between CSF and chorioretinal thickness in VKH patients with and without sunset glow fundus (SGF).
Methods
This is a cross-sectional study. Seventy-six eyes of VKH patients and 56 eyes of normal controls were evaluated. Patients were divided into SGF and non-SGF groups. The best-corrected visual acuity (BCVA) of all the participants was ≤0.0 logMAR. Their CSF and macular chorioretinal thickness were measured with quantitative CSF (qCSF) and Optical Coherence Tomography (OCT) and compared using repeated measures analysis of variance at the group level. Relationships between CSF and macular chorioretinal thickness were evaluated using generalized estimating equations.
Results
The CSF was significantly impaired in the SGF group compared to that in the control group (p = 0.001), especially at medium and high spatial frequencies. No significant CSF difference was found between the non-SGF group and control group, nor between the SGF and non-SGF groups. Compared to the controls, outer retinal thickness (ORT) in both VKH subgroups was significantly reduced (P < 0.001 or 0.005, respectively), although their outer nuclear layer thickness (ONLT) and choroidal thickness (CT) were not significantly different (both P = 1.000, P = 0.829 or 0.112, respectively). We found no significant correlation between CSF metrics and outer retinal thickness.
Conclusions
Despite good recovery of visual acuity, reduced CSF and outer retina thickness were found in convalescent VKH patients. CSF may be an important and sensitive metric to evaluate functional vision in VKH disease.
Subject terms: Autoimmune diseases, Uveal diseases
Background
Vogt-Koyanagi-Harada (VKH) disease is an autoimmune disorder that affects the eyes, auditory systems, meninges and skin [1, 2]. It is one of the most common uveitis entities in China, accounting for about 12.7% of uveitis patients [3]. The chronic recurrent inflammation caused by VKH can lead to serious visual impairments [4–7]. With adequate systemic immunosuppressive therapy, VKH disease can usually develop into the convalescent stage and exhibit a good prognosis [1]. For most patients, vision and retinal structure can be restored as inflammation subsides [8, 9]. However, even after adequate systemic immunosuppressive therapy, some patients in the convalescent stage develop sunset glow fundus (SGF) [10, 11], characterized by chorioretinal depigmentation [2]. Recent research found that subclinical inflammation persisted in convalescent VKH disease [12]. Clinically, patients with recovered visual acuity still complain about poor visual quality [13]. A more complete evaluation of visual function in convalescent VKH disease is necessary.
Visual acuity, a measure of spatial resolution of central vision at high contrast, is commonly used to evaluate functional vision in VKH patients [9]. However, it poorly reflects accurate visual functions in real life [14, 15]. Studies have found that although visual acuity returned to normal, recovery of other vision functions, including electrophysiology parameters, visual field and colour vision were delayed or incomplete in VKH patients [16, 17].
The contrast sensitivity function (CSF), a measure of visual sensitivity to patterns of a wide range of spatial frequencies at threshold contrast levels, provides a more comprehensive assessment of spatial vision and is correlated with daily visual functions in real life [18]. Many eye diseases exhibited contrast sensitivity function deficits in their early stage even when visual acuity was normal or nearly normal [19–21] or after recovery of visual acuity [22]. Whether CSF deficits remain in patients in the convalescent stage of VKH, especially those with SGF, is not clear [23].
In addition, it is well known that the integrity of chorioretinal structure was associated with the prognosis of VKH disease [24–26]. Previous studies reported that the subclinical inflammation in VKH disease destroyed the protective barrier formed by retinal pigment epithelium (RPE) [27], which may lead to damaged fundus microstructures, including discontinuous outer retina, RPE or focal atrophy and choroid thinning [28–31]. Lee et al. [4] reported that ocular complications, longer disease duration and uveitis recurrences led to higher degrees of SGF. Others suggested that chorioretinal thickness was critically correlated with ocular inflammation and macular function [28, 32]. Takahashi et al. [30] demonstrated that choroidal thickness (CT) in eyes with no or mild SGF was close to normal eyes, but eyes with severe SGF had thinner choroid than in controls. Zhou et al. [28] found that patients with inactive VKH but impaired outer retina and thickened RPE had poorer best-corrected visual acuity (BCVA) and worse retinal sensitivity. We hypothesized that convalescent VKH patients, even those with good visual acuity, may have CSF deficits, and their CSF may be correlated with SGF and chorioretinal thickness.
Methods
Subjects
The study protocol was approved by the institutional review board of the Affiliated Eye Hospital of Wenzhou Medical University and adhered to the tenets of the Declaration of Helsinki. Written informed consent including consent to publish photographs was obtained from every participant before the study. A total of 38 subjects (mean age 41.1 ± 9.7 years, 42% male) with diagnosis of convalescent VKH disease and 28 normal controls (mean age 38.5 ± 11.9 years, 32% male) participated in the study between June 2020 and September 2021 in the Affiliated Eye Hospital of Wenzhou Medical University. The normal controls were staff members and graduate students recruited from the eye hospital.
