Abstract
c-type cytochromes are characterized by covalent attachment of haem to the protein by two thioether bonds formed between the haem vinyl groups and the cysteine sulphurs in a CXXCH peptide motif. In Escherichia coli and many other Gram-negative bacteria, this post-translational haem attachment is catalysed by the Ccm (cytochrome c maturation) system. The features of the apocytochrome substrate required and recognized by the Ccm apparatus are uncertain. In the present study, we report investigations of maturation of cytochrome b562 variants containing CXXCR, CXXCK or CXXCM haem-binding motifs. None of them showed any evidence for correct maturation by the Ccm system. However, we have determined, for each variant, that the proteins (i) were expressed in large amounts, (ii) could bind haem in vivo and/or in vitro and (iii) were not degraded in the cell. Together with previous observations, these results strongly suggest that the apocytochrome substrate feature recognized by the Ccm system is simply the two cysteine residues and the histidine of the CXXCH haem-binding motif. Using the same experimental approach, we have also investigated a cytochrome b562 variant containing the special CWSCK motif that binds the active-site haem of E. coli nitrite reductase NrfA. Whereas a CWSCH analogue was matured by the Ccm apparatus in large amounts, the CWSCK form was not detectably matured either by the Ccm system or by the dedicated Nrf biogenesis proteins, implying that the substrate recognition features for haem attachment in NrfA may be more extensive than the CWSCK motif.
Keywords: c-type cytochrome biogenesis, cytochrome b562, cytochrome c maturation (Ccm), haem-binding motif, NrfA, post-translational modification
Abbreviations: Ccm, cytochrome c maturation; ESI, electrospray ionization
INTRODUCTION
c-type cytochromes are characterized by covalent attachment of haem to the protein by two thioether bonds formed between the haem vinyl groups and the cysteine sulphurs in a CXXCH peptide motif [1–3]. Their haem iron is typically ligated by two amino-acid side chains from the protein, with the proximal ligand being the histidine from the CXXCH haem-binding motif and the distal ligand being provided from elsewhere on the polypeptide. In b-type cytochromes, haem is non-covalently bound. Cytochrome b562 is a 12 kDa soluble protein, of uncertain function, from the periplasm of Escherichia coli [4]. It has a four-α-helix structure with the haem iron co-ordinated by Met7 and His102 [5]. Cytochrome b562 has been mutated at Arg98 and Tyr101 to create a CXXCH c-type cytochrome haem attachment motif [6]. This variant protein can form stable holocytochrome c when expressed in the periplasm of E. coli in conjunction with the periplasmically functioning E. coli cytochrome c biogenesis system [the Ccm (cytochrome c maturation) apparatus] and is, under those conditions, a correctly matured, homogeneous c-type cytochrome [7]. In contrast, when the protein was expressed in the absence of the Ccm system, a heterogeneous mixture of incorrectly matured c-type cytochromes was observed [6,7]. Notably, in each case, a large amount of stable apo (haem-free) protein was also obtained.
Remarkably, three distinct cytochrome c biogenesis systems have been identified in different cell types [3,8] and at least one more is anticipated [9]. One of these, the Ccm system, found in many Gram-negative bacteria and plant mitochondria, acts on a wide variety of apocytochromes with differing structures, folds and origins [10,11]. As yet, the precise features in the apocytochrome recognized by the Ccm system are unknown, but the localized region around the CXXCH motif has been strongly implicated [10,12,13] and the system will not act on either natural or variant apocytochromes containing only one cysteine residue, rather than the usual two, in the haem-binding motif [9,14]. The role of the conserved histidine remains of particular interest in this context. Nitrite reductase (NrfA) has, in E. coli and many other Gram-negative bacteria, a unique CWXCK haem-binding motif at its active site (X=S, T, V or N in cases identified so far) [15], and haem attachment to this centre requires additional dedicated biogenesis proteins homologous with two components of the Ccm system [16]. We have recently investigated maturation by the Ccm system of several cytochromes with variant (CXXCM or CXXCK) haem-binding motifs, but negative results were obtained [17]. However, it was not clear whether that was due to a failure to mature the cytochromes or, for example, proteolysis after haem attachment.
