Abstract
In Escherichia coli, DnaC is essential for loading DnaB helicase at oriC (the origin of chromosomal DNA replication). The question arises as to whether this model can be generalized to other species, since many eubacterial species fail to possess dnaC in their genomes. Previously, we have reported the characterization of HpDnaB (Helicobacter pylori DnaB) both in vitro and in vivo. Interestingly, H. pylori does not have a DnaC homologue. Using two different E. coli dnaC (EcdnaC) temperature-sensitive mutant strains, we report here the complementation of EcDnaC function by HpDnaB in vivo. These observations strongly suggest that HpDnaB can bypass EcDnaC activity in vivo.
Keywords: DNA replication, helicase, loader, oriC (origin of chromosomal DNA replication)
Abbreviations: DTT, dithiothreitol; dnaBts, dnaB temperature-sensitive; dnaCts, dnaC temperature-sensitive; EcDnaB/C, Escherichia coli DnaB/C; GST, glutathione S-transferase; HpDnaB(wt/mut), wild-type or mutant Helicobacter pylori DnaB respectively; oriC, origin of chromosomal DNA replication; ORF, open reading frame; PaDnaB, Ps. aeruginosa DnaB
INTRODUCTION
The classical model of DNA replication initiation at oriC (origin of chromosomal DNA replication) in Escherichia coli starts with the binding of bacterial replication initiator protein DnaA to the DnaA boxes, followed by unwinding of the nearby AT-rich sequences to form an open complex [1]. Subsequently, a hexameric (DnaB–DnaC)6 complex is recruited at the oriC, with the help of the DnaA protein [2]. However, DnaB cannot be loaded at the oriC alone. After loading, DnaC protein is released, with the concomitant hydrolysis of ATP [3]. Finally, DnaB exerts its 5′→3′ helicase activity with the help of ATP, which results in strand separation [4]. For detailed molecular mechanisms behind helicase recruitment and loading in prokaryotes, see a recent review [5].
Although DnaB is conserved in bacteria, only HpDnaB (Helicobacter pylori DnaB) has been characterized both in vitro and in vivo to date [6], in addition to EcDnaB (the E. coli DnaB helicase). Cloning and characterization of DnaB has been reported from Thermus aquaticus [7], Pseudomonas putida and Pseudomonas aeruginosa [8]. However, these dnaB genes fail to complement EcdnaBts (E. coli dnaB temperature-sensitive) mutant strains. In contrast, HpdnaB can complement EcdnaBts strains efficiently [6].
Unlike DnaB, DnaC is not as well conserved among bacteria. Some bacteria, such as Salmonella typhimurium, Neisseria gonorrhoeae and Klebsiella pneumoniae, contain a dnaC-like gene [8]. A homologue of dnaC is absent from many other recently sequenced bacterial genomes, suggesting that DnaC function may not be universal. It is important to note that there are many examples of proteins that perform similar functions at the replication forks without sharing significant sequence homology [9]. The DnaC and λP accessory proteins having no homology can recruit DnaB helicase at the replication origins of E. coli and bacteriophage λ respectively [9]. In bacteriophage T4, gp59 protein serves as a helicase loader for gp41 helicase [10]. In higher eukaryotes, Cdc6 has been proposed to load MCM (mini-chromosome maintenance) complex [9]. One aspect common to these helicase loaders is that all of them contain nucleotide binding and hydrolysis domains.
It will be misleading to assume that an accessory protein is always required to load helicases, based on the E. coli model; no other bacterium has been studied thoroughly in vitro and in vivo in this regard. A recent study in Pseudomonas nicely demonstrated that, although the E. coli and Pseudomonas helicases share approx. 80% similarity at the amino acid level, EcDnaC (E. coli DnaC) is not required to load Pseudomonas DnaB at the origin of plasmid RK2 in vitro [8]. However, this study failed to show any in vivo data.
To investigate the issue of the requirement of the loader protein, we have employed a combined biochemical and genetic approach using H. pylori and E. coli systems. Unlike Pseudomonas dnaB, HpdnaB complements a dnaBts strain of E. coli [6]. Investigation of the H. pylori genomic database fails to identify a DnaC homologue, or an ORF (open reading frame) with a nucleotide-binding domain of similar molecular mass to that of DnaC, suggesting that H. pylori might not require an accessory protein. Conditional lethal mutants of replication proteins in H. pylori are not available yet. We used the E. coli mutants for the complementation studies, and we hypothesized that, if a DnaC loader function is not required for the loading of HpDnaB, HpdnaB should be able to complement EcdnaCts (E. coli dnaC temperature-sensitive) strains in vivo.
Here we show that, although HpDnaB can functionally complement EcDnaB function in vivo, it does not interact physically with EcDnaC in a GST (glutathione S-transferase) pull-down experiment or in yeast two-hybrid analysis. Furthermore, HpDnaB can complement two different EcdnaCts strains, suggesting that HpDnaB can bypass EcDnaC function in vivo. The presence of an intact HpdnaB gene, and the expression of the protein in the dnaCts strains at the restrictive temperature, ruled out the possibility of any recombination effect. These results strongly suggest that a DnaC-like accessory protein may not be universally required for loading bacterial replicative helicases.
MATERIALS AND METHODS
Bacterial strains and plasmid construction
H. pylori (strain 26695) genomic DNA and specific primers (as shown in Table 1) were used for PCR in order to amplify the coding regions of wild-type HpdnaB (HpdnaBwt; 1.5 kb). The amplified products were cloned at the BamHI site of pET28a expression vector, as described previously [6]. HpdnaB was also cloned into pGEX2T to produce a GST-tagged protein. The coding regions of the EcdnaB and EcdnaC genes were PCR-amplified using E. coli genomic DNA and specific primers (Table 1), and subsequently cloned at the BamHI site of pGEX2T or pET28a respectively. Primers for the PCR reactions and the strains and plasmids used in the present study are shown in Tables 1 and 2.
Table 1. Primers list.