VKH disease was diagnosed according to revised diagnostic criteria established by the International Nomenclature Committee [33, 34]. All patients were treated with a 1–2 weeks initial course of oral corticosteroid (1–1.2 mg/kg/day) and tapered dosage following the resolution of inflammation. The treatment was usually discontinued after at least 12 months. In this study, we only recruited patients with good visual acuity, who had mild disease and were sensitive to systemic steroids therapy. Immunomodulatory agents were not used in this study according to the standard practice for acute VKH [35]. The convalescent stage was defined as: disease duration > 3 months, no acute anterior inflammation, and absence of posterior active inflammatory signs such as retinal detachment, cystoid macula oedema, or disc oedema. The duration was defined as the interval between symptom onset and the examination time. The inclusion criteria for the VKH patients were: (1) age from 18 to 65 years, (2) clinical diagnosis of convalescent VKH disease, (3) no obvious cataract or other ocular diseases, and (4) BCVA ≤ 0.0 logMAR. Exclusion criteria for the participants were: (1) any evidence of systemic disease or cognitive disorder that may impair vision, (2) −6.00 dioptres (D) >spherical equivalent refractive error (SE) > +3.00 D. The diagnosis of SGF was determined by two experienced uveitis clinicians (DL and MLD) based on fundus photographs (CR-2AF, Canon Inc., Japan). The colour fundus photographs were commonly used in the diagnosis of SGF [30, 36]. Any disagreement was adjudicated by a third specialist (YQW).
All participants received a complete ocular examination, including BCVA, slit-lamp examination, CSF (MCVM; Adaptive Sensory Technology, San Diego, California, USA), optical coherence tomography (OCT) (Heidelberg Engineering, Heidelberg, Germany) and axial length (Lenstar LS 900, Haag Streit, Koeniz, Switzerland). BCVA was measured with the standard logarithmic visual acuity chart. [37] All participants had a duochrome (red-green) test.
Contrast sensitivity function
The CSF was measured with the Manifold Contrast Vision Meter (Adaptive Sensory Technology, San Diego, California, USA), which uses quantitative CSF, a Bayesian active learning algorithm, to measure the entire CSF curve [38]. Bandpass-filtered Sloan digits at varying sizes were used in the ten-alternative forced digit identification task [39]. Participants identified the digits monocularly with their best refractive correction in a dark room, at a viewing distance of 3 m. Both eyes were tested. No feedback was provided.
The MCVM reported CS at six spatial frequencies (1, 1.5, 3, 6, 12, 18 cycles per degree [cpd]), the area under log CSF (AULCSF), and CSF acuity. AULCSF was used as the summary statistic of the CSF [40, 41]. CSF acuity represented the high spatial frequency cutoff of the visual system [42].
Optical coherence tomography
Chorioretinal images were taken by a trained ophthalmic photographer using the Heidelberg Spectralis OCT instrument (Heidelberg Engineering, Heidelberg, Germany). The enhanced depth imaging (EDI) mode, centred on the fovea and comprising 1536 A-scans per B-scan, was used, with the eye tracking system monitoring eye positions for clear images. All scans were performed by an experienced operator, who was blinded to the clinical data. Chorioretinal thickness was manually measured by two independent investigators (XNG, YSL) using the virtual ruler tool provided by the Heidelberg Spectralis. Manual measurements were commonly used for quantifying chorioretinal thickness [36, 43]. Subfoveal outer nuclear layer thickness (ONLT) was measured from the internal limiting membrane to the upper boundary of the outer limiting membrane. Subfoveal outer retinal thickness (ORT) was measured from the upper boundary of the outer limiting membrane to the inferior boundary of Bruch’s membrane. Subfoveal CT was measured from the inferior boundary of the Bruch’s membrane to the choroid–scleral interface (Fig. 1). The average thicknesses of two measurements from the OCT horizontal and vertical images were used for statistical analysis.
Fig. 1. Schematic representation of macular structures.
The right panels show the horizontal EDI-OCT image of the left eye of a 40-year-old healthy female. The yellow line indicates the subfoveal outer nuclear layer thickness, the red line indicates the subfoveal outer retinal thickness and the blue line represents the subfoveal choroidal thickness.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS Science, Chicago, IL, USA). Continuous descriptive statistics were represented as mean ± standard deviation. Categorical variables were compared via the Chi-Squared test. Both eyes of the participants were included. Considering the possible correlation between the two eyes, group comparisons were performed using repeated measures analysis of variance (ANOVA) followed by post hoc analysis with Bonferroni correction. For the CSF, the effects of eye, spatial frequency (SF) and group were considered; For the other measures, the effects of eye and group were considered. The correlations between functional and structural metrics were evaluated using generalized estimating equations (GEE) with eye as a within-subject factor. P-value < 0.05 was considered statistically significant.
Results
Clinical characteristics
Seventy-six eyes of 38 VKH patients in the convalescent phase with BCVA ≤ 0.0 logMAR were examined in this study. Patients were divided into two subgroups, the SGF group with 34 eyes from 17 patients and the non-SGF group with 42 eyes from 21 patients. In addition, 56 eyes of 28 normal controls, matched for gender, age, visual acuity, and axial length, were examined. The baseline demographics and characteristics of the participants are described in Table 1. There was no significant difference between the control and VKG groups, nor among the control, non-SGF and SGF groups.
Table 1.
