Abstract
The A subclass of the ABC (ATP-binding cassette) transporter superfamily has a structural feature that distinguishes it from other ABC transporters, and is proposed to be involved in the transmembrane transport of endogenous lipids. Here we have cloned mouse and rat full-length cDNAs of ABCA17, a novel ABC transporter belonging to the A subclass. Mouse and rat ABCA17 proteins comprise 1733 and 1773 amino acid residues respectively, having 87.3% amino acid identity; mouse ABCA17 has amino acid identities of 55.3% and 36.7% with mouse ABCA3 and sea urchin ABCA respectively. RNA blot and quantitative real-time PCR analyses showed that ABCA17 mRNA is expressed exclusively in the testis. Examination of testis by in situ hybridization showed that ABCA17 mRNA is expressed in germ cells, mainly spermatocytes, in the seminiferous tubule. Immunoblot analysis using a specific antibody showed that ABCA17 is a protein of 200 kDa, and immunohistochemical analysis demonstrated that the protein is detected in the anterior head of sperm and elongated spermatids. ABCA17 was localized in the endoplasmic reticulum in transiently transfected HEK293 cells. Metabolic labelling analysis showed that intracellular esterified lipids, including cholesteryl esters, fatty acid esters and triacylglycerols, were significantly decreased in HEK293 cells stably expressing ABCA17 compared with untransfected cells. These results suggest that ABCA17 may play a role in regulating lipid composition in sperm.
Keywords: acrosomal reaction, capacitation, cholesterol efflux, esterified lipid, sperm, testis
Abbreviations: ABC, ATP-binding cassette; suABCA, sea urchin ABCA; apoA-I, apolipoprotein A-I; DAPI, 4′,6-diamidino-2-phenylindole; DIG, digoxigenin-11-UTG; DMEM, Dulbecco's modified Eagle's medium; Endo H, endoglycosidase H; ER, endoplasmic reticulum; FCS, fetal calf serum; HA, haemagglutinin; PNGaseF, peptide N-glycosidase F; RT-PCR, reverse transcription–PCR
INTRODUCTION
Capacitation is a functional maturation process of sperm that occurs in the female tract and confers on sperm the ability to fertilize an egg. Capacitation involves changes in both the sperm's ability to undergo acrosomal exocytosis and its acquisition of a hyperactivated pattern of motility [1]. Although the molecular mechanism of capacitation is poorly understood, a decrease in sperm cholesterol is thought to be essential for a series of molecular events in capacitation [2–6]. Serum albumin is an essential component of the media used in in vitro experiments mimicking capacitation, functioning as an acceptor of cholesterol under the experimental conditions [4,7–9], but the role of albumin in physiological situations is uncertain.
The ABC (ATP-binding cassette) transporters constitute one of the largest gene families, encoding highly conserved proteins involved in the energy-dependent transport of a variety of substrates across membranes. ABCA1, ABCA3, ABCA4 and ABCA12 have been determined to be the causative genes in Tangier disease [10–12], fatal surfactant deficiency [13], Stargardt's macular dystrophy [14] and lamellar ichthyosis type 2 [15] respectively. ABCA1 functions to promote cellular phospholipid and cholesterol efflux to apoA-I (apolipoprotein A-I), initiating the formation of high-density lipoprotein [10–12,16], and ABCA4 is proposed to be a transmembrane transporter for intracellular N-retinylidene-phosphatidylethanolamine [17]. Although the functions of ABCA3 and ABCA12 are not known, pulmonary surfactant is composed mainly of lipids, and disorders in lipid metabolism are found in the stratum corneum in lamellar ichthyosis, suggesting that ABCA3 and ABCA12 are involved in lipid transport [18]. In addition, ABCA2 and ABCA7 mRNA levels are up-regulated by sustained cholesterol influx mediated by modified low-density lipoproteins [19,20], and ABCA7 has been reported recently to mediate cellular phospholipid efflux through binding to apoA-I [21,22], although the involvement of ABCA7 in cholesterol efflux is controversial [21,22]. Thus the A subclass of ABC transporters, to which 15 functional mammalian genes have been assigned to date (ABCA11 is a pseudogene), is believed to be involved in the transport of endogenous lipids [23].
In the present study, we cloned mouse and rat full-length cDNAs for a novel ABC transporter, ABCA17, which is expressed exclusively in testis. We generated a specific antibody against mouse ABCA17, and found ABCA17 protein to be expressed exclusively in the anterior head of sperm. Metabolic labelling analysis using [14C]acetate revealed that intracellular levels of esterified lipids, including cholesteryl esters, fatty acid esters and triacylglycerols, were significantly decreased in HEK293 cells stably expressing ABCA17 compared with untransfected cells, suggesting that ABCA17 may regulate lipid composition in sperm.
EXPERIMENTAL
Cloning of ABCA17 cDNA
The experimental procedures used to obtain full-length mouse and rat ABCA17 cDNAs are described in Supplemental online experimental procedure 1 (available at http://www.BiochemJ.org/bj/389/bj3890577add.htm).
RNA blot analysis
RNA blot analysis was carried out using a 32P-labelled DNA fragment (corresponding to nucleotides +2479 to +3342) as a probe under high-stringency hybridization conditions as described previously [24,25].
Quantitative real-time PCR
Quantitative real-time RT-PCR (reverse transcription–PCR) analysis was performed using a TaqMan Core Reagent Kit (Applied Biosystems). PCR primers were designed to amplify DNA between the 29th and 30th exons of the total of 30 exons covering the coding region based on the DNA database. The forward primer was 5′-AGCACCAAAACATGCTTCAATATTAC-3′ (corresponding to nucleotides +4883 to +4908 relative to the putative translation start site of the mouse ABCA17 cDNA) and the reverse primer was 5′-GATAGCTGGCTGATGGAGTAGTCTT-3′ (corresponding to nucleotides +4982 to +5006). The TaqMan probe was 5′-ACATGTAATCCTTCTTGGCTTGTTCCA-3′ (corresponding to nucleotides +4952 to +4978) for exon 30, labelled with reporter dye (FAM) at the 5′-end and with quencher dye (TAMRA) at the 3′-end. PCR was performed using the TaqMan Universal PCR Master Mix (Applied Biosystems), with cDNAs derived from 0.1 μg of total RNA, 250 nM probe and 900 nM primers in a 50 μl final reaction mixture. The PCR conditions were denaturing at 95 °C for 15 s and annealing plus extension at 60 °C for 1 min (40 cycles). Signals were analysed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems), and the quantity of ABCA17 mRNA was normalized to that of 18 S rRNA.