FW, forward primer; RV, reverse primer.
| Primers | Primer sequence |
|---|---|
| HpDnaB FW | 5′-GCGGATCCATGAAAAACGTTGGCGACCTG-3′ |
| HpDnaB RV | 5′-GCGGATCCTCAAGTTGTAACTATATCATAA-3′ |
| EcDnaB FW | 5′-CGGGATCCAATGGCAGGAAATAAACCCTTC-3′ |
| EcDnaB RV | 5′-CGGGATCCTCATTCGTCGTCGTACTGCGGCC-3′ |
| EcDnaC FW | 5′-GCGGATCCATGAAAAACGTTGGCGACCTG-3′ |
| EcDnaC RV | 5′-GCGGATCCATACTCTTTACCTGTTACCCG-3′ |
Table 2. Bacterial strains and plasmids used in this work.
| Strain/plasmid | Genotype/relevant characteristics | Reference |
|---|---|---|
| E. coli DH5α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Sambrook et al. [13] |
| DJ58 (EcdnaBts) | Temperature-sensitive mutant of EcdnaB | Gift from Dr Dhruba K. Chattoraj [6] |
| BR1701 (EcdnaCts) | dnaC2 thyA47 leu6 deoC3 strr | Gift from Dr Dhruba K. Chattoraj [19] |
| MG1655dnaC2 (EcdnaCts) | thr:: Tn10 dnaT2dnaC2(Ts) | Gift from Dr Santunu DasGupta [20] |
| pdnaBC | Plasmid for overproduction of EcDnaB and DnaC | Gift from Dr Dhruba K. Chattoraj |
| pdnaB | Plasmid for overproduction of EcDnaB | The present study |
| pET28a | T7, his, kanR | Novagen |
| pET28a HpdnaB (wild-type/mutant) | pET28a derivative containing 1.5 kb of HpdnaB/point mutation in the | Soni et al. [6] |
| Walker A motif | ||
| pGEX2T EcdnaB | pGEX2T derivative containing 1.4 kb of EcdnaB | This work |
| pGEX2T HpdnaB | pGEX2T derivative containing 1.5 kb of HpdnaB | This work |
| pET28a EcdnaC | pET28a derivative containing 753bp EcdnaC | This work |
| pBR322 | tetR, ampR | Bolivar et al. [12] |
| BL21 (DE3) | F− ompT hsdSB (rB− mB) gal dcm BL21 (DE3) | Novagen |
| pBR-HpdnaB (wild-type/mutant) | pBR derivative containing 1.5 kb of HpdnaB/point mutation in the Walker A motif | Soni et al. [6] |
pdnaBC and pdnaB plasmids were kindly given by Dr Dhruba K. Chattoraj (National Institutes of Health, Bethesda, MD, U.S.A.). In brief, in pdnaBC, the coding regions of EcdnaC and EcdnaB were cloned under the control of bacteriophage λ promoters PR and PL (PR–PL) [11] to direct co-transcription of these genes in a synthetic operon. This plasmid construct contains a short 23 bp linker separating the ochre stop codon of the dnaC gene and the synthetic ribosome-binding site fused upstream of dnaB. pdnaB contains the coding region of EcdnaB under the same PR–PL promoters.
pBR-HpdnaBwt and pBR-HpdnaBmut (containing a point mutation in the Walker A nucleotide-binding domain of HpDnaB) [6] plasmid constructs were made by subcloning the respective genes (including the His6-tag) from the pET28a recombinant clones into pBR322 downstream of the Bla-P2 promoter [12]. The details of the cloning strategies are described elsewhere [6].
Protein purification and helicase assay
GST and His6-tagged proteins were purified as described previously [6]. GST-EcDnaB was purified in the presence of 2 mM ATP and 5 mM MgCl2. Helicase assays were performed as described previously [6]. The substrates for the helicase assays were obtained by annealing a radiolabelled 5′-tailed 23-mer oligonucleotide (5′-CCAAAACCCAGTCACGACGTTGTAAAACG-3′) to M13mp18 DNA, followed by purification of the annealed products, as described elsewhere [6].
Gel-filtration chromatography
Gel-filtration chromatography was performed essentially following the protocol described previously [6]. Samples for gel-filtration chromatography of an equimolar mixture of His6-HpDnaB and His6-EcDnaC were prepared as follows: 200 μg of His6-HpDnaB and 100 μg of His6-EcDnaC were mixed and dialysed against buffer A [50 mM Tris/HCl (pH 7.4)/50 mM NaCl/1mM DTT (dithiothreitol)/1 mM ATP/2 mM MgCl2] at 4 °C. The dialysed sample was centrifuged at 15000 g for 15 min. The supernatant was transferred to a fresh tube, and 200 μl of the supernatant (containing 40 μg of HpDnaB and 20 μg of EcDnaC) was subjected to size-exclusion chromatography on a Bio-Sil SEC 250-5 (Bio-Rad) column. His6-HpDnaB and His6-EcDnaC were also dialysed separately in buffer A for individual gel-filtration chromatography experiments.
Western blot analysis and antibodies
Western blot analysis was performed following standard procedures [13]. Anti-His6 and anti-GST rabbit polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, U.S.A.). Anti-HpDnaB polyclonal antibodies were raised in rabbits using purified HpDnaB as the antigen. Anti-EcDnaC rabbit polyclonal antibodies were obtained from Professor Kenneth Marians (Memorial Sloan-Kettering Cancer Center, New York, NY, U.S.A.).
GST pull-down assay
GST pull-down assays were performed by incubating purified His6-EcDnaC (2 μg) in the presence of either GST–HpDnaB or GST–EcDnaB beads in the binding buffer [50 mM Tris/HCl (pH 7.5)/1 mM DTT/4% (v/v) glycerol/0.1 mg of BSA/5 mM MgCl2/1 mM ATP/50 mM NaCl] at 4 °C for 1 h with gentle rotation. The beads were then washed three times with binding buffer containing 250 mM NaCl, and the bound proteins were analysed by SDS/PAGE followed by Western blot analysis.