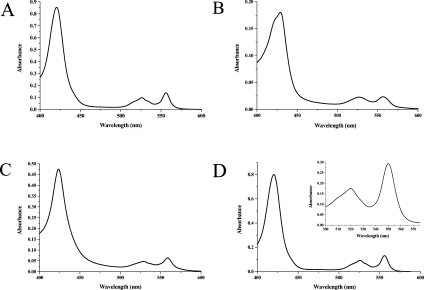
We have therefore extended our experiments using the cytochrome b562-derived c-type cytochrome [6,7], which has the following major advantages. (i) The apoprotein and various forms of the holoprotein are, unlike almost all other apocytochromes c, stable. This allows, in every case, for the positive detection of expressed proteins and confirmation of the correct mutations by MS. (ii) Correctly and incorrectly matured c-type holocytochrome products can be readily assessed and distinguished using absorption spectroscopy [6,7]. c-type cytochromes with reduced low-spin haem iron give rise to three absorption peaks in the visible region: the γ (or Soret), β and α-bands (Figure 1A). For cytochromes c with correct haem attachment through two thioether bonds, the α-band occurs in the range 549–556 nm. For the CXXCH variant of cytochrome b562 matured by the Ccm system, which has been shown by NMR to have the haem attached through two thioether bonds with the correct (universally conserved) stereochemistry, the α-peak is at 556 nm (Figure 1A) [6,7]. However, as mentioned above, b562 CXXCH can also form holocytochromes with incorrect covalent haem attachment, e.g. where the haem is inverted and attached through only one thioether bond [7]. This occurs in vivo when the Ccm apparatus is not expressed and also in vitro, i.e. when the attachment is not enzyme-catalysed and is apparently uncontrolled. In these cases, the α-peak of the absorption spectrum is significantly red-shifted, from 556 nm to 558–560 nm [6,7]. Reduced pyridine haemochrome spectra are highly indicative of the nature of covalent haem attachment to a protein, while being independent of that protein. Thus they provide a further means of investigating cytochrome maturation. In such experiments, the protein is saturated with pyridine/hydroxide solution, resulting in haem iron with bis-pyridinyl co-ordination. Non-covalently bound haem (as in haemoglobin or wild-type cytochrome b562) gives rise to an α-peak at 556 nm when so treated; c-type cytochromes with two thioether linkages (e.g. mitochondrial cytochrome c and Ccm matured b562 CXXCH) diagnostically have this peak at 550 nm (e.g. [7]), whereas natural cytochromes c with one thioether bond (e.g. from mitochondria of the Euglenozoa) have the peak at 552–3 nm (e.g. [9]). Thus there are very reliable spectral characteristics that can be used to assess the nature of haem attachment and hence the maturation of c-type cytochromes, including variants of cytochrome b562 (e.g. [6,7]).
Figure 1. Absorption spectra of c-type cytochrome variants of E. coli cytochrome b562.
(A) Unpurified periplasmic extracts of E. coli transformed with plasmids for the E. coli Ccm system and cytochrome b562 with a CNACH variant haem-binding motif (after Allen et al. [7]). The spectrum was recorded at 25 °C after the addition of a few grains of dithionite to the periplasmic proteins in 100 mM Tris/HCl (pH 8.0), 0.5 mM EDTA and 0.25 M sucrose. The spectral maxima were 420, 526 and 556 nm. On the basis of the data and molar absorption coefficients given in [6,7], the holocytochrome concentration was 5.8 μM. Such a holocytochrome when purified was independently examined by NMR spectroscopy and found to be properly matured, i.e. homogeneous, with haem covalently attached to the protein through two thioether bonds with the ‘correct’, universally conserved, stereochemistry [7]. (B) E. coli holocytochrome b562 with a CXXCM variant haem-binding motif. This protein was purified from periplasmic extracts of E. coli using Q-Sepharose chromatography resin and was subsequently reduced with disodium dithionite. The spectrum was recorded at 25 °C in 50 mM Tris/HCl (pH 8.0). Observed spectral maxima were 429, 526 and 557 nm, with a prominent shoulder at approx. 422 nm. There is no molar absorption coefficient available for this protein, but based on the data in [6], the holocytochrome concentration can be estimated as approx. 1.2 μM. (C) Unpurified periplasmic extracts of E. coli transformed with the plasmid for E. coli cytochrome b562 with a CNACK variant haem-binding motif. The spectrum was recorded at 25 °C after the addition of a few grains of dithionite to the periplasmic proteins in 100 mM Tris/HCl (pH 8.0), 0.5 mM EDTA and 0.25 M sucrose. The spectral maxima were 423, 529 and 559 nm. There is no molar absorption coefficient available for this protein, but based on the data in [6], the holocytochrome concentration can be estimated as approx. 3.3 μM. (D) Unpurified periplasmic extracts of E. coli transformed with plasmids for the E. coli Ccm system and cytochrome b562 with a CWSCH variant haem-binding motif. The spectrum was recorded at 25 °C after the addition of a few grains of dithionite to the periplasmic proteins in 20 mM Tris/HCl (pH 8.0), 0.1 mM EDTA and 0.1 M sucrose. The spectral maxima were 420, 526 and 556 nm. On the basis of the data and molar absorption coefficients given in [6,7], the holocytochrome concentration was 5.5 μM. Inset: reduced pyridine haemochrome spectrum of the same periplasmic extract. Final concentrations of hydroxide and pyridine were 0.2 M and 30% (v/v) respectively. The peak maximum was at 550.0 nm, characteristic of haem attachment to the protein through two thioether bonds.