In situ hybridization analysis
C57BL/6N mice (2 months old) were anaesthetized and killed by decapitation, and the testes and lungs were quickly removed. Paraffin sections of thickness 3.5 μm were pretreated by microwave heating at 95 °C for 3 min and incubated with 1 μg/ml proteinase K for 5 min at room temperature. The 390 bp 3′-translated and untranslated region of mouse ABCA17 cDNA (corresponding to nucleotides +5024 to +5412) was inserted into a Bluescript SK(−) plasmid. Antisense and sense cRNA probes were synthesized in vitro using DIG (digoxigenin-11-UTG) with T7 and T3 RNA polymerases respectively (DIG-labelling system; Boehringer Mannheim), followed by alkaline treatment for 3 min. Hybridization signals were detected using the GenPoint™ Catalyzed Signal Amplification System (DAKO) and horseradish peroxidase-labelled anti-DIG F(ab')2 (DAKO) according to the manufacturer's instructions.
Cell culture and transfection
The culture and transfection of HEK293 cells and COS-1 cells were carried out as described previously [24,26]. Briefly, 2 μg of mouse ABCA17 expression vector (pCMVmABCA17), pCMVmABCA17-HA [in which DNA encoding the human influenza HA (haemagglutinin) tag (YPYDVPDYA) was introduced into the 3′-end of the coding region of mABCA17 using PCR] or the empty expression vector (pCMV) was transfected into cells in 35 mm culture dishes using Lipofectamine 2000 and Opti-MEM I (Invitrogen), according to the manufacturer's instructions. To establish HEK293 cells stably expressing ABCA17, cells were transfected with mABCA17-HA expression plasmid (pIREShyg3/mABCA17-HA) containing a hygromycin selection marker as well as mABCA17-HA, and were selected by 400 μg/ml hygromycin for 2 weeks. Single colonies were isolated, and expression of mABCA17-HA was confirmed by immunoblot analysis using high-affinity anti-HA rat monoclonal antibody (Roche). HEK293 cells stably expressing ABCA1 were established as described previously [22]. To observe ER (endoplasmic reticulum) and mitochondria, DsRed2 expression plasmid containing either an ER localization signal (pDsRed2-ER; Clontech) or a mitochondria localization signal (pDsRed2-Mito; Clontech) was transfected.
Preparation of specific antibody
An antibody specific for ABCA17 was raised in rabbits against a synthetic peptide corresponding to 19 C-terminal amino acid residues (SSPTPKPLPSPPPSEPILL) of mouse ABCA17, which shows no similarity to the other members of the ABCA subfamily or any other proteins in sequence databases. The antibody was purified using affinity chromatography (MabTrap Protein G; Amersham Biosciences).
Immunoblot analysis
Crude membranes were prepared and immunoblot analysis was performed as described previously [25]. After blocking, the PVDF membrane was incubated with 0.1 μg/ml anti-HA rat antibody or 1:1000-diluted anti-(mouse ABCA17) rabbit antibody for 2 h at room temperature. As the secondary antibody, 1:5000-diluted horseradish peroxidase-conjugated anti-rat F(ab')2 (Chemicon) or anti-(rabbit Ig) (Amersham Biosciences) respectively was used. Proteins were detected using an enhanced chemiluminescence system (ECL®; Amersham Biosciences).
Immunocytochemistry
HEK293 cells grown on poly(L-lysine)-coated cover glasses were fixed and permeabilized. The cover glasses were incubated overnight at 4 °C with 1:1000-diluted anti-(mouse ABCA17) rabbit antibody and anti-HA rat antibody at a concentration of 0.1 μg/ml in PBS containing 1% (v/v) normal goat serum and 3% (w/v) BSA, and then incubated for 1 h at room temperature with 1:2000-diluted Cy3-conjugated goat anti-rabbit IgG (Amersham Biosciences) and 1:40-diluted FITC-conjugated sheep anti-rat IgG (Chemicon). Nuclear counterstaining was done for 30 min at room temperature with DAPI (4′,6-diamidino-2-phenylindole). Cover glasses were mounted with PermaFluor Aqueous Mounting Medium (Immunon). DAPI staining and the fluorescences of Cy3, FITC and DsRed2 were observed as described in Supplemental online experimental procedure 2 (available at http://www.BiochemJ.org/bj/389/bj3890577add.htm).
Immunohistochemistry
After the testes had been permeated with 20% (w/v) sucrose for 1 day, they were frozen in an embedding OCT compound (Sakura Finetechnical) and cut into 10 μm-thick sections on a cryostat (Leica). Immunofluorescence analysis was performed as described previously [27]. The sections were incubated overnight at 4 °C with anti-(mouse ABCA17) antibody diluted 1:1000 in PBS containing 1% (v/v) normal goat serum and 3% (w/v) BSA, rinsed, and incubated for 1 h at room temperature with 1:2000-diluted Cy3-conjugated anti-rabbit IgG. Nuclei were counterstained, and Cy3 fluorescence and DAPI were observed as described above.
Cholesterol and choline-phospholipid efflux assay
Untransfected HEK293 cells and HEK293 cells stably expressing ABCA17 or ABCA1 were cultured in six-well plates at a density of 1×106 cells per well in a 1:1 (v/v) mixture of DMEM (Dulbecco's modified Eagle's medium) and Ham's F12 medium supplemented with 10% (v/v) FCS (fetal calf serum). After incubation for 48 h, the cells were washed with PBS and incubated in the same medium supplemented with 0.1% fatty acid-free BSA (Sigma) and 10 μg/ml apoA-I. The lipid content in the medium was determined after a 24 h incubation as described previously [22].