Complementation assay
E. coli temperature-sensitive mutant strains DnaC2 and BR1701 (dnaCts) and DJ 58 (dnaBts) were transformed with pBR322, pBR-HpdnaBwt, pBR-HpdnaBmut, pdnaB and pdnaBC. The transformed cells were grown either at the permissive (30 °C) or non-permissive (37 °C for DnaC2 and BR1701 cells; 40 °C for DJ58 cells) temperature.
Yeast two-hybrid system
The GAL4 Two-hybrid Phagemid System (Stratagene) was used to perform the yeast two-hybrid experiments. The EcdnaC gene, encoding EcDnaC, was subcloned into the plasmid encoding the transcription activation domain (pAD) of GAL4, and the EcdnaB and HpdnaB genes were subcloned into the DNA-binding domain (pBD) of GAL4 at the BamHI site, generating plasmids pADEcdnaC, pBDEcdnaB and pBDHpdnaB. Recombinant clones were confirmed by restriction digestion and sequencing.
Yeast strain AH109 was co-transformed with 500 ng of plasmids (either a combination of pADEcdnaC and pBDEcdnaB, or pADEcdnaC and pBDHpdnaB, using the lithium acetate method [14]). The transformed yeast cells were then plated on SD agar medium containing 2% (w/v) glucose and either lacking tryptophan and leucine (SD–Trp, –Leu; 2D plates) or lacking leucine, tryptophan, histidine and adenine (SD–Leu, –Trp, –His, –Ade; 4D plates), followed by incubation at 30 °C for 3 days, as described in the GAL4 Two-hybrid Phagemid manual (Stratagene).
To confirm protein–protein interactions, β-galactosidase colony lift filter assays were performed using the yeast β-galactosidase assay kit, as described by the manufacturer (Stratagene). Briefly, colonies were lifted from the 4D plates (see above) using a pre-soaked sterile Whatman #5 filter, by placing it in 2.5–5 ml of β-galactosidase assay buffer [110 mM Na2HPO4/35 mM NaH2PO4 (pH 7.0)/10 mM KCl/1 mM MgSO4,7H2O/20 mM 2-mercaptoethanol)] solution containing X-gal (5-bromo-4-chloroindol-3-yl β-D-galactopyranoside; 20 mg/ml) in a clean 150 mm plate. The filter paper was then frozen completely using liquid nitrogen to permeabilize the cells, followed by incubation of the filter paper at 30 °C for 30 min for the appearance of the blue colour.
RESULTS
Primary structure comparison of HpDnaB with the E. coli and PaDnaB (Ps. aeruginosa DnaB) proteins
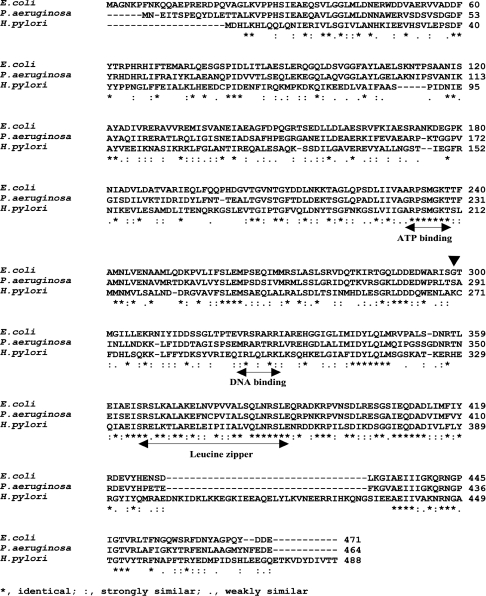
In vitro, PaDnaB was reported to be loaded at the plasmid RK2 ori in the absence of DnaC, but it failed to complement the EcDnaB function in a temperature-sensitive strain [8]. In contrast, HpDnaB, without having an obvious DnaC homologue in the H. pylori genome, can complement EcdnaBts strains. In order to investigate whether HpDnaB has some unique features, the amino acid sequences of HpDnaB, EcDnaB and PaDnaB were aligned (Figure 1). HpDnaB shows 32% identity and 53% similarity with EcDnaB, whereas PaDnaB shows 61% identity and approx. 80% similarity with EcDnaB. The similarity in amino acid residues between EcDnaB and PaDnaB is spread from the N-terminus through to the C-terminus, whereas the C-terminus of HpDnaB shares more homology with EcDnaB than does the N-terminus. The C-terminus of EcDnaB contains important features such as the ATPase domain, DNA-binding domain and leucine zipper motif, which are all conserved in HpDnaB [15].
Figure 1. Primary sequence analysis of HpDnaB.
Alignment of the H. pylori, Ps. aeruginosa and E. coli DnaB primary structures using the CLUSTALW multiple alignment programme. The ATP binding, DNA binding and leucine zipper domains are also shown. ‘▼’ indicates the position of the 299th amino acid residue (glycine) of EcDnaB.
It has been shown previously that the overexpression of EcdnaC can overcome the temperature-sensitive phenotype of the mutant dnaB252 in vivo [16]. Mutation of residue 299 from glycine to aspartic acid was found to be responsible for this effect, suggesting that this residue would be important for DnaB–DnaC interaction [17]. Comparison of this region among the three different DnaB sequences reveals a high degree of divergence, failing to identify any conserved residue that might be essential for DnaB–DnaC interaction. Although the C-terminus of HpDnaB is highly similar to that of EcDnaB, there is a unique insertion of 34 amino acid residues (residues 400–433) that is not present in any DnaB known so far. These differences could make HpDnaB unique and different from EcDnaB.