In this paper, we report investigations of the expression and Ccm-dependent maturation of CXXCR, CXXCM, CXXCK and CWSCK variants of cytochrome b562, each thus being mutated in the proximal haem iron ligand His102 (the histidine of the CXXCH motif). Methionine is, in common with histidine, frequently a strong field ligand to the haem iron of c-type cytochromes. For example, in mitochondrial cytochrome c, the haem iron has a histidine residue from the CXXCH motif as its proximal ligand and a methionine residue as the distal ligand. Thus it is interesting and important to determine whether Met can substitute for the histidine in the CXXCH motif during c-type cytochrome maturation. A cytochrome b562 variant in which His102 was replaced by methionine, creating a bis-Met-co-ordinated b-type haem, has been characterized previously [18,19]. Barker et al. [18,19] also produced a b562 variant with bis-Met haem co-ordination and (non-Ccm-dependent) covalent attachment through one thioether bond. The present study investigates a protein capable of bis-Met haem iron co-ordination and formation of two thioether bonds from protein to haem. This is the appropriate variant to assess the ability of the Ccm system to mature a CXXCM-containing c-type cytochrome. The CXXCK and CWSCK b562 variants in the present work are models for the active-site haem of NrfA. In that case, the covalently attached haem has a lysine as the proximal iron ligand, but there is no distal ligand bound (i.e. the haem is penta-co-ordinate and capable of binding the substrate). However, several CXXCH c-type cytochromes with penta-co-ordinate haem are matured by the Ccm apparatus and it therefore seems apparent that the covalent haem attachment process is independent of a distal ligand to the iron ([17] and references therein). Two groups have investigated Saccharomyces cerevisiae cytochrome c where the invariant axial histidine ligand has been mutated to an arginine to give a CXXCR haem-binding motif [20,21]. In one case, the cytochrome c H18R (His18→Arg) variant [20] grew on non-fermentable carbon sources, implying the maturation of the cytochrome by the c-type cytochrome biogenesis apparatus of yeast (which is different from that of E. coli [22]). Thus the effect of mutation of the proximal histidine residue to an arginine on cytochrome c biogenesis is intriguing and requires investigation in the context of the Ccm system.
EXPERIMENTAL
Plasmid construction
Site-directed mutagenesis was performed by the Quik Change method (Stratagene, Cambridge, U.K.) using Pfx DNA polymerase (Invitrogen) according to the manufacturer's instructions. The plasmid for cytochrome b562 R98C/Y101C (CNACH haembinding motif) was as described by Barker et al. [6] and was used as the template for further mutagenesis. Three His102 mutants (CXXCH→M, CXXCH→R and CXXCH→K), a CWSCH double mutant and a CWSCK triple mutant were constructed. Mutagenesis primers were (i) CNACH to CNACK: 5′-CTGAAAACGACCTGCAACGCCTGTAAGCAGAAGTATCGT and 5′-ACGATACTTCTGCTTACAGGCGTTGCAGGTCGTTTTCAG; (ii) CNACH to CNACM: 5′-GCAACTGAAAACGACCTGCAACGCCTGTATGCAGAAGTATCGT and 5′-ACGATACTTCTGCATACAGGCGTTGCAGGTCGTTTTCAGTTGC; (iii) CNACH to CNACR: 5′-CTGAAAACGACCTGCAACGCCTGTCGCCAGAAGTATCGT and 5′-ACGATACTTCTGGCGACAGGCGTTGCAGGTCGTTTTCAG; (iv) CNACH to CWSCH: 5′-GAGCAACTGAAAACGACCTGCTGGTCCTGTCACCAGAAGTATCG and 5′-CGATACTTCTGGTGACAGGACCAGCAGGTCGTTTTCAGTTGCTC; (v) CNACK TO CWSCK: 5′-GCAGAGCAACTGAAAACGACCTGCTGGTCCTGTAAGCAGAAGTATCG and 5′-CGATACTTCTGCTTACAGGACCAGCAGGTCGTTTTCAGTTGCTCTGC. The Ccm system was expressed from the plasmid pEC86 [23], which contains the E. coli genes ccmABCDEFGH. pKK223-3 (Amersham Biosciences) was used as supplied by the manufacturer. Cytochrome variant plasmids were used only after DNA sequencing demonstrated the presence of the desired mutation(s), the correct periplasmic targeting sequence and the absence of any secondary mutation in the cytochrome gene.