Metabolic labelling of cellular lipids
Untransfected HEK293 cells and HEK293 cells stably expressing ABCA17 or ABCA1 were grown in 100 mm culture dishes in the presence of 0.25 μCi/ml [14C]acetate for 72 h in 10 ml of DMEM supplemented with 10% (v/v) FCS. Lipids in the cells or medium were extracted by the method of Bligh and Dyer [28], and then resuspended in chloroform/methanol (2:1, v/v). For neutral lipid analysis, aliquots of the solubilized lipids were developed by HPTLC on Silica gel 60 HPTLC plates (Merck) with appropriate standards, using hexane/diethyl ether/acetic acid (80:20:1, by vol.). For phospholipid analysis, aliquots of the solubilized lipids were developed on Silica gel 60 TLC plates (Merck; 20 cm×20cm) using two-dimensional solvent systems; the first and the second chromatographic runs were performed with chloroform/methanol/7 M ammonia (65:30:4, by vol.) and with chloroform/methanol/acetic acid/water (170:25:25:4, by vol.) respectively, with the second run carried out at 90° to the original direction. The HPTLC and two-dimensional TLC plates were dried, and the radiolabelled lipid signals were analysed by autoradiography using FLA-5000 (Fujifilm).
Statistics
Data, normalized with regard to the protein content (for cholesterol and choline-phospholipid efflux) or the number of cells (for metabolic labelling), are represented as means±S.D. Statistical analysis was performed by the Bonferroni/Dunn procedure for post hoc testing.
RESULTS
Cloning of ABCA17 cDNA
Using a 1455-bp radiolabelled human ABCA3 cDNA fragment corresponding to the first nucleotide-binding domain as a probe, a total of 7.2×105 plaques of a mouse genomic library were screened under high-stringency hybridization conditions, and two clones, λmABCX-1 and λmABCX-2, encoding a novel ABC transporter, and four clones encoding the mouse ABCA3 gene, were obtained. As the nucleotide sequence of the putative exons in the novel ABC transporter gene had high similarity with the cDNA sequence of ABCA3, which belongs to the ABCA subclass, we designated the gene ABCA17. To obtain the full-length mouse ABCA17 cDNA, oligonucleotide primers were designed based on the exon sequence, and RT-PCR was carried out using total RNAs extracted from various mouse tissues. Since the RT-PCR products were obtained from testis, a mouse testis cDNA library was screened using the radiolabelled partial cDNAs as probes, and 3 λ clones were isolated from 7.2×105 plaques. Two overlapping clones, λmABCA17-1 and λmABCA17-2, were subcloned and sequenced. To obtain the 3′-end of the mouse ABCA17 cDNA, 3′-RACE (rapid amplification of cDNA ends) was performed using the total RNA extracted from mouse testis, and an approx. 650 bp DNA fragment was obtained and sequenced. The sequence of the 3′-end was confirmed by amplification of the mouse testis cDNA library by PCR and by an EST (expressed sequence tag) clone on the database (AI626925).
The composite sequence of 5199 bp contains a single open reading frame beginning with the first ATG conforming to the Kozak consensus sequence in the cDNA sequence, which predicts the amino acid sequence of a 1733-residue protein (with a calculated molecular mass of 195970.85 Da) (Figure 1). A termination codon was found in the 27 bp of DNA upstream of the first ATG. The predicted amino acid sequence of mouse ABCA17 is most similar to those of mouse ABCA3 (GenBank accession no. AY083616) and suABCA (sea urchin ABCA) (accession no. AF529424), with amino acid identities of 55.3% and 36.7% respectively (Figure 1). Hydropathy analysis predicts that 12 hydrophobic segments (H1–H12 in Figure 1), six in the first membrane-spanning domain (H1–H6) and six in the second membrane-spanning domain (H7–H12), are well conserved among mouse ABCA17, mouse ABCA3 and suABCA. The amino acid sequences of these proteins are conserved in the nucleotide-binding domains and the membrane-spanning domains, but not in the large extracellular loops between H1 and H2 and between H7 and H8. In addition, an approx. 40-amino-acid sequence at the N-terminus of these proteins is well conserved, further affirming that the first ATG of ABCA17 is a translation start site. Based on the DNA database, a mouse ABCA17 gene containing at least 30 exons covering the coding region is located at chromosome 17A3.3 [29,30], the same locus as the ABCA3 gene.
Figure 1. Comparison of the amino acid sequences of rat ABCA17, mouse ABCA17, mouse ABCA3 and suABCA.
Amino acids are indicated in single-letter code. Identical amino acid residues among these proteins are shown in bold type. Predicted hydrophobic segments (H1–H12) and consensus Walker A and Walker B motifs conserved among these proteins are indicated. Potential N-linked glycosylation sites in ABCA17 are indicated by dots. The prefixes r and m denote rat and mouse respectively.
To obtain the full-length cDNA of rat ABCA17, oligonucleotide primers were designed based on the rat genomic DNA database corresponding to the mouse ABCA17 cDNA sequence. RT-PCR was carried out using total RNAs extracted from rat testis, and the PCR products obtained were subcloned and sequenced. The sequence of 5319 bp contains a single open reading frame, which predicts the amino acid sequence of a 1773-residue protein (with a calculated molecular mass of 199854.60 Da) with amino acid identity of 87.3% with mouse ABCA17, suggesting a rat orthologue (Figure 1). Based on the rat genomic DNA database, the rat ABCA17 gene also contains at least 30 exons covering the coding region, and is located at chromosome 10q12 [31], the same locus as the ABCA3 gene.
Tissue distribution of ABCA17 mRNA
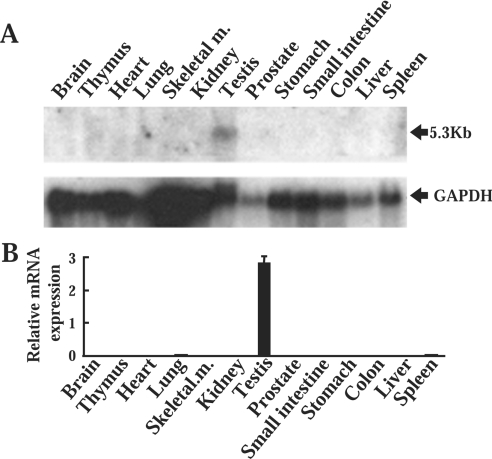
An RNA blotting study revealed a 5.3 kb transcript expressed exclusively in the testis and not in the other tissues, including lung, indicating that the probe was not cross-hybridized to ABCA3 mRNA (Figure 2A). To confirm specificity of the RNA blotting, a quantitative real-time PCR analysis was conducted using ABCA17-specific primers and TaqMan probe; ABCA17 cDNA was amplified exclusively in the testis, and ABCA17 mRNA levels were much lower in the other tissues (Figure 2B).