HpDnaB does not interact with EcDnaC in vitro
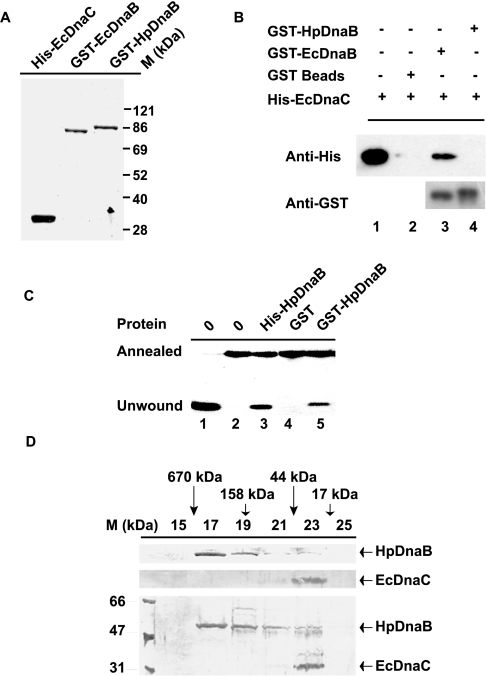
HpdnaB complements EcdnaBts strain at the restrictive temperature [6]. It would be interesting to know whether EcDnaC mediates this rescue event. The non-availability of a dnaC-like gene in the H. pylori genome already suggests that EcDnaC might not be a partner of HpDnaB. To test whether HpDnaB interacts with EcDnaC, we performed GST pull-down experiments, which are often used for the demonstration of direct protein–protein interactions. For this purpose, GST–EcDnaB and GST–HpDnaB proteins were purified through binding to GST beads, and His6-EcDnaC was purified as a soluble protein (Figure 2A). His6-EcDnaC was incubated in the presence of either the GST–EcDnaB or GST–HpDnaB beads. After washing the beads carefully, proteins were released by boiling them in loading buffer, and analysed by SDS/PAGE followed by Western blotting analysis using anti-His6 antibodies. We found that the binding of EcDnaC to GST–EcDnaB beads was very specific compared with the control GST or GST–HpDnaB beads. The equal loading of GST–HpDnaB and GST–EcDnaB was shown by probing the same blot with anti-GST antibodies (Figure 2B).
Figure 2. HpDnaB does not interact with E. coli DnaC in vitro.
(A) Purification of GST–EcDnaB, GST–HpDnaB and His6–EcDnaC. EcDnaB and HpDnaB were purified as GST fusion proteins, and EcDnaC was purified as a His6 fusion protein. The purity of the proteins was checked by SDS/PAGE analysis. (B) GST pull-down experiment: GST (lane 2) or GST–EcDnaB (lane 3) or GST–HpDnaB beads (lane 4) were incubated in the presence of purified His6-EcDnaC. Proteins bound to the beads were subjected to SDS/PAGE, followed by Western blot analysis using anti-His6 and anti-GST antibodies. GST beads were used as a control. Lane 1 contains purified His6-EcDnaC protein as the input (5%). (C) Helicase activity of the purified proteins, as indicated on the top of the panel. The details of the substrates used for the helicase assay have been described in the materials and methods section. All the proteins were tested for helicase activity following the protocol described previously [6]. Lane 1 contains heated sample to show the complete unwinding of the radiolabelled oligonucleotides. Lanes 1–2 do not contain protein; lanes 3–5 contain His6-HpDnaB, GST and GST–HpDnaB respectively. (D) Gel-filtration chromatography of His6-HpDnaB, His6-EcDnaC and an equimolar mixture of His6-HpDnaB and His6-EcDnaC. The proteins were passed through a Bio-Sil SEC 250-5 column, and 0.5 ml fractions were collected in each case, followed by SDS/PAGE and silver staining of the gel containing alternate fractions (15–25), as shown along the top. The positions of the gel-filtration standards are shown along the top as follows: thyroglobulin (670 kDa; fraction 16), bovine γ-globulin (158 kDa; fraction 19), chicken ovalbumin (44 kDa; fraction 22) and horse myoglobin (17 kDa; fraction 24).
The question arises as to whether the inability of GST–HpDnaB to interact with EcDnaC in the pull-down experiments is due to the differential effect on the HpDnaB–EcDnaC interaction due to the addition of GST tags at the N-terminus. In order to find out whether GST–HpDnaB is functionally active, we checked the helicase activity of GST–HpDnaB. GST–HpDnaB showed helicase activity with no activity in the GST control lane, suggesting that the GST tag at the N-terminus did not affect the conformation related to the in vitro activity of the protein (Figure 2C). It is unlikely that the addition of the GST tag has specifically protected the EcDnaC-interacting domain of HpDnaB, but not that of EcDnaB, while these proteins have been treated the same way. It is important to note in this regard that His6-HpDnaB was used for both the in vitro helicase assay (Figure 2C) and in vivo rescue experiments (see Figures 4A and 4B), and His6-HpDnaB could rescue the temperature-sensitive phenotypes, effectively suggesting that the addition of the tags did not affect the in vivo activity of these proteins.
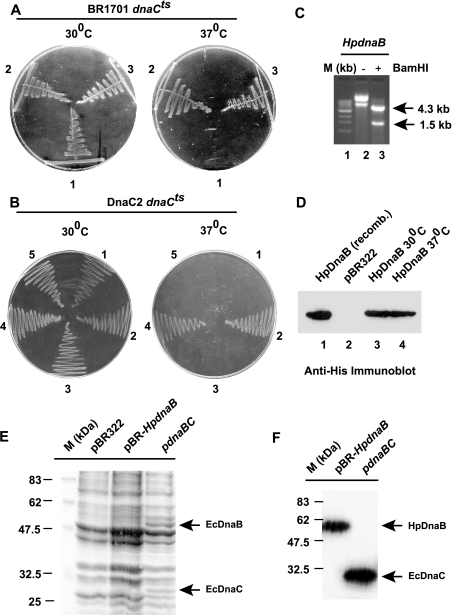
Figure 4. Complementation of E. coli dnaCts strains.