Baseline demographics and characteristics of the eyes.
| Parameters | Control | VKH | Non-SGF | SGF | Pa value | Pb value |
|---|---|---|---|---|---|---|
| Eyes | 56 | 76 | 42 | 34 | ||
| Age (years) | 38.5 ± 11.9 | 41.1 ± 9.7 | 42.5 ± 7.7 | 39.2 ± 11.7 | 0.351 | 0.324 |
| Male/Female | 9/19 | 16/22 | 9/12 | 7/10 | 0.410 | 0.708 |
| Disease duration (months) | NA | 21.6 ± 27.6 | 19.4 ± 19.2 | 24.4 ± 35.8 | NA | 0.977 |
| BCVA (logMAR) | −0.03 ± 0.05 | −0.04 ± 0.06 | −0.04 ± 0.06 | −0.03 ± 0.05 | 0.851 | 0.860 |
| SE | −0.97 ± 1.67 | −1.12 ± 1.72 | −1.02 ± 1.93 | −1.25 ± 1.44 | 0.472 | 0.148 |
| Axial length (mm) | 23.8 ± 1.0 | 23.5 ± 1.1 | 23.5 ± 1.3 | 23.5 ± 0.8 | 0.257 | 0.514 |
SGF sunset glow fundus, BCVA best-corrected visual acuity, SE spherical equivalent refractive error, NA not applicable.
aP = P-value between the control and VKH groups.
bP = P-value among the control, non-SGF and SGF groups.
Contrast sensitivity dysfunction in VKH patients
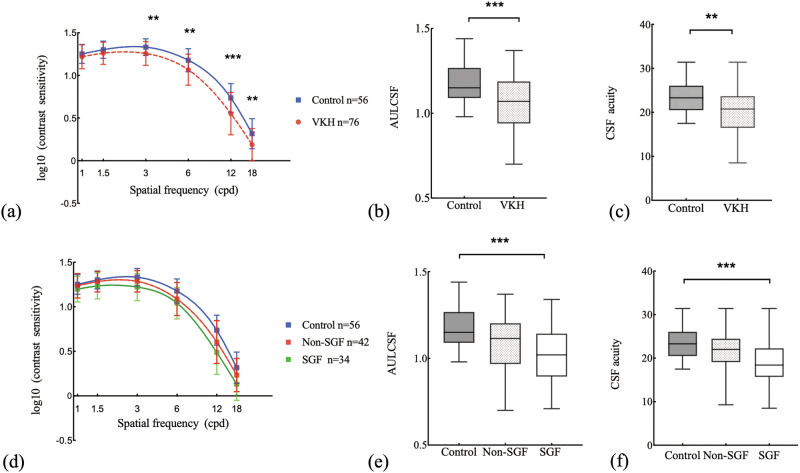
The average CSFs of the VKH patients and controls are plotted in Fig. 2a. Repeated measures ANOVA with eye, group and spatial frequency (SF) as factors revealed significant effects of group (F(1,64) = 11.46, P = 0.001), SF × group interaction (F(1.98, 126.76) = 7.08, P = 0.001), and SF × eye interaction (F(2.19, 140.31) = 5.70, P = 0.03), but no significant effects of eye (F(1, 64) = 0.01, P = 0.921), eye × group interaction (F(1,64) = 0.15, P = 0.703), and eye × group × SF interaction (F(2.19,140.31) = 0.79, P = 0.464). VKH patients had worse CSF than controls, especially at 3, 6, 12 and 18 cpd (Bonferroni corrected pairwise comparisons, all Ps < 0.05).
Fig. 2. Contrast sensitivity functions of the convalescent VKH patients and controls.
a Average contrast sensitivity functions of VKH patients and controls. VKH patients had lower CS, especially in medium and high spatial frequencies. b, c AULCSF and CSF acuity of the two groups. d Average contrast sensitivity functions of the SGF, non-SGF and control groups. e, f AULCSF and CSF acuity of the three groups. The central lines of the box plots represent medians. The boxes encompass the 25th–75th percentile, with whiskers representing the minimum and maximum. Error bars: standard deviation. *P < 0.05, **P < 0.01, and ***P < 0.001.
There were significant effects of group on AULCSF (F(1,64) = 12.60, P < 0.001) and CSF acuity (F(1,64) = 10.31, P = 0.002) in the VKH group. The mean values of both metrics were markedly decreased in the VKH group than those of the controls (Bonferroni corrected pairwise comparisons, P < 0.05) (Fig. 2).
The average CSFs of the SGF, non-SGF and control groups are shown in Fig. 2. Repeated measures ANOVA revealed significant effects of group (F(2,63) = 7.79, P < 0.001), SF × group interaction (F(3.96,124.58) = 4.22, P = 0.003), and SF × eye interaction (F(2.17,136.53) = 5.67, P = 0.003), but no significant effects of eye (F(1,63) = 0.06, P = 0.815), eye × group interaction (F(2,63) = 0.08, P = 0.921), eye × group × SF interaction (F(4.33,136.53) = 0.76, P = 0.563). As shown in Fig. 2, patients with SGF had the worst CSF. Compared with the controls, the SGF group had the lower CSF, especially at 3, 6, 12 and 18 cpd (Bonferroni corrected post hoc tests, all Ps < 0.05). No statistically significant difference was found between the non-SGF group and controls, nor between the SGF and non-SGF groups.