Figure 2. RNA blot and quantitative real-time PCR analyses of ABCA17 mRNA in various mouse tissues.
(A) RNA blot analysis of various mouse tissues. Total RNA (20 μg) was denatured and electrophoresed in a 1% (w/v) agarose gel, blotted on a nylon membrane, and hybridized with a 32P-labelled mouse ABCA17 cDNA probe. The membrane was exposed to X-ray film with an intensifying screen at −80 °C for 4 days. The lower panel shows the same blot probed with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as the internal control to evaluate differences among various tissues. (B) Comparison of ABCA17 mRNA levels in various mouse tissues as determined by quantitative real-time PCR analysis of reverse-transcribed total RNA. Data are shown as the RNA expression levels normalized to 18 S rRNA controls, and are means±S.D. obtained from four independent experiments.
Expression of ABCA17 mRNA in testis
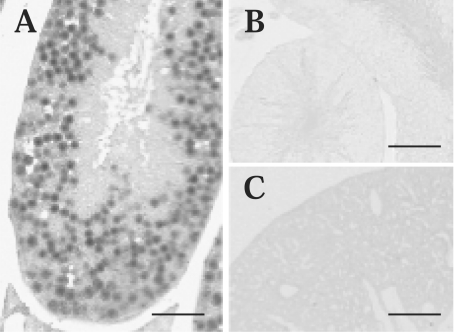
To identify the cells that express ABCA17 mRNA in the testis, we performed in situ hybridization analysis using a DIG-labelled antisense cRNA probe. Expression of ABCA17 mRNA was clearly detected in spermatocytes in seminiferous tubules (Figure 3A), although the possibility that Sertoli cells were stained cannot be completely excluded by this experiment. Elongated spermatids representing late-stage spermatids as well as sperm appeared not to be stained. ABCA17 mRNA expression was undetectable in peritubular cells, including Leydig cells. No cells in the testis were stained in a negative control using a DIG-labelled sense cRNA probe (Figure 3B). To confirm the specificity of the probe, lung tissue was hybridized with the antisense cRNA probe (Figure 3C), and no signal was detected.
Figure 3. Localization of ABCA17 mRNA by in situ hybridization.
(A, B) In situ hybridization of mouse testis with DIG-labelled antisense (A) and sense (B) cRNA probes. Signals were detected mainly in spermatocytes in testis with the antisense probe (A), but not with the sense probe (B). (C) In situ hybridization of mouse lung with a DIG-labelled antisense cRNA probe. No staining was detected in the lung. Scale bar=50 μm.
Localization of ABCA17 protein
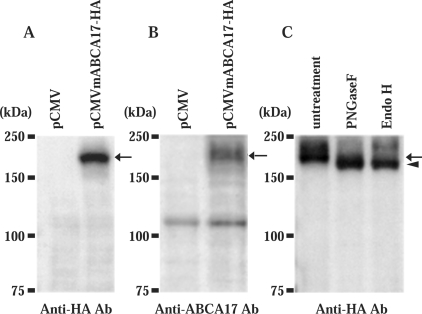
To characterize the ABCA17 protein, the HA epitope (YPYDVPDYA) was introduced into the C-terminus of the protein, and immunoblot analysis was performed using anti-HA antibody. The total membrane fraction of COS-1 cells transfected with the ABCA17–HA fusion protein expression vector (pCMVmA-BCA17-HA) revealed a band of 200 kDa (Figure 4A, arrow), while that of COS-1 cells mock-transfected with pCMV vector showed no such band (Figure 4A). We then generated a specific antibody against a peptide corresponding to the 19 C-terminal amino acid residues of ABCA17 protein. Using the anti-ABCA17 antibody, the 200 kDa protein was again detected in the total membrane fraction of COS-1 cells transfected with the pCMVmA-BCA17-HA plasmid (Figure 4B, arrow), affirming the specificity of the antibody. Glycosylation of ABCA17 was examined by treatment with PNGaseF (peptide N-glycosidase F) and Endo H (endoglycosidase H). Endo H cleaves two proximal N-acetylglucosamine residues of the high-mannose type but not of the complex type, while PNGaseF cleaves sugar chains of both types. Treatment with either Endo H or PNGaseF increased the electrophoretic mobility of ABCA17 protein from 200 kDa (Figure 4C, arrow) to 180 kDa (Figure 4C, arrowhead), which represents the deglycosylated form, indicating that ABCA17 is modified with high-mannose-type sugar chains.
Figure 4. Immunoblot analysis of ABCA17 protein expressed in COS-1 cells.
(A, B) Membrane proteins (30 μg) prepared from COS-1 cells transfected with pCMV vector alone or pCMVmABCA17-HA were electrophoresed on a SDS/8% (w/v)-polyacrylamide gel and immunoblotted with anti-HA antibody (A) and with anti-ABCA17 antibody (B). Both anti-HA and anti-ABCA17 antibodies detected the 200 kDa ABCA17–HA fusion protein. (C) Glycosylation of ABCA17. Membrane proteins (30 μg) prepared from COS-1 cells transfected with pCMVmABCA17-HA were incubated with 1 unit of PNGaseF (Roche) and 0.005 unit of Endo H (Roche) for 1 h at 37 °C, followed by immunoblot analysis with anti-HA antibody. Molecular masses of the marker proteins are indicated on the left, and the detected 200 kDa and 180 kDa bands are indicated by the arrow and arrowhead respectively.