Bacterial growth
E. coli strain JCB387 (a ΔnirB ΔlacF− derivative of strain RV) [24], a gift from Professor J. Cole (University of Birmingham, Birmingham, U.K.), was transformed with the appropriate plasmids and was used in all growth experiments. This strain was chosen because we have found in previous studies (e.g. [7,14,17]) that it reliably produces large amounts of holocytochromes c (including b562 CXXCH) and also apocytochrome b562 CXXCH. Expression of the endogenous E. coli Ccm apparatus was repressed by the use of aerobic growth conditions [25]. Transformants were initially plated on LB (Luria–Bertani) agar containing the appropriate antibiotics (100 μg/ml ampicillin, and 34 μg/ml chloramphenicol where pEC86 was co-transformed). For aerobic growth, single colonies were picked from these plates into 500 ml of 2×TY medium (16 g/l bacteriological peptone, 10 g/l yeast extract and 5 g/l NaCl) supplemented with 1 mM isopropyl β-D-thiogalactoside and antibiotics. Such cultures were grown in 2 litre flasks for 20–24 h at 37 °C with shaking at 200 rev./min before harvesting and fractionation. Periplasmic fractions of the cell cultures were obtained exactly as described in [7,14]. For anaerobic growth of E. coli, the medium described by Sambongi and Ferguson [26] was used. Such cultures were grown at 37 °C for 24–48 h in 50 ml tubes or 5 litre bottles filled to the top with antibiotic-supplemented medium. Periplasmic fractions from such cultures were obtained using essentially the same method as described in [14], but scaled in volume to reflect the cell mass.
Biochemical procedures
Cytochrome content of cell extracts was determined by recording the absorption spectra of samples to which a few grains of disodium dithionite had been added to reduce any cytochrome present. It was necessary to correct the total absorbance for low-level background (endogenous) cytochrome expression; see [17] for details of such corrections. Cytochrome b562 variants were purified using a DEAE-Sepharose or Q-Sepharose anion-exchange column, as described in [7]. SDS/PAGE analysis of protein expression used gel staining by one of two methods, either detecting the protein (Coomassie stain) or haem that was covalently attached to protein [27]. Reduced pyridine haemochrome spectra were obtained as described by Bartsch [28]. ESI–MS (electrospray ionization MS) was performed as described in [7] and was used to verify the identity, integrity and presence of appropriate mutations in all the cytochromes in the present study. For apoproteins containing a CXXC(H/R/K/M) motif, the expected mass given in the text, calculated from the peptide sequence, assumes formation of a disulphide bond between the cysteine residues (see [7]); masses quoted are for the apo (haem-free) proteins.
RESULTS AND DISCUSSION
Effect of proximal haem ligand residue change from histidine to arginine on cytochrome c biosynthesis
Cells were transformed with the plasmid encoding cytochrome b562 R98C/Y101C/H102R (containing the putative covalent haem attachment motif CXXCR). This variant was expressed in the presence of the Ccm apparatus expressed from plasmid pEC86. Absorption spectra of periplasmic extracts from such cells showed that there was no increase in holocytochrome production relative to expected endogenous (background) cytochrome expression levels (see the Experimental section). However, SDS/PAGE showed substantial periplasmic expression of protein of similar molecular mass to wild-type cytochrome b562, indicating the presence of large amounts of apo (i.e. haem-free) protein. Electrospray mass spectra of the purified apoprotein showed that the observed mass of the variant (11684 Da) was in agreement with the expected mass (11684 Da) and thus confirmed the presence of the desired mutation and that the protein was intact.