(A) Complementation of BR1701 E. coli strain dnaCts. BR1701 dnaCts cells were transformed with either pBR322 (1), or pdnaBC (E. coli) (2) or pBR-HpdnaBwt (3). Cells were plated on to LB agar plates and incubated either at the permissive temperature (30 °C) or at a non-permissive temperature (37 °C). (B) Complementation of the DnaC2 mutant strain. E. coli DnaC2 mutant cells were transformed with either pBR322 (1) or pdnaBC (2) or pdnaB (E. coli) (3), or pBR-HpdnaBwt (4) or pBR-HpdnaBmut (5). Cells were grown either at 30 °C or at 37 °C. (C) Plasmid DNA isolated from DnaC2 cells grown at 37 °C was subjected to BamHI restriction enzyme digestion, followed by agarose gel-electrophoresis analysis. Lane 1 (M) shows a 1 kb ladder used as a marker; lane 2 shows undigested plasmid DNA and lane 3 indicates DNA digested with BamHI. (D) Detection of HpDnaB expression in DnaC2 cells by immunoblotting using anti-His6 antibody. Lane 1, purified HpDnaB; lanes 2 and 3, extract from pBR322- and HpdnaB-transformed cells respectively, grown at 30 °C; lane 4, extract from HpdnaB-transformed cells grown at 37 °C. (E) SDS/PAGE analysis of bacterial lysate (100 μg each lane) from DnaC2 temperature-sensitive cells transformed with pBR322 (grown at 30 °C) or pBR-HpdnaB or pdnaBC (grown at 37 °C). The gel was subsequently stained with Coomassie Brilliant Blue to visualize the proteins. Molecular mass (M) markers are also shown. (F) Detection of EcDnaC and HpDnaB expression in DnaC2 cells transformed with either pBR-HpdnaB or pdnaBC (grown at 37 °C), followed by SDS/PAGE analysis and immunoblotting using rabbit polyclonal anti-EcDnaC and anti-HpDnaB antibodies.
Previously, we claimed that HpDnaB might have an affinity towards EcDnaC, since His6-HpDnaB and His6-EcDnaC co-eluted in a fraction following gel-filtration chromatography [6]. The co-elution of HpDnaB and EcDnaC was detected by Western blot analysis using anti-His6 antibodies. However, following repeated gel-filtration chromatography and critical evaluation of the results, we found that the amount of EcDnaC present in the same fraction containing HpDnaB was not stoichiometric. We have already shown that HpDnaB exists as a hexamer in solution [6]. In general, six monomers of EcDnaC bind to the hexameric EcDnaB to form a (EcDnaB–EcDnaC)6 complex. In the present study, by mixing His6-HpDnaB and His6-EcDnaC in an equimolar ratio, followed by gel-filtration and silver staining of the gel containing alternate fractions from the gel-filtration chromatography, we found that the peak fractions of HpDnaB and EcDnaC do not co-elute (Figure 2D, bottom panel). Although we see the presence of both HpDnaB and EcDnaC in fractions 21–23, we believe that this is not due to the formation of a stoichiometric HpDnaB–EcDnaC complex, since the peak fractions of hexameric HpDnaB (fraction 17) and monomeric EcDnaC (fraction 23) are far apart. When His6-HpDnaB and His6-EcDnaC were subjected to gel-filtration chromatography separately, the majority of these proteins were found to be present in fractions 17 and 23 respectively (Figure 2D, upper two panels). It has been reported previously [8] that the peak fractions of EcDnaB and EcDnaC overlap with each other when a mixture of EcDnaB and EcDnaC is subjected to gel-filtration chromatography under the same experimental conditions as those described here. These results taken together suggest that HpDnaB does not interact with EcDnaC in vitro.
EcDnaB, but not HpDnaB, interacts specifically with EcDnaC in a yeast two-hybrid system
To investigate the potential interactions between EcDnaB and HpDnaB with EcDnaC in an in vivo environment, we used a yeast two-hybrid system (the GAL4 Two-hybrid Phagemid ssytem; Stratagene). In this system, the yeast GAL4 transcription activator has been divided into the following two separate functional domains: (i) the transcription activation domain (AD) present on plasmid pAD-GAL4-2.1 (pAD), which encodes the LEU2 gene as a selectable marker; and (ii) the DNA-binding domain (BD) present on the plasmid pBD-GAL4-Cam (pBD), which encodes the TRP1 gene as a selectable marker [18]. If two fusion proteins interact in this system, they will bring into close proximity the GAL4-transcription activation domain and the GAL4 DNA-binding domain to the GAL4 promoters, which in turn will initiate the transcription of the HIS3, ADE2 and lacZ reporter genes. Protein–protein interactions are then detected by the ability of the co-transformed yeast cells to grow in selective medium lacking leucine, tryptophan, adenine and histidine (SD–Leu, –Trp, –His and –Ade), and by production of β-galactosidase activity.