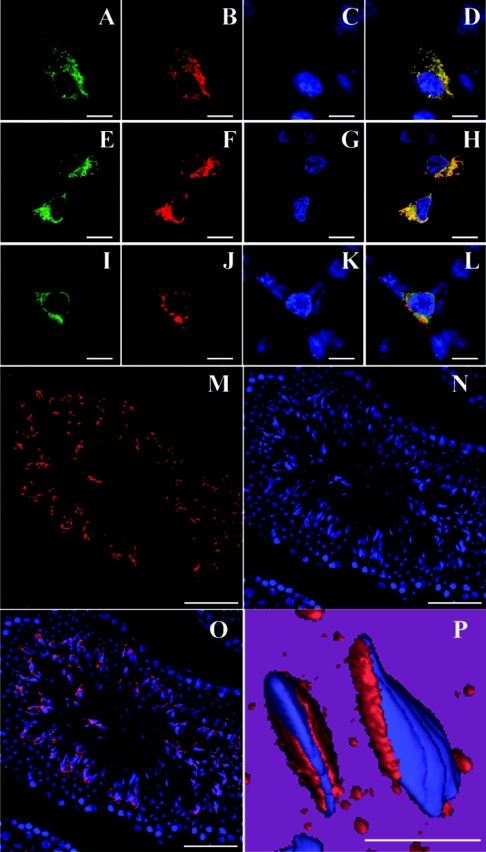
To further confirm the specificity of the anti-ABCA17 antibody, HEK293 cells were transfected with pCMVmABCA17-HA or pCMV plasmids, and immunolabelled using anti-ABCA17 and anti-HA antibodies. Immunolabelling with anti-ABCA17 and anti-HA antibodies was completely merged (Figures 5A–5D), while no staining was observed with either of the antibodies in cells mock-transfected with pCMV (results not shown), indicating that the anti-ABCA17 antibody specifically stains ABCA17 protein.
Figure 5. Immunofluorescence labelling of ABCA17 protein in ABCA17-transfected cells and mouse testis.
(A–D) Confocal microscopic images of HEK293 cells expressing ABCA17–HA fusion protein. Cells were double-labelled with anti-HA antibody (A; green) and anti-ABCA17 antibody (B; red), and visualized with FITC and Cy3 respectively. Nuclei were counterstained by DAPI (C; blue). A merged picture of (A), (B) and (C) is shown in (D). (E–H) Confocal microscopic images of HEK293 cells co-expressing ABCA17–HA fusion protein and DsRed2-ER. ABCA17–HA fusion protein immunolabelled with anti-HA antibody was visualized with FITC (E; green), and the ER was observed with DsRed2 fluorescence (F; red). Nuclei were counterstained by DAPI (G; blue). A merged picture of (E), (F) and (G) is shown in (H). (I–L) Confocal microscopic images of HEK293 cells co-expressing ABCA17–HA fusion protein and DsRed2-Mito. ABCA17–HA fusion protein immunolabelled with anti-HA antibody was visualized with FITC (I; green) and mitochondria were observed with DsRed2 fluorescence (J; red). Nuclei were counterstained by DAPI (K; blue). A merged picture of (I), (J) and (K) is shown in (L). (M–O) Confocal microscopic images of mouse testis. A frozen mouse testis section immunolabelled with anti-ABCA17 antibody was visualized with Cy3 (M; red). Nuclei were counterstained by DAPI (N; blue). A merged picture of (M) and (N), and its magnified three-dimensional image, are shown in (O) and (P) respectively. The scale bar represents 5 μm (A–L), 50 μm (M–O) and 10 μm (P).
To determine the subcellular localization of ABCA17 in HEK293 cells, the cells were co-transfected with pCMVmA-BCA17-HA and pDsRed2-ER (DsRed2 expression plasmid containing an ER localization signal) or pDsRed2-Mito (DsRed2 expression plasmid containing a mitochondrial localization signal). Immunostaining with anti-HA antibody was merged with the DsRed2 fluorescence of the ER (Figures 5E–5H) but not with that of mitochondria (Figures 5I–5L), suggesting that ABCA17 protein is localized in intracellular organelles, especially the ER, when expressed in HEK293 cells.
To determine the localization of ABCA17 protein in the mouse adult testis, immunohistochemical analysis was performed using the anti-ABCA17 antibody. Immunoreactivity for ABCA17 was detected predominantly in elongated spermatids at the late stage of germ cell development and in sperm (Figures 5M–5O). No signal was detected in immature germ cells such as spermatogonia and spermatocytes, or in somatic cells such as Sertoli cells (Figures 5M–5O) and Leydig cells (results not shown). In the magnified three-dimensional image, subcellular localization of ABCA17 was observed in the anterior head region (Figure 5P). Immunoreactivity for ABCA17 was adsorbed by the synthetic peptide corresponding to 19 C-terminal amino acid residues of ABCA17 in mouse testis (results not shown).
Involvement of ABCA17 in the regulation of cellular neutral lipid content
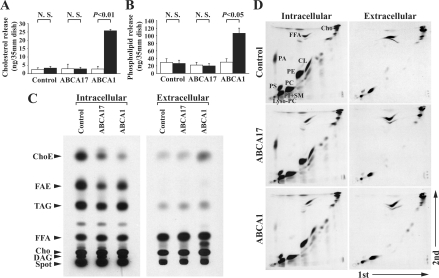
To determine the functional significance of ABCA17, we examined its involvement in cellular cholesterol and phospholipid efflux. Untransfected control HEK293 cells and cells stably expressing ABCA1 or ABCA17 were cultured in medium supplemented with 0.1% fatty acid-free BSA in the presence of 10 μg/ml apoA-I for 24 h, and the lipids in the medium were measured. The amounts of total cholesterol and choline-phospholipids released from ABCA17-expressing cells were not altered compared with control cells, while amounts released from ABCA1-expressing cells were dramatically increased by 11.3-fold and 3.6-fold respectively, as reported previously [10–12,16,22] (Figures 6A and 6B).
Figure 6. Lipid metabolism of HEK293 cells stably expressing ABCA17 or ABCA1.
(A, B) Release of cholesterol (A) and choline-phospholipids (B) from ABCA17- and ABCA1-expressing cells and untransfected control cells. Cells were cultured in the absence (open bars) or presence (closed bars) of 10 μg/ml apoA-I and 0.1% fatty acid-free BSA for 24 h, and released cholesterol and choline-phospholipids were measured. Values represent means±S.D. of three independent experiments; N.S., not significant. (C, D) Metabolic labelling of neutral lipids (C) and phospholipids (D) of ABCA17- and ABCA1-expressing cells and untransfected control cells. Intracellular 14C-labelled lipids and extracellular 14C-labelled lipids released from cells incubated with [14C]acetate for 72 h were separated by TLC, and representative autoradiographs are shown. Similar results were obtained from at least three independent experiments, and the radioactivity of each of the 14C-labelled lipid species quantified by FLA-5000 is shown in Table 1. ChoE, cholesteryl esters; FAE, fatty acid esters; TAG, triacylglycerols; FFA, non-esterified (free) fatty acids; Cho, cholesterol; DAG, diacylglycerols; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SM, sphingomyelin; PS, phosphatidylserine; PA, phosphatidic acid; CL, cardiolipin; Lyso-PC, lyso-phosphatidylcholine.