Previous evidence is inconclusive as to whether a proximal arginine residue can ligate the haem iron of a c-type cytochrome in vivo [20,21]. Work on S. cerevisiae iso-1-cytochrome c showed that a variant protein with a CXXCR haem-binding motif still possessed electron-transfer function, allowing cells to grow on non-fermentable carbon sources (i.e. where functional cytochrome c is required for respiration), albeit at retarded rates, when the wild-type gene was removed [20]. This implies that the variant c-type cytochrome was matured by the cytochrome c biogenesis system of yeast, the enzyme haem lyase [22]. However, expression levels of the variant protein were low and rigorous determination of its identity, e.g. by MS, was not performed. In contrast, another study with an S. cerevisiae cytochrome c H18R variant [21] suggested that arginine cannot act as the proximal haem ligand since not only did the transformed cells exhibit no growth on a non-fermentable medium (indicating loss of function), but also showed no evidence of the product cytochrome c. The present study, with a different c-type cytochrome and cytochrome c biogenesis apparatus, shows that, even in the presence of large amounts of stable apoprotein, Ccm-catalysed holocytochrome formation with a CXXCR haem-binding motif does not occur. The side chain of arginine can fold to a similar shape as that of histidine [20], so it could, in principle, be accommodated in the cytochrome.
Formation of the c-type R98C/Y101C/H102M variant of E. coli cytochrome b562
A plasmid encoding cytochrome b562 R98C/Y101C/H102M (i.e. with a CXXCM haem-binding motif) was transformed into cells with or without the ccm plasmid pEC86. Absorption spectra of the reduced periplasmic extracts from cells expressing the CXXCM protein showed the presence of a product c-type (covalent) cytochrome at well above background levels in each case. Covalent attachment of the haem to b562 CXXCM was confirmed by activity staining of SDS/PAGE gels with purified holocytochrome b562 CXXCH as a marker (results not shown). Coomassie-stained SDS/polyacrylamide gels of the periplasmic extracts from b562 CXXCM-transformed cells showed high levels of apoprotein expression. The apoprotein was purified and analysed by MS, which indicated the presence of the desired mutation (calculated and observed masses 11659 and 11658 Da respectively).
A major aim of the present study was to assess whether the Ccm system can mature cytochromes with variant (e.g. CXXCM) haem-binding motifs. Thus the CXXCM holocytochrome product produced in the presence and absence of pEC86 was analysed in more detail. It is insufficient to look only at the crude periplasmic extracts because cells grown with pEC86 produce more of the endogenous soluble E. coli c-type cytochromes (NrfA, NapB) than those without; naturally, this makes the spectra of the crude extracts look more like bona fide c-type cytochromes. Thus the CXXCM holocytochromes were purified by anion-exchange chromatography. Following this step, on either a DEAE-Sepharose or a Q-Sepharose column, no distinct absorption, pyridine haemochrome or electrophoretic differences, within the limits of detection and errors of the experiment, were observed in holocytochrome b562 CXXCM produced with or without the Ccm apparatus. In both cases, the product CXXCM protein has absorption and pyridine haemochrome spectra characteristic of an improperly matured c-type cytochrome derivative of b562 (Figure 1B), i.e. red-shifted maxima compared with Ccm matured cytochrome b562 CXXCH (see the Introduction section and Figure 1A). We conclude that, although the CXXCM variant of b562 can bind haem covalently to form a holocytochrome, the Ccm system is unable to facilitate its maturation.