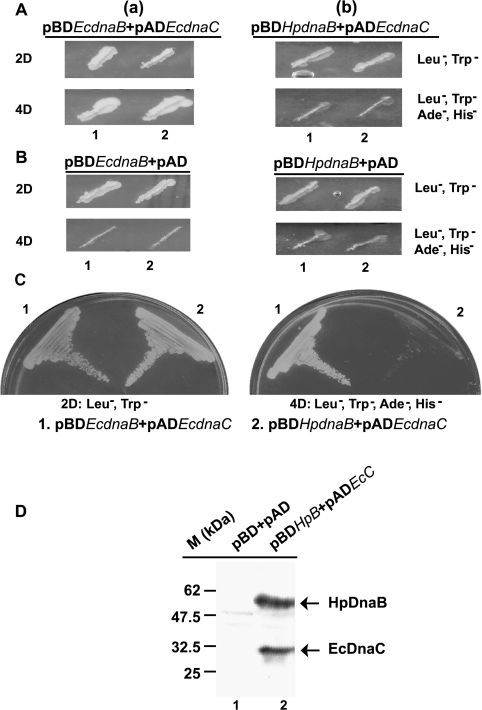
In order to test whether interactions between EcDnaB, HpDnaB with EcDnaC could be detected in this system, we first subcloned EcdnaC at the BamHI site of pAD. EcdnaB and HpdnaB genes were cloned at the BamHI site of pBD. The yeast reporter strain AH109 was subsequently co-transformed either with: (case a) pADEcdnaC and pBDEcdnaB, or (case b) pADEcdnaC and pBDHpdnaB, followed by plating them on 2D plates (see the Materials and methods section). Growth of the co-transformed yeast cells on these plates confirmed that the transformation had been successful, and provided an indication of the relative number of co-transformed yeast cells. Furthermore, 20 colonies in each case (either a or b) were re-streaked on 2D plates or 4D plates (i.e. SD agar medium lacking leucine, tryptophan, histidine and adenine; see the Materials and methods section for further details). In the case of (a), all the colonies grew on the 2D as well as the 4D plates. On the other hand, in the case of (b), all the colonies grew on 2D plates, but none grew on 4D plates. A representation of two colonies streaked on such plates is shown in Figure 3(A). These observations strongly suggest that EcDnaC interacts with EcDnaB, but not with HpDnaB. As a control, yeast strains were co-transformed either with pBDEcdnaB and pAD (empty vector) or with pBDHpdnaB and pAD, followed by plating them on 2D and 4D plates (Figure 3B). As expected, all grew on 2D plates, but none were found to grow on 4D plates, suggesting that the yeast cells growing in the 4D plates [Figure 3A(a)] are the outcome of the real interaction between EcDnaB and EcDnaC. In order to consolidate the results from Figure 3(A), yeast cells [shown in Figures 3A(a) and 3A(b), ‘2D’ panels, lanes 2] were re-streaked further on to 2D and 4D plates (Figure 3C).
Figure 3. EcDnaB but not HpDnaB interacts with EcDnaC.
(A) Yeast reporter strain AH 109 was co-transformed with either (a) pADEcdnaC and pBDEcdnaB or (b) pADEcdnaC and pBDHpdnaB, and the transformed cells were plated in an SD agar minimal-media plate (2D plate). Two representative colonies (the numbers are indicated along the bottom of the respective Figure parts) in each case were streaked further in SD minimal 2D or 4D plates, followed by incubation at 30 °C for 2–3 days. All the colonies grew on 2D plates. Both the colonies grew on 4D plates in the case of (a), whereas none grew on 4D plates in the case of (b). (B) Yeast reporter strain AH 109 was co-transformed with either pAD (empty vector) and pBDEcdnaB, or pAD and pBDHpdnaB, and two colonies (the numbers are indicated along the bottom of the respective Figure parts) grown on 2D plates were streaked further in 2D or 4D plates. All of them grew on 2D plates, but none grew on 4D plates. (C) In order to confirm the results, colony 2 from 2D plates in A(a) and A(b) was re-streaked in 2D and 4D plates. While both grew on 2D plates, only one (a) grew on the 4D plate. (D) Western blot analysis of the cell lysate obtained from yeast strain AH 109 co-transformed either with empty vectors pAD and pBD (lane 1) or pADEcdnaC (pADEcC) and pBDHpdnaB (pBDHpB) (lane 2) using anti-HpDnaB and anti-EcDnaC antibodies.
The EcDnaB–EcDnaC interaction observed in co-transformed yeast cells was confirmed further by analysis of β-galactosidase activity, which reflects the activation of the lacZ reporter gene (results not shown). The expression of HpDnaB and EcDnaC from the respective plasmids pBDHpdnaB and pADEcdnaC was also confirmed by SDS/PAGE followed by Western blot analysis of the yeast cell lysate [Figure 3A(b), ‘2D’ panel] using anti-HpDnaB and anti-EcDnaC antibodies (Figure 3D). These results strongly suggest that, even though HpDnaB and EcDnaC were expressed in the yeast reporter strain AH109, the reporter genes were not activated due to the failure of their interaction.
HpdnaB rescues the temperature-sensitive phenotype of the EcdnaCts strains
Absence of the DnaC homologue in the H. pylori genome, and the failure of HpDnaB to interact with EcDnaC in vitro and in the yeast two-hybrid assay, raised the issue of whether HpDnaB needs a loader. We reasoned that, if the above results were truly reflected in vivo, HpDnaB would be able to perform its function in the absence of a functional DnaC protein. This hypothesis was experimentally proved by complementing two different EcdnaCts strains with HpDnaB at the non-permissive temperature.
The E. coli system allows us the opportunity to study gene function due to the availability of conditional lethal mutants. To carry out the complementation analysis, a His6-tagged HpdnaB gene was subcloned into the low-copy pBR322 plasmid under the control of the Bla-P2 promoter [12], and E. coli strain BR1701 dnaCts [19] was transformed with the recombinant plasmids. HpdnaB was found to complement the defective dnaC gene in E. coli at 37 °C, whereas pBR322 failed to do so (Figure 4A). pdnaBC containing wild-type dnaC gene under the control of the bacteriophage λ promoters (PR–PL) also rescued the defective gene function. These results suggest that HpDnaB is capable of functioning as a helicase in vivo where DnaC is not functional. It is possible that the defect in the E. coli strain BR1701 dnaCts will allow HpDnaB to still interact with EcDnaC, and rescue the temperature-sensitive phenotype. However, in order to rule out the possibility of any allele specificity in the BR1701 dnaCts strain, we performed the rescue experiment in another dnaCts strain, MG1655dnaC2 [20] using a wide range of plasmids. It is very unlikely that HpDnaB would rescue two different dnaCts strains, having mutations in two different places in the dnaC gene, purely by chance, whereas the wild-type EcDnaB cannot rescue either of these strains. pBR-HpdnaBwt, pBR-HpdnaBmut, pdnaB (containing the EcdnaB gene) and pdnaBC (containing the wild-type EcdnaC gene) were used for complementation. Only HpdnaBwt and pdnaBC could rescue the temperature-sensitive phenotype of the DnaC2 cells (Figure 4B). These results clearly suggest that HpDnaB can bypass the EcDnaC function in vivo. The failure of wild-type EcDnaB to complement the dnaCts strain strengthens the notion that a functional DnaC is absolutely essential for EcDnaB to be loaded at the E. coli oriC.