To determine if ABCA17 is involved in the regulation of lipid metabolism, untransfected control HEK293 cells and cells stably expressing ABCA1 or ABCA17 were cultured in medium supplemented with 10% (v/v) FCS in the presence of [14C]acetate for 72 h, and the amounts of 14C-labelled intracellular neutral lipids and extracellular lipids in the medium (i.e. released from the cells) were measured. Intracellular 14C-labelled cholesteryl esters in ABCA17-expressing cells were markedly decreased by 77.5% compared with control cells, similar to the situation in ABCA1-expressing cells (Figure 6C and Table 1). In addition, amounts of 14C-labelled fatty acid esters (such as fatty acid methyl esters and fatty acid ethyl esters) and triacylglycerols in ABCA17-expressing cells were also decreased by 73.5% and 41.5% respectively, while levels in ABCA1-expressing cells were not altered (Figure 6C and Table 1). On the other hand, the amount of extracellular 14C-labelled cholesteryl esters released from ABCA17-expressing cells was similar to that released from control cells, whereas release from ABCA1-expressing cells was dramatically increased by 14.5-fold, consistent with the results for cholesterol efflux shown in Figure 6(A) (Figure 6C and Table 1). The amounts of 14C-labelled triacylglycerols, non-esterified fatty acids and cholesterol in the medium released from ABCA17-expressing cells were slightly but significantly increased by 1.3-fold, 1.4-fold and 1.3-fold respectively compared with control cells, but the increment in cholesterol was much smaller than in ABCA1-expressing cells. In addition, the amounts of 14C-labelled intracellular phospholipids and extracellular lipids in the medium were measured (Figure 6D and Table 1). The intracellular 14C-labelled phospholipids examined (except for phosphatidylethanolamine, which was slightly decreased) were not altered in ABCA17-expressing cells compared with control cells. The levels of phosphatidylcholine, lyso-phosphatidylcholine and sphingomyelin plus phosphatidylinositol released from ABCA17-expressing cells were similar to those from control cells, while the release of these phospholipids from ABCA1-expressing cells was dramatically increased, consistent with the results for choline-phospholipid efflux shown in Figure 6(B) (Figure 6D and Table 1). These results indicate that ABCA17 plays a distinct role in lipid metabolism by decreasing the levels of intracellular esterified neutral lipids, such as cholesteryl esters, fatty acid esters and triacylglycerols.
Table 1. Quantification of metabolically 14C-labelled neutral lipids and phospholipids of ABCA17- and ABCA1-expressing and untransfected control HEK293 cells.
The radioactivity of each of 14C-labelled lipid species, separated by TLC as shown in Figures 6(C) and 6(D), was counted, and values are represented as means±S.D. Significance: *P<0.05, **P<0.005 compared with control. ChoE, cholesteryl esters; FAE, fatty acid esters; TAG, triacylglycerols; NEFA, non-esterified fatty acids; Chol, cholesterol; DAG, diacylglycerols; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SM, sphingomyelin; PS, phosphatidylserine; PA, phosphatidic acid; CL, cardiolipin; Lyso-PC, lyso-phosphatidylcholine; N.D., not detected.
| Intracellular (d.p.m./1×104 cells) | Extracellular (d.p.m./1×104 cells) | |||||
|---|---|---|---|---|---|---|
| Lipids | Control | ABCA17 | ABCA1 | Control | ABCA17 | ABCA1 |
| Neutral lipids | (n=11) | (n=10) | (n=4) | (n=11) | (n=10) | (n=4) |
| ChoE | 14.72±4.91 | 3.31±1.20** | 2.06±0.65** | 0.57±0.25 | 0.75±0.41 | 8.25±2.15** |
| FAE | 17.72±6.57 | 4.69±1.67** | 12.55±4.37 | N.D.b | N.D. | N.D. |
| TAG | 17.69±6.48 | 10.35±2.99** | 25.11±6.73 | 0.91±0.15 | 1.17±0.12** | 2.50±0.37** |
| NEFA | 11.92±1.89 | 12.61±2.29 | 16.20±1.79** | 30.18±5.26 | 43.75±6.62** | 52.81±13.28** |
| Chol | 57.83±12.34 | 70.60±17.27 | 112.51±27.24** | 44.68±6.49 | 58.08±5.90** | 181.16±13.15** |
| DAG | 15.69±5.18 | 19.51±7.58 | 22.30±4.07* | N.D. | N.D. | N.D. |
| Phospholipids | (n=4) | (n=4) | (n=3) | (n=4) | (n=4) | (n=3) |
| PC | 394.49±58.34 | 354.38±64.84 | 375.73±62.98 | 12.79±2.62 | 12.57±1.95 | 41.40±3.89** |
| PE | 147.38±20.07 | 103.48±24.53* | 117.36±26.44 | 0.23±0.07 | 0.11±0.03* | 0.26±0.04 |
| PI+SM | 70.96±12.03 | 65.19±13.70 | 91.51±18.04 | 4.38±0.87 | 3.36±0.67 | 11.14±1.23** |
| PS | 39.15±4.42 | 34.66±7.44 | 32.98±8.39 | 0.53±0.11 | 0.21±0.05** | 0.18±0.02** |
| PA | 6.67±4.57 | 6.32±5.32 | 1.07±0.40 | N.D. | N.D. | N.D. |
| CL | 34.97±8.64 | 23.79±6.83 | 12.57±5.98* | N.D. | N.D. | N.D. |
| Lyso-PC | 3.50±0.56 | 4.22±1.01 | 3.17±0.80 | 0.12±0.02 | 0.12±0.01 | 0.58±0.05** |
DISCUSSION
We have cloned a novel transporter, ABCA17, which belongs to the A subclass of the ABC transporter family. ABCA17 mRNA is expressed exclusively in spermatocytes at the early stage of spermatogenesis, and the protein is expressed predominantly in elongated spermatids at the later stage of spermatogenesis and in sperm in the seminiferous tubule of testis. The amino acid identity between mouse ABCA17 and mouse ABCA3 is 55.3%, and that between mouse ABCA17 and suABCA [32] is 36.7%. The amino acid identity between suABCA and mouse ABCA3 is 39.7%, indicating that suABCA has similar identity to ABCA3 as to ABCA17. Since the ABCA17 gene is at the same locus as the ABCA3 gene in mouse and rat, the suABCA gene could well be a common ancestor of both genes. However, suABCA is expressed in sperm [32], as is ABCA17, while ABCA3 is expressed predominantly in lung alveolar type II cells [26], indicating a function more similar to that of suABCA for ABCA17. Even so, the localization of suABCA and ABCA17 in sperm differs: suABCA is expressed in the entire membrane of sperm in a punctate pattern [32], while ABCA17 is shown in the present study to be expressed at the anterior head of sperm. In addition, the orthologue of ABCA17 has not been isolated in humans.