Considering the SGF, non-SGF and control groups, there were significant effects of the group on AULCSF (F(2,63) = 8.04, P < 0.001) and CSF acuity (F(2,63) = 7.62, P = 0.001). The mean values of both metrics in the SGF group were significantly lower than those of the controls (Bonferroni corrected post hoc tests, both Ps < 0.001). The mean values of both metrics in the non-SGF group were lower than those of the controls, but the differences were not statistically significant (Bonferroni corrected post hoc tests, both Ps > 0.05). No statistically significant difference of either metric was found between the SGF and non-SGF groups (Bonferroni corrected post hoc tests, both Ps > 0.05).
Chorioretinal thickness in VKH patients
Chorioretinal thickness of the VKH patients and controls were analysed with repeated measures ANOVA with eye and group as factors. For the ORT, there was significant effect of group (F(1,64) = 23.13, P < 0.001). However, no significant effects of group in ONLT (F(1,64) = 0.29, P = 0.592) or CT (F(1,64) = 3.89, P = 0.053) were found. That is, VKH patients had significantly decreased ORT compared to the controls (Bonferroni corrected pairwise comparisons, P < 0.001) (Table 2).
Table 2.
Chorioretinal thickness of the convalescent VKH patients and controls.
| Parameters | Control | VKH | Non-SGF | SGF | P-value | Pa-value | Pb-value | Pc-value |
|---|---|---|---|---|---|---|---|---|
| Outer nuclear layer thickness (μm) | 106.31 ± 13.38 | 107.97 ± 13.84 | 107.89 ± 14.21 | 108.07 ± 13.59 | 0.592 | 1.000 | 1.000 | 1.000 |
| Outer retinal thickness (μm) | 112.59 ± 5.41 | 105.55 ± 7.49 | 107.06 ± 5.03 | 103.69 ± 9.47 | <0.001 | 0.005 | <0.001 | 0.237 |
| Choroidal thickness (μm) | 299.94 ± 73.17 | 334.69 ± 78.51 | 343.44 ± 80.89 | 323.88 ± 75.24 | 0.053 | 0.112 | 0.829 | 1.000 |
P = P-value between the control and VKH groups.
aP = P-value between the control and non-SGF groups.
bP = P-value between the control and SGF groups.
cP = P-value between the SGF and non-SGF groups.
To evaluate the influence of SGF, we compared the chorioretinal thickness of the SGF, non-SGF and control groups. Repeated measures ANOVA revealed a significant ORT difference between the groups (F(2,63) = 13.55, P < 0.001), but no significant ONLT (F(2,63) = 0.14, P = 0.866) and CT (F(2,63) = 2.29, P = 0.109) difference. Bonferroni corrected post hoc tests revealed that patients with (P < 0.001) or without (P = 0.005) SGF had thinner outer retina compared with the controls, but no significant difference between the two patient groups (P = 0.237) (Table 2).
Relationship between CSF and chorioretinal thickness
Correlations between CSF metrics and chorioretinal thickness were evaluated using GEE with eye as a within-subject factor. No significant correlation was found between CSF metrics (CS at six spatial frequencies, AULCSF and CSF acuity) and chorioretinal thickness (ONLT, ORT and CT) in the VKH, SGF, and non-SGF groups (Table 3). When we considered the VKH patients and controls as a whole, we found significant correlation between CSF metrics (AULCSF and CSF Acuity) and ORT (β = 0.005, 95%CI = 0.001, 0.008, P = 0.005; β = 0.176, 95%CI = 0.065, 0.287, P = 0.002 respectively).
Table 3.
Correlations between CSF metrics and chorioretinal thickness.
| Parameters | Groups | Outer nuclear layer thickness (μm) | Outer retinal thickness (μm) | Choroid thickness (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | (95%CI) | P-value | β | (95%CI) | P-value | β | (95%CI) | P-value | ||
| AULCSF | VKH | <0.001 | (−0.004, 0.003) | 0.973 | 0.001 | (−0.002, 0.005) | 0.448 | <0.001 | (−0.0004, <0.001) | 0.740 |
| SGF | −0.001 | (−0.006, 0.005) | 0.853 | 0.001 | (−0.003, 0.006) | 0.495 | <0.001 | (−0.0005, 0.001) | 0.417 | |
| Non−SGF | 0.001 | (−0.003, 0.004) | 0.759 | −0.004 | (−0.009, 0.002) | 0.215 | −0.0002 | (−0.001, <0.001) | 0.476 | |
| CSF Acuity | VKH | 0.041 | (−0.048, 0.130) | 0.370 | 0.103 | (−0.022, 0.228) | 0.106 | 0.004 | (−0.010, 0.018) | 0.558 |
| SGF | 0.069 | (−0.098, 0.236) | 0.420 | 0.137 | (−0.036, 0.310) | 0.120 | 0.009 | (−0.014, 0.032) | 0.455 | |
| Non-SGF | 0.027 | (−0.050, 0.104) | 0.493 | −0.159 | (−0.339, 0.022) | 0.084 | −0.003 | (−0.020, 0.014) | 0.707 | |
Discussion
In this study, we measured CSF and chorioretinal structure in convalescent VKH patients as well as normal participants. We found reduced CSF and thinned outer retina in convalescent VKH patients. Eyes with SGF had more reduced CSF and chorioretinal thickness compared to those without SGF. Compared with controls, CSF was significantly impaired in eyes with SGF, especially at medium and high spatial frequencies. No significant correlation was found between CSF metrics and chorioretinal thickness.