It is possible that the complementation event could be the outcome of recombination of the HpdnaB gene with the E. coli genome. To resolve this issue, plasmid DNA was isolated from the transformed DnaC2 cells (grown at 37 °C), followed by restriction enzyme digestion using BamHI. A 1.5 kb HpdnaB DNA fragment was observed in the HpdnaB-transformed DnaC2 cells (Figure 4C). The expression of HpDnaB was also confirmed by Western blotting using anti-His6 antibodies in HpdnaB-transformed DnaC2 cells at 37 °C (Figure 4D). The presence of an approx. 55 kDa band in the Western blot rules out the possibility of the recombination of HpdnaB gene with the E. coli genome.
Furthermore, we wanted to compare experimentally the efficiency of the Bla-P2 promoter in the pBR-HpdnaB plasmid construct with the bacteriophage λ PR–PL promoters in pdnaBC or pdnaB plasmids, since the level of expression of proteins is an important issue for the complementation experiments. To compare the efficiency of these promoters, DnaC2 cells transformed with either pBR-HpdnaB or pdnaBC were grown at 37 °C (Figure 4B), and the bacterial cell extracts were subjected to SDS/PAGE analysis, followed by Coomassie staining of the gel to visualise the proteins. Bacterial cell extract from pBR322-transformed DnaC2 cells (grown at 30 °C) was used as control. The expression of EcDnaB and EcDnaC could be detected at the restrictive temperature easily, but the expression of HpDnaB was not very obvious by this method (Figure 4E), suggesting that PR–PL promoters are stronger than the Bla-P2 promoter. The expression levels of HpDnaB and EcDnaC were confirmed further by Western blot analysis using rabbit polyclonal antibodies against HpDnaB and EcDnaC. Anti-HpDnaB antibodies do not cross-react with EcDnaC, and vice versa (results not shown). We observed that both HpDnaB and EcDnaC were expressed in the DnaC2 cells at the non-permissive temperature (Figure 4F). Owing to the unavailability of anti-EcDnaB antibodies, we could not check the expression of EcDnaB by Western blot analysis. In vivo, both pdnaBC and pdnaB plasmid constructs complemented the EcdnaBts strain DJ58 [6] at the non-permissive temperature efficiently (results not shown), suggesting that EcDnaB was indeed expressed from these plasmid constructs.
DISCUSSION
Analysis of the genome sequences of H. pylori reveals many unique features related to DNA replication. The typical conserved eubacteria gene cluster rnpA-rmpH-dnaA-dnaN-recF-gyrB is absent, and the dnaA gene is located approx. 600 kb away from the dnaN-gyrB genes [21]. The helicase loader DnaC is also absent. Here, we have shown that HpDnaB can complement two different EcdnaCts strains, citing the first evidence of a bypassing DnaC function in vivo. Recently, in vitro, a DnaC-independent mechanism for Ps. aeruginosa replicative helicase loading at the broad host range plasmid RK2 ori was reported [8]. Our finding is important and relevant, since several eubacterial species, including Ps. aeruginosa and H. pylori, lack a DnaC homologue, suggesting that an accessory-protein-independent loading of helicases might be possible in some bacteria.
How does HpDnaB work at the E. coli oriC in the absence of a functional EcDnaC? We believe that DNA replication initiation at the oriC in the dnaCts strains at the non-permissive temperature is due to the HpDnaB activity only, and does not require a helicase loader. However, functional EcDnaB is present in the dnaCts strains. Therefore it cannot be ruled out that the rescue phenomenon is the result of a DnaC-like activity of HpDnaB that may assist the loading of EcDnaB at the oriC in the dnaCts strains.
In the present study, we have shown that HpDnaB does not require a functional DnaC-like protein in a heterologous system. The question arises of whether HpDnaB needs a helicase loader in H. pylori itself. It is possible that a functional homologue of DnaC, without sharing any sequence homology with EcDnaC, is present in H. pylori. In fact, according to the H. pylori protein–protein interaction map [22], two unknown ORFs (Hp0897 and Hp0340) were reported to be interacting with HpDnaB. Interestingly, these ORFs do not contain nucleotide binding and hydrolysis domains, and Hp0897 does not have a counterpart in a similar H. pylori strain, J99. These observations strongly argue against their conserved DnaC-like role in H. pylori. Additional experiments are required to unveil their functional roles in H. pylori. Furthermore, according to this protein–protein interaction map, HpDnaB does not interact with HpDnaA, in contrast with EcDnaB, which needs EcDnaA to be loaded at the oriC. These observations suggest that HpDnaB might have some unique features, compared with its E. coli counterpart.
It has been reported that the overexpression of dnaC can rescue the temperature sensitivity of the E. coli dnaB252 allele [16]. Mutation of residue 299 of EcDnaB, from glycine to aspartic acid, was found to be responsible for this defect [17]. DnaB252 protein showed identical ATPase, helicase and single-strand DNA binding activities, both at the permissive and restrictive temperature, suggesting that the defect in the dnaB252 gene does not affect the gene function as such. However, this mutation must affect the interaction between EcDnaB and EcDnaC, since overexpression of dnaC can abrogate this defect. It has been strongly suggested that the glycine residue at position 299 of DnaB is critical for the DnaB–DnaC interaction. We chose to examine the status of this residue in the DnaB helicases shown in Figure 1, and we found that the glycine residue is absent in both H. pylori and Ps. aeruginosa. Moreover, the amino acid residues immediately adjacent to the residue at position 299 are also divergent in both the cases. It is important to note in this regard that, although PaDnaB shows 80% overall homology with EcDnaB at the amino acid level, it lacks a DnaC homologue. We believe that these observations may shed some light on to our efforts to find a region in DnaB that might be important for the presence or absence of DnaC. However, further detailed studies are needed before we can assert that this region is an absolute determinant of the presence or absence of DnaC.
Another interesting feature of HpDnaB is the presence of a unique insertion of 34 residues at its C-terminus (residues 400–433). This region contains as many as ten lysine and arginine residues, with an overall positive charge. Further detailed mutational analysis of these residues will be required to understand their biological function.