It is well known that testicular sperm in mammals is morphologically differentiated, yet not able to fertilize the egg, and that sperm acquire the ability to fertilize during resistance in the lumen of the female reproductive tract. This process, capacitation, involves biochemical and physiological changes in the sperm that prepare the cell to undergo the acrosome reaction. Although capacitation occurs in the female reproductive tract in vivo, it can be achieved in vitro, serum albumin being the essential component of in vitro capacitation media [7,33]. Davis et al. [9] found that a decrease in the cholesterol/phospholipid ratio destabilizes sperm membrane, promoting the acrosomal reaction and capacitation. Although the role of albumin in in vitro capacitation is not well understood, it is possible that albumin binds lipids, modulating the cholesterol/phospholipid ratio in sperm in a non-specific manner. Go and Wolf [8] reported that the sterol-releasing activity of albumin accounts for this effect in the mouse. However, the mechanism responsible for the decrease in cholesterol in sperm in the physiological situation is unknown.
Recently, the function of ABCA1 and ABCA7 was proposed to be promotion of cellular cholesterol and phospholipid efflux to apoA-I, although the involvement of ABCA7 in cholesterol efflux is controversial [21,22]. In the present study, we investigated the promotion by ABCA17 of cellular lipid efflux to albumin or apolipoproteins, and found that efflux of neither cholesterol nor phospholipid to albumin and apoproteins, including apoA-I, apoE (results not shown) and apoJ (results not shown), was facilitated by ABCA17. Metabolic labelling analysis using [14C]acetate showed that expression of ABCA17 in HEK293 cells reduced the content of intracellular esterified neutral lipids, such as cholesteryl esters, fatty acid esters and triacylglycerols, which suggests that ABCA17 could be involved in capacitation through regulation of the cholesterol/phospholipid ratio in sperm. If this is the case, because capacitation also is observed in human sperm, other ABC transporters, such as ABCA1 and ABCG1, might substitute for ABCA17 in humans, as a functional ABCA17 gene has not yet been identified in humans or other non-human primates. On the other hand, in mice, ABCA1 mRNA is expressed abundantly in Sertoli cells, but at low levels in isolated germ cells, and ABCA1 mediates lipid efflux from Sertoli cells, which affects male fertility [34]. In additon, ABCG1 mRNA is reported not to be expressed in human testis [35]. We have confirmed in mice by immunohistochemical analysis that ABCA1 and ABCG1 proteins are not expressed in germ cells or sperm, but are expressed mainly in interstitial cells in testis (Supplemental Figure 1; available at http://www.BiochemJ.org/bj/389/bj3890577add.htm). In this context, ABC transporters other than ABCA1 and ABCG1 might be involved in the decrease in cholesterol levels observed in human sperm during capacitation.
The mechanism by which ABCA17 decreases the levels of intracellular esterified neutral lipids is not known. Since ABCA17 is localized on the membrane of the ER when expressed in HEK293 cells, and since esterified neutral lipids such as cholesteryl esters, fatty acid esters and triacylglycerols are synthesized in ER and microsomes [36–40], ABCA17 might be involved in sorting esterified neutral lipids from the ER to other intracellular compartments. On the other hand, ABCA17 expression in HEK293 cells may not reflect its function in sperm, which have a unique structure quite distinct from that of HEK293 cells, and the component in the anterior head of sperm corresponding to the ER is uncertain. Further investigations using sperm are required in order to clarify the physiological role of ABCA17.
Online data
Acknowledgments
This work was supported by Scientific Research Grants and a Grant-in-Aid for Creative Scientific Research (15GS0301) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Dr Sumiko Abe-Dohmae and Dr Shinji Yokoyama (Nagoya City University, Nagoya, Japan) for their helpful advice on cholesterol and phospholipid efflux assay, Dr Toyoshi Fujimoto (Nagoya University) and Dr Katsuya Yamada (Akita University) for helpful discussions, and Mr Hiroki Sato and Ms Mai Hirakawa for technical assistance.