The visual system contains many spatial frequency channels [44]. Whereas low and medium spatial frequency channels are important for object recognition in the real world (objects faces and road signs) [15, 45], high spatial frequency channels are critical for visual details discrimination [18, 45]. Previous research found that, for patients with uveitis, CS measured by the Pelli-Robson chart showed a negative correlation with the Vision Quality of Life questionnaire [46, 47]. The observed CSF deficits in medium and high spatial frequencies suggest VKH patients, especially those with SGF, may adapt to the basic visual demands, but have limitations in resolving the fine details of everyday objects.
We found significant CSF reduction only in eyes with SGF. The results suggest that patients with SGF were more likely to retain CSF dysfunction, despite good recovery of visual acuity. The presence of SGF may be linked to inflammatory recurrence [4, 10, 12]. The RPE cells provide nutrients to photoreceptors and participate in the visual retinoid cycle, which plays an important role in contrast detection [48]. SGF reflects depigmentation of the RPE and choroid, and the deficient protective barriers may affect the function of the foveal cone system [49, 50]. Okamoto et al. [49] found that photopigment regeneration was impaired, and its recovery took more time than focal macular electroretinogram parameters, colour vision and visual field in VKH patients with good visual acuity and intact macular morphology. Nakamura et al. thought the inflammation and serous retinal detachment caused the injury of outer segments of the photoreceptors [51]. Yang et al. [16] inferred that photoreceptor dysfunction may be a direct consequence of VKH disease or a secondary result of damage to the choroid and/or RPE. The impairments of visual function have also been observed in other depigmentary conditions. For example, typical retinitis pigmentosa patients may have potentially good acuity but poor contrast sensitivity [52]. Study have shown that, due to the absence of RPE melanin granules, albino neural retina optical absorption was at least 30% higher than that of normal retina [53]. Albinos are linked with poor visual function and photophobia with persistent photon damage [54]. We speculate that chorioretinal depigmentation may have led to reduced CSF in patients with SGF.
We found that the outer retina of patients with or without SGF was significantly thinner than that of the controls. Moreover, eyes with SGF had the thinnest outer retina, with disease progression. No significant ONLT difference was found among the groups. Although early retinal detachment had recovered in convalescent VKH patients, there may be other microstructural changes. Nakamura et al. evaluated the macular cone density in VKH patients using adaptive optics fundus camera, and they reported that the outer segment of the photoreceptor was damaged, but the final visual acuity was well maintained after 12 months therapy [51]. Zhu et al. reported the outer nuclear layer attenuation, interruption of myoid zone, ellipsoid zone, and outer segments of photoreceptors in convalescent VKH patients [55]. Our results showed that the changes predominantly occurred in ORT. No significant difference in CT was found among patients with or without SGF and healthy controls, consistent with previous studies [30, 56]. Jaisankar et al. showed the choroid remodelled and tended to normalize in VKH patients after systemic therapy [57]. However, Takahashi et al. found that CT was significantly reduced in patients with severe SGF [30]. One possible reason for this discrepancy is that different types of patients were recruited in the two studies. Takahashi et al. included patients with long durations of the disease (more than 3 years) and most patients with severe SGF also exhibited peripapillary atrophy. In this study, we only recruited patients with good visual acuity, whose choroidal impairment and fundus depigmentation were relatively mild.
Nakamura et al. indicated that the presence of subretinal fluid did not delay the functional recovery of photoreceptors in patients with VKH disease [51]. Abu et al. found no association between exudative retinal detachment and the final mean retinal sensitivity of 7.0 dB or better with logistic regression analysis [5]. In addition, the previous macular involvement in retinal detachment during the active phase of our participants was determined by the optical coherence tomography images or the medical record. There were missing data for 10 eyes in the SGF group and for 6 eyes in the non-SGF group. Macula-involving retinal detachment was found in 17 eyes in the SGF group and 32 eyes in the non-SGF group. No macula-involving retinal detachment was found in 7 eyes in the SGF group and 4 eyes in the non-SGF group. The data were analysed by Chi-square test (X2 = 2.05, P = 0.15). There was no significant difference between the two groups. Thus, it is less likely that the previous macular involvement in retinal detachment during the active phase contributed to our results.
Contrast detection relies on intact chorioretinal structure [24, 26, 58]. Correlations between CSF metrics and macular structure have been reported in other eye diseases [19, 24, 26, 59]. In a cross-sectional study, late-stage VKH patients with intact cone outer segment tips line had better retinal sensitivity measured with MP-1 microperimeter [28]. However, our study did not find any significant correlation between CSF metrics (AULCSF and CSF acuity) and structural thickness (ORT, ONLT and CT) in eyes with or without SGF. The relationship between CSF metric and chorioretinal thickness might be more complicated than a simple linear relationship [60]. Although more detailed structure stratification was not performed in this study, we did notice ellipsoid zone loss, discontinuous RPE signal or RPE folds in several patients. Recently, studies showed fundus structure damages in VKH disease at the cellular and ultrastructural levels, or in terms of microvascular circulation [55, 61–63]. More detailed multidimensional approaches might be necessary to further evaluate the relationships between CSF and retinal structure in patients with VKH disease.