The unavailability of the dnaC gene in the H. pylori genome, the inability of HpDnaB to interact with EcDnaC, and finally the rescue of two different dnaC temperature-sensitive strains using HpDnaB, clearly suggest that HpDnaB can bypass E. coli DnaC function in vivo efficiently.
Acknowledgments
The authors acknowledge Dr Santanu Dasgupta (Uppsala University, Sweden) and Dr Dhruba K. Chattoraj (National Institutes of Health, Bethesda, MD, U.S.A.) for providing the dnaCts and dnaBts strains, and the pdnaBC construct. The authors also acknowledge Dr Sunil Mukherjee, Dr Nirupam Roy Choudhury and Mr Dharmendra K. Singh of ICGEB (the International Centre for Genetic Engineering and Biotechnology), New Delhi, India for their help with the yeast two-hybrid assay. This work is supported by grants awarded to S.K.D. by the University Grant Commission (Excellence programme) and CSIR (the Council of Scientific and Industrial Research). R.K.S. and P.M. acknowledge CSIR for fellowships.
References
- 1.Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E coli. chromosome. Cell. 1988;52:743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- 2.Marszalek J., Kaguni J. M. DnaA protein directs the binding of DnaB protein in initiation of DNA replication in Escherichia coli. J. Biol. Chem. 1994;269:4883–4890. [PubMed] [Google Scholar]
- 3.Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J. Biol. Chem. 1987;262:10327–10334. [PubMed] [Google Scholar]
- 4.LeBowitz J. H., McMacken R. The Escherichia coli DnaB replication protein is a DNA helicase. J. Biol. Chem. 1986;261:4738–4748. [PubMed] [Google Scholar]
- 5.Konieczny I. Strategies for helicase recruitment and loading in bacteria. EMBO Reports. 2003;4:37–41. doi: 10.1038/sj.embor.embor703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soni R. K., Mehra P., Choudhury N. R., Mukhopadhyay G., Dhar S. K. Functional characterization of Helicobacter pylori DnaB helicase. Nucleic Acids Res. 2003;31:6828–6840. doi: 10.1093/nar/gkg895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan D. L., Steitz T. A. DnaB from Thermus aquaticus unwinds forked duplex DNA with an asymmetric tail length dependence. J. Biol. Chem. 1999;274:6889–6897. doi: 10.1074/jbc.274.11.6889. [DOI] [PubMed] [Google Scholar]
- 8.Caspi R., Pacek M., Consiglieri G., Helinski D. R., Toukdarian A., Konieczny I. A broad host range replicon with different requirements for replication initiation in three bacterial species. EMBO J. 2001;20:3262–3271. doi: 10.1093/emboj/20.12.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker T. A., Bell S. P. Polymerases and the replisome: machines within machines. Cell. 1998;92:295–305. doi: 10.1016/s0092-8674(00)80923-x. [DOI] [PubMed] [Google Scholar]
- 10.Kreuzer K. N., Morrical S. W. Initiation of DNA replication. In: Karam J. D., editor. Molecular Biology of Bacteriophage T4. Washington DC: ASM Press; 1994. pp. 28–42. [Google Scholar]
- 11.Elvin C. M., Thompson P. R., Argall M. E., Hendry P., Stamford N. P., Lilley P. E., Dixon N. E. Modified bacteriophage lambda promoter vectors for overproduction of proteins in Escherichia coli. Gene. 1990;87:123–126. doi: 10.1016/0378-1119(90)90503-j. [DOI] [PubMed] [Google Scholar]
- 12.Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 13.Sambrook J. F., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 14.Woods R. A., Gietz R. D. High efficiency transformation of plasmid DNA into yeast. Methods Mol. Biol. 2001;177:85–97. doi: 10.1385/1-59259-210-4:085. [DOI] [PubMed] [Google Scholar]
- 15.Biswas E. E., Biswas S. B. Mechanism of DnaB helicase of Escherichia coli: structural domains involved in ATP hydrolysis, DNA binding, and oligomerization. Biochemistry. 1999;38:10919–10928. doi: 10.1021/bi990048t. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani R. A., Wechsler J. A. Deoxyribonucleic acid initiation mutation dnaB252 is suppressed by elevated dnaC+ gene dosage. J. Bacteriol. 1981;146:418–421. doi: 10.1128/jb.146.1.418-421.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saluja D., Godson G. N. Biochemical characterization of Escherichia coli temperature-sensitive dnaB mutants dnaB8, dnaB252, dnaB70, dnaB43, and dnaB454. J. Bacteriol. 1995;177:1104–1111. doi: 10.1128/jb.177.4.1104-1111.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James P. Yeast two hybrid vectors and strains. Methods Mol. Biol. 2001;177:41–84. doi: 10.1385/1-59259-210-4:041. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol. Gen. Genet. 1971;113:273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- 20.Maisnier-Patin S., Nordstrom K., Dasgupta S. Replication arrests during a single round of replication of the Escherichia coli chromosome in the absence of DnaC activity. Mol. Microbiol. 2001;42:1371–1382. doi: 10.1046/j.1365-2958.2001.02718.x. [DOI] [PubMed] [Google Scholar]
- 21.Zawilak A., Cebrat S., Mackiewicz P., Krol-Hulewicz A., Jakimowicz D., Messer W., Gosciniak G., Zakrzewska-Czerwinska J. Identification of a putative chromosomal replication origin from Helicobacter pylori and its interaction with the initiator protein DnaA. Nucleic Acids Res. 2001;29:2251–2259. doi: 10.1093/nar/29.11.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rain J. C., Selig L., De Reuse H., Battaglia V., Reverdy C., Simon S., Lenzen G., Petel F., Wojcik J., Schachter V., et al. The protein–protein interaction map of Helicobacter pylori. Nature (London) 2001;409:211–215. doi: 10.1038/35051615. [DOI] [PubMed] [Google Scholar]