References
- 1.Kopf G. S., Visconti P. E., Galantino-Homer H. Capacitation of the mammalian spermatozoon. Adv. Dev. Biochem. 1999;5:83–107. [Google Scholar]
- 2.Davis B. K. Timing of fertilization in mammals: Sperm cholesterol/phospholipid ratio as a determinant of the capacitation interval. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7560–7564. doi: 10.1073/pnas.78.12.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross N. L. Role of cholesterol in sperm capacitation. Biol. Reprod. 1998;59:7–11. doi: 10.1095/biolreprod59.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Visconti P. E., Westbrook V. A., Chertihin O., Demarco I., Sleight S., Diekman A. B. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002;53:133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 5.Benoff S. Preliminaries to fertilization. The role of cholesterol during capacitation of human spermatozoa. Hum. Reprod. 1993;8:2001–2008. doi: 10.1093/oxfordjournals.humrep.a137971. [DOI] [PubMed] [Google Scholar]
- 6.Travis A. J., Kopf G. S. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 2002;110:731–736. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis B. K. Influence of serum albumin on the fertilizing ability in vitro of rat spermatozoa. Proc. Soc. Exp. Biol. Med. 1976;151:240–243. doi: 10.3181/00379727-151-39182. [DOI] [PubMed] [Google Scholar]
- 8.Go K. J., Wolf D. P. Albumin-mediated changes in sperm sterol content during capacitation. Biol. Reprod. 1985;32:145–153. doi: 10.1095/biolreprod32.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Davis B. K., Byrne R., Bedigian K. Studies on the mechanism of capacitation: Albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1546–1550. doi: 10.1073/pnas.77.3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden M. R., Clee S. M., Brooks-Wilson A., Genest J., Jr, Attie A., Kastelein J. J. P. Cholesterol efflux regulatory protein, Tangier disease and familial high-density lipoprotein deficiency. Curr. Opin. Lipidol. 2000;11:117–122. doi: 10.1097/00041433-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Oram J. F., Vaughan A. M. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr. Opin. Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz G., Langmann T. Structure, function and regulation of the ABC1 gene product. Curr. Opin. Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Shulenin S., Nogee L. M., Annilo T., Wert S. E., Whitsett J. A., Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N. Engl. J. Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 14.Allikmets R. Simple and complex ABCR: genetic predisposition to retinal disease. Am. J. Hum. Genet. 2000;67:793–799. doi: 10.1086/303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefèvre C., Audebert S., Jobard F., Bouadjar B., Lakhdar H., Boughdene-Stambouli O., Blanchet-Bardon C., Heilig R., Foglio M., Weissenbach J., et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum. Mol. Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- 16.Orsó E., Broccardo C., Kaminski W. E., Böttcher A., Liebisch G., Drobnik W., Götz A., Chambenoit O., Diederich W., Langmann T., et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 17.Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 18.Nagata K., Yamamoto A., Ban N., Tanaka A. R., Matsuo M., Kioka N., Inagaki N., Ueda K. Human ABCA3, a product of a responsible gene for abca3 for fatal surfactant deficiency in newborns, exhibits unique ATP hydrolysis activity and generates intracellular multilamellar vesicles. Biochem. Biophys. Res. Commun. 2004;324:262–268. doi: 10.1016/j.bbrc.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Klucken J., Büchler C., Orsó E., Kaminski W. E., Porsch-Özcürümez M., Liebisch G., Kapinsky M., Diederich W., Drobnik W., Dean M., Allikmets R., Schmitz G. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. U.S.A. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminski W. E., Piehler A., Püllmann K., Porsch-Özcürümez M., Duong C., Bared G. M., Büchler C., Schmitz G. Complete coding sequence, promoter region, and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 2001;281:249–258. doi: 10.1006/bbrc.2001.4305. [DOI] [PubMed] [Google Scholar]
- 21.Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., Chen W., Martinez L. O., Tall A. R. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 22.Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., Yokoyama S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 2004;279:604–611. doi: 10.1074/jbc.M309888200. [DOI] [PubMed] [Google Scholar]
- 23.Dean M., Hamon Y., Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid. Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 24.Inagaki N., Gonoi T., Clement J. P., IV, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L.-X., Zhou C.-J., Tanaka A., Nakata M., Hirabayashi T., Amachi T., Shioda S., Ueda K., Inagaki N. Cloning, characterization and tissue distribution of the rat ATP-binding cassette (ABC) transporter ABC2/ABCA2. Biochem. J. 2000;350:865–872. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamano G., Funahashi H., Kawanami O., Zhao L.-X., Ban N., Uchida Y., Morohoshi T., Ogawa J., Shioda S., Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y., Yamada K., Zhou C.-J., Ban N., Shioda S., Inagaki N. Temporal and spatial profiles of ABCA2-expressing oligodendrocytes in the developing rat brain. J. Comp. Neurol. 2003;455:353–367. doi: 10.1002/cne.10493. [DOI] [PubMed] [Google Scholar]
- 28.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Waterston R. H., Lindblad-Toh K., Birney E., Rogers J., Abril J. F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., et al. Initial sequencing and comparative analysis of the mouse genome. Nature (London) 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki Y., Furuno M., Kasukawa T., Adachi J., Bono H., Kondo S., Nikaido I., Osato N., Saito R., Suzuki H., et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature (London) 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs R. A., Weinstock G. M., Metzker M. L., Muzny D. M., Sodergren E. J., Scherer S., Scott G., Steffen D., Worley K. C., Burch P. E., et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature (London) 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 32.Mengerink K. J., Vacquier V. D. An ATP-binding cassette transporter is a major glycoprotein of sea urchin sperm membranes. J. Biol. Chem. 2002;277:40729–40734. doi: 10.1074/jbc.M207184200. [DOI] [PubMed] [Google Scholar]
- 33.Yanagimachi R. In vitro capacitation of golden hamster spermatozoa by homologous and heterologous blood sera. Biol. Reprod. 1970;3:147–153. doi: 10.1093/biolreprod/3.2.147. [DOI] [PubMed] [Google Scholar]
- 34.Selva D. M., Hirsch-Reinshagen V., Burgess B., Zhou S., Chan J., McIsaac S., Hayden M. R., Hammond G. L., Vogl A. W., Wellington C. L. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J. Lipid Res. 2004;45:1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Chen H., Rossier C., Lalioti M. D., Lynn A., Chakravarti A., Perrin G., Antonarakis S. E. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am. J. Hum. Genet. 1996;59:66–75. [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce C. W., Shelness G. S., Davis M. A., Lee R. G., Skinner K., Anderson R. A., Rudel L. L. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol. Biol. Cell. 2000;11:3675–3687. doi: 10.1091/mbc.11.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaphalia B. S., Fritz R. R., Ansari G. A. S. Purification and characterization of rat liver microsomal fatty acid ethyl and 2-chloroethyl ester synthase and their relationship with carboxylesterase (pI 6.1) Chem. Res. Toxicol. 1997;10:211–218. doi: 10.1021/tx960079e. [DOI] [PubMed] [Google Scholar]
- 38.Kaphalia B. S., Ansari G. A. S. Purification and characterization of rat hepatic microsomal low molecular weight fatty acid ethyl ester synthase and its relationship to carboxylesterases. J. Biochem. Mol. Toxicol. 2001;15:165–171. doi: 10.1002/jbt.14. [DOI] [PubMed] [Google Scholar]
- 39.Kaphalia B. S., Ansari G. A. S. Purification and characterization of rat pancreatic fatty acid ethyl ester synthase and its structural and functional relationship to pancreatic cholesterol esterase. J. Biochem. Mol. Toxicol. 2003;17:338–345. doi: 10.1002/jbt.10097. [DOI] [PubMed] [Google Scholar]
- 40.Owen M. R., Corstorphine C. C., Zammit V. A. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 1997;323:17–21. doi: 10.1042/bj3230017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.