Conclusions
To conclude, despite good recovery of visual acuity, reduced CSF and outer retina thickness were found in convalescent VKH patients. CSF may be an important and sensitive metric to evaluate functional vision in VKH disease.
Summary
What was known before
Clinically, patients with recovered visual acuity still complain about poor visual quality.
What this study adds
Despite good recovery of visual acuity, reduced contrast sensitivity function and outer retina thickness were found in convalescent Vogt-Koyanagi-Harada patients.
Author contributions
Design and conduct of the study (YSL, FH, YQW); Data collection (YSL, FYZ, XLY, RRL, XNG, KW, JQW); Analysis and interpretation of data (FH, DL, MLD); Draw pictures (XH, YSL); Manuscript preparation (YSL, FH, ZLL, YQW); Manuscript review (LL, ZLL). All authors read and approved the final manuscript.
Funding
This work was supported by the Scientific Research project of Zhejiang Provincial Education Department of China under Grant Y202045475; Zhejiang Province Natural Science Foundation of China under Grant LY21H180004, LQ20H180006 and LQ21H180011; and the Key R&D Program of Zhejiang Province under Grant 2021C04019.
Data availability
The data used during this study are available from the corresponding author on reasonable request.
Competing interests
LL and ZLL own intellectual property interests on qCSF technology and financial interests in Adaptive Sensory Technology, Inc. LL holds employment at Adaptive Sensory Technology, Inc. The rest of the authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the institutional review board of the Affiliated Eye Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China (No. 2020-037-K-32-01) and adhered to the tenets of the Declaration of Helsinki
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi-Sha Li, Xia Hu.
Contributor Information
Zhong-Lin Lu, Email: zhonglin@nyu.edu.
Yu-Qin Wang, Email: wangyuqin@eye.ac.cn.
References
- 1.Sakata VM, Da Silva FT, Hirata CE, De Carvalho JF, Yamamoto JH. Diagnosis and classification of Vogt-Koyanagi-Harada disease. Autoimmun Rev. 2014;13:550–555. [DOI] [PubMed] [Google Scholar]
- 2.O’keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017;62:1–25. [DOI] [PubMed] [Google Scholar]
- 3.Yang P, Zhong Z, Du L, Li F, Chen Z, Zhu Y, et al. Prevalence and clinical features of systemic diseases in Chinese patients with uveitis. Br J Ophthalmol. 2021;105:75–82. [DOI] [PubMed] [Google Scholar]
- 4.Lee EK, Lee SY, Yu HG. A clinical grading system based on ultra-wide field retinal imaging for sunset glow fundus in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2015;253:359–368. [DOI] [PubMed] [Google Scholar]
- 5.Abu El-Asrar AM, Al Mudhaiyan T, Al Najashi AA, Hemachandran S, Hariz R, Mousa A, et al. Chronic recurrent Vogt-Koyanagi-Harada disease and development of ‘sunset glow fundus’ predict worse retinal sensitivity. Ocul Immunol Inflamm. 2017;25:475–485. [DOI] [PubMed] [Google Scholar]
- 6.Chee SP, Luu CD, Cheng CL, Lim WK, Jap A. Visual function in Vogt-Koyanagi-Harada patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:785–790. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2009;30:943–948. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–292. [DOI] [PubMed] [Google Scholar]
- 9.Abu El-Asrar AM, Al Tamimi M, Hemachandran S, Al-Mezaine HS, Al-Muammar A, Kangave D. Prognostic factors for clinical outcomes in patients with Vogt-Koyanagi-Harada disease treated with high-dose corticosteroids. Acta Ophthalmol. 2013;91:e486–493. [DOI] [PubMed] [Google Scholar]
- 10.Keino H, Goto H, Usui M. Sunset glow fundus in Vogt-Koyanagi-Harada disease with or without chronic ocular inflammation. Graefes Arch Clin Exp Ophthalmol. 2002;240:878–882. [DOI] [PubMed] [Google Scholar]
- 11.Yang P, Ye Z, Du L, Zhou Q, Qi J, Liang L, et al. Novel treatment regimen of Vogt-Koyanagi-Harada disease with a reduced dose of corticosteroids combined with immunosuppressive agents. Curr Eye Res. 2018;43:254–261. [DOI] [PubMed] [Google Scholar]
- 12.Murata T, Sako N, Takayama K, Harimoto K, Kanda K, Herbort CP Jr, et al. Identification of underlying inflammation in Vogt-Koyanagi-Harada disease with sunset glow fundus by multiple analyses. J Ophthalmol. 2019;2019:3853794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang P, Sun M, Liu X, Zhou H, Fang W, Wang L, et al. Alterations of color vision and central visual field in patients with Vogt-Koyanagi-Harada syndrome. J Ophthalmic Inflamm Infect. 2012;2:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wai KM, Vingopoulos F, Garg I, Kasetty M, Silverman RF, Katz R, et al. Contrast sensitivity function in patients with macular disease and good visual acuity. Br J Ophthalmol. 2022;106:839–844. [DOI] [PubMed]
- 15.Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br J Ophthalmol. 1987;71:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Fang W, Wang L, Wen F, Wu W, Kijlstra A, et al. Study of macular function by multifocal electroretinography in patients with Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 2008;146:767–771. [DOI] [PubMed] [Google Scholar]
- 17.Sakata VM, Lavezzo MM, Da Silva FT, Rodriguez EEC, Morita C, Abdallah SF, et al. Full-field electroretinogram behavior in Vogt-Koyanagi-Harada disease: a 24-month longitudinal study in patients from acute onset evaluated with multimodal analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257:2285–2295. [DOI] [PubMed] [Google Scholar]
- 18.Wood RL, Wood JM. The role of contrast sensitivity charts and contrast letter charts in clinical practice. Clin Exp Optom. 2010;78:43–57.
- 19.Joltikov KA, De Castro VM, Davila JR, Anand R, Khan SM, Farbman N, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:BIO277–BIO290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raming K, Gliem M, Charbel Issa P, Birtel J, Herrmann P, Holz FG, et al. Visual dysfunction and structural correlates in Sorsby Fundus Dystrophy. Am J Ophthalmol. 2022;234:274-284. [DOI] [PubMed]
- 21.Tu Y, Jin H, Xu M, Liu W, Hu X, Wang M, et al. Reduced contrast sensitivity function correlated with superficial retinal capillary plexus impairment in early stage of dysthyroid optic neuropathy. Eye Vis. 2023;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oner O, Akca Bayar S, Oto S, Gokmen O, Tekindal MA. Contrast sensitivity in microtropic and anisometropic eyes of successfully treated amblyopes. Turk J Ophthalmol. 2017;47:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souto FMS, Missaka R, Lavezzo MM, Nobrega PFC, Sakata VM, Oyamada MK, et al. Quality of Life (QoL) in Non-Acute Vogt-Koyanagi-Harada Disease (VKHD) at Two Time Points 24 Months Apart. Ocul Immunol Inflamm. 2024;32:384–390. [DOI] [PubMed]
- 24.Wang J, Cui Y, Vingopoulos F, Kasetty M, Silverman RF, Katz R, et al. Disorganisation of retinal inner layers is associated with reduced contrast sensitivity in retinal vein occlusion. Br J Ophthalmol. 2022;106:241–245. [DOI] [PubMed]
- 25.Fatehi N, Nowroozizadeh S, Henry S, Coleman AL, Caprioli J, Nouri-Mahdavi K. Association of structural and functional measures with contrast sensitivity in glaucoma. Am J Ophthalmol. 2017;178:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joltikov KA, Sesi CA, De Castro VM, Davila JR, Anand R, Khan SM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5481–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000;19:41–68. [DOI] [PubMed] [Google Scholar]
- 28.Zhou M, Jiang C, Gu R, Sun Z, Huynh N, Chang Q. Correlation between retinal changes and visual function in late-stage Vogt-Koyanagi-Harada disease: an optical coherence tomography study. J Ophthalmol. 2015;2015:916485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai K, Gomi F, Ikuno Y, Yasuno Y, Nouchi T, Ohguro N, et al. Choroidal observations in Vogt-Koyanagi-Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250:1089–1095. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Takase H, Ishizuka A, Miyanaga M, Kawaguchi T, Ohno-Matsui K, et al. Choroidal thickness in convalescent Vogt-Koyanagi-Harada disease. Retina. 2014;34:775–780. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M, Gu RP, Sun Z, Jiang CH, Chang Q, Xu GZ. Differences in photoreceptor recovery among patients and between different parts of the posterior pole in Vogt-Koyanagi-Harada disease. Eye. 2018;32:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jap A, Chee SP. The role of enhanced depth imaging optical coherence tomography in chronic Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2017;101:186–189. [DOI] [PubMed] [Google Scholar]
- 33.Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652. [DOI] [PubMed] [Google Scholar]
- 34.Yang P, Zhong Y, Du L, Chi W, Chen L, Zhang R, et al. Development and evaluation of diagnostic criteria for Vogt-Koyanagi-Harada disease. JAMA Ophthalmol. 2018;136:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama M, Keino H, Watanabe T, Okada AA. Clinical features and visual outcomes of 111 patients with new-onset acute Vogt-Koyanagi-Harada disease treated with pulse intravenous corticosteroids. Br J Ophthalmol. 2019;103:274–278. [DOI] [PubMed] [Google Scholar]
- 36.Hirooka K, Saito W, Namba K, Mizuuchi K, Iwata D, Hashimoto Y, et al. Early post-treatment choroidal thickness to alert sunset glow fundus in patients with Vogt-Koyanagi-Harada disease treated with systemic corticosteroids. PLoS ONE 2017;12:e0172612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang QM, Wanf CX, Ye TT. Standard for logarithmic visual acuity charts. Beijing: Standards Press of China. 2012;56:1–4.
- 38.Lu LesmesLA, Baek ZL, Albright J. TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vision. 2010;10:11–21. 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Shen M, He X, Cui R, Lesmes LA, Lu ZL, et al. Comparing spatial contrast sensitivity functions measured with digit and grating stimuli. Transl Vis Sci Technol. 2019;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou F, Huang CB, Lesmes L, Feng LX, Tao L, Zhou YF, et al. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest Ophthalmol Vis Sci. 2010;51:5365–5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshika T, Okamoto C, Samejima T, Tokunaga T, Miyata K. Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes. Ophthalmology. 2006;113:1807–1812. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vis Res. 2006;46:739–750. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Z, Zhang N, Ji H, Zhu M, Zhou M, Dong J. Correlation between serum immunoglobulin levels and retinal structure in patients with newly diagnosed Vogt‑Koyanagi‑Harada disease. Mol Med Rep. 2022;26:291–296. [DOI] [PMC free article] [PubMed]
- 44.Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968;197:551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonova A, Pokorny J, Smith VC. Spatial frequency processing in inferred PC- and MC-pathways. Vis Res. 2003;43:2133–2139. [DOI] [PubMed] [Google Scholar]
- 46.Gardiner AM, Armstrong RA, Dunne MC, Murray PI. Correlation between visual function and visual ability in patients with uveitis. Br J Ophthalmol. 2002;86:993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy CC, Hughes EH, Frost NA, Dick AD. Quality of life and visual function in patients with intermediate uveitis. Br J Ophthalmol. 2005;89:1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto Y, Miyake Y, Horio N, Takakuwa H, Yamamoto E, Terasaki H. Delayed regeneration of foveal cone photopigments in Vogt-Koyanagi-Harada disease at the convalescent stage. Invest Ophthalmol Vis Sci. 2004;45:318–322. [DOI] [PubMed] [Google Scholar]
- 50.Seagle BL, Rezai KA, Gasyna EM, Kobori Y, Rezaei KA, Norris JR Jr. Time-resolved detection of melanin free radicals quenching reactive oxygen species. J Am Chem Soc. 2005;127:11220–11221. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T, Hayashi A, Oiwake T. Recovery of macular cone photoreceptors in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2018;256:387–394. [DOI] [PubMed] [Google Scholar]
- 52.Lepri BP. Is acuity enough? Other considerations in clinical investigations of visual prostheses. J Neural Eng. 2009;6:035003. [DOI] [PubMed] [Google Scholar]
- 53.Guo Y, Yao G, Lei B. Monte Carlo model for studying the effects of melanin concentrations on retina light absorption. J Opt Soc Am A. 2008;25:304–311. [DOI] [PubMed] [Google Scholar]
- 54.Neveu MM, Padhy SK, Ramamurthy S, Takkar B, Jalali S, Cp D, et al. Ophthalmological manifestations of oculocutaneous and ocular albinism: current perspectives. Clin Ophthalmol 2022;16:1569–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu R, Zhang J, Qiao L, Zhang Y, Gu X, Yang L. Photoreceptor cell injury starts in the initial stage of Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 2018;26:934–942. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Huang J, Chen Y, Ying GS. Evaluation of approaches to analyzing continuous correlated eye data when sample size is small. Ophthalmic Epidemiol. 2018;25:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaisankar D, Raman R, Sharma HR, Khandelwal N, Bhende M, Agrawal R, et al. Choroidal and retinal anatomical responses following systemic corticosteroid therapy in Vogt-Koyanagi-Harada disease using swept-source optical coherence tomography. Ocul Immunol Inflamm. 2019;27:235–243. [DOI] [PubMed] [Google Scholar]
- 58.Mcanany JJ, Park JC, Liu K, Liu M, Chen YF, Chau FY, et al. Contrast sensitivity is associated with outer-retina thickness in early-stage diabetic retinopathy. Acta Ophthalmol. 2020;98:e224–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baldwin G, Vingopoulos F, Garg I, Moon JY, Zeng R, Cui Y, et al. Structure-function associations between contrast sensitivity and widefield swept-source optical coherence tomography angiography in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2023;261:3113–3124. [DOI] [PubMed]
- 60.Zhang Y, Yang L, Gao Y, Zhang D, Tao Y, Xu H, et al. Choroid and choriocapillaris changes in early-stage Parkinson’s disease: a swept-source optical coherence tomography angiography-based cross-sectional study. Alzheimers Res Ther. 2022;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan S, Lin D, Hu J, Cao J, Wu K, Li Y, et al. Evaluation of microvasculature alterations in convalescent Vogt-Koyanagi-Harada disease using optical coherence tomography angiography. Eye. 2021;35:1993–1998. [DOI] [PMC free article] [PubMed]
- 62.Huang Y, Yang YT, Lin B, Huang SH, Sun ZH, Zhou R, et al. Melanin change of retinal pigment epithelium and choroid in the convalescent stage of Vogt-Koyanagi-Harada disease. Int J Ophthalmol. 2020;13:1928–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miura M, Makita S, Yasuno Y, Azuma S, Mino T, Yamaguchi T, et al. Objective evaluation of choroidal melanin loss in patients with Vogt-Koyanagi-Harada disease using polarization-sensitive optical coherence tomography. Sci Rep. 2022;12:3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used during this study are available from the corresponding author on reasonable request.