Curiously, the Soret absorption band of the b562 CXXCM holocytochrome, purified using either DEAE-Sepharose or Q-Sepharose, is a doublet of roughly equal intensities with maxima at approx. 422 and 429 nm (Figure 1B). Resolved α- and β-absorption maxima show that the haem iron is low-spin. A bis-Met co-ordinated b-type haem in cytochrome b562 has its Soret peak at 431 nm [19]; for a c-type variant with haem attachment through two vinyl groups, one would expect a blue shift of approx. 6 nm for this peak. Thus the doublet observed for the CXXCM protein may at first sight be interpreted as a mixture of covalent and non-covalent bis-Met-co-ordinated cytochromes. Several lines of evidence indicate that this is not the case: (i) the pyridine haemochrome α-band maximum (approx. 551 nm) is not consistent with large quantities of non-covalently bound haem in the cytochrome; (ii) the absorption maxima did not change after treatment of the holocytochrome with acidified butanone, a procedure that removes non-covalently bound haem [29]; and (iii) haem-stained SDS/polyacrylamide gels of preparations of the purified holocytochrome indicate the presence of only one cytochrome (rather than two) with covalently bound haem. Thus we conclude that the doublet arises from two isoforms of holocytochrome b562 CXXCM with haem covalently bound. The pyridine haemochrome spectrum of this material has a typical (singlet) Soret band, thus the absorption band doublet may well represent mixed co-ordination of the haem iron.
Effect of mutation of the proximal axial ligand to lysine on biosynthesis of c-type cytochromes
A cytochrome b562 variant was generated containing the mutation H102K [b562 R98C/Y101C/H102K (CXXCK motif)]. Absorption spectra revealed periplasmic formation of a c-type holocytochrome of this variant (Figure 1C), which was confirmed by staining of SDS/polyacrylamide gels for proteins with covalently bound haem. Absorption and pyridine haemochrome data did not indicate any difference in the product cytochrome produced with or without co-expression of the Ccm apparatus from pEC86. Further purification of the CXXCK holocytochrome products by anion-exchange chromatography confirmed this initial analysis; thus we conclude that the Ccm system did not mature the CXXCK cytochrome. In each case, the averaged reduced pyridine haemochrome maximum was at approx. 551.5 nm and the absorption α-band at approx. 558 nm, red-shifted from the values expected for a properly matured c-type cytochrome with haem attachment through two thioether bonds. As for each of the other b562 variants described in the present study, SDS/PAGE analysis of periplasmic fractions from cells transformed with the CXXCK plasmid showed the presence of a large amount of apoprotein. ESI–MS analysis indicated the presence of the expected mutation (calculated and observed masses 11656 and 11654 Da respectively).
In the absence of oxygen, E. coli can grow anaerobically using nitrate or nitrite as terminal electron acceptors. This requires the expression of a number of proteins from the Nrf pathway, including NrfA, the pentahaem nitrite reductase enzyme. This enzyme has four haems covalently attached through typical CXXCH motifs and one active-site haem attached by an unusual CWSCK motif [16]. Covalent attachment of this active-site haem requires the presence of the CcmFH orthologues, NrfEFG, in addition to the Ccm apparatus [16], although it is not yet clear what the specific recognition factors for the Nrf biogenesis apparatus are or precisely why these proteins are required. Therefore we also constructed a b562 variant with a CWSCK haem-binding motif (b562 R98C/N99W/A100S/Y101C/H102K; the motif till now described as CXXCK is actually CNACK) and, as a control, a CWSCH variant. Cells transformed with these plasmids were first grown aerobically (as for all the other variants in the present study). Apoprotein was shown to be plentiful in each case by SDS/PAGE, and MS indicated the presence of the desired mutations (for b562 CWSCH, the calculated mass was 11753 Da and the observed mass was 11754 Da; for b562 CWSCK, both the calculated and observed masses were 11744 Da). Cytochrome b562 CWSCH expressed in the presence of the Ccm apparatus (from pEC86) was a correctly matured c-type cytochrome, indicated particularly (Figure 1D) by the absorption and pyridine haemochrome α-band maxima at 556 and 550 nm respectively. These values are the same as for b562 CNACH (‘CXXCH’), which has been extensively analysed (by NMR) [7], and are essentially diagnostic of covalent haem attachment to the b562 variant through two thioether bonds. SDS/PAGE of crude periplasmic extracts stained for covalently bound haem showed the dominant band at the same molecular mass as that for purified b562 CXXCH. The yield of holocytochrome b562 CWSCH was approx. 4 mg/g of wet cells, using a molar absorption coefficient of 146 mM/cm at 420 nm (as reported for Ccm matured b562 CNACH) [6,7]. In contrast, cytochrome b562 CWSCK behaved very similarly to the CNACK variant (see data above), i.e. there was a population of improperly matured holocytochrome and no evidence for such a holoprotein being different in the presence of the Ccm apparatus.
E. coli JCB387 cells transformed with either the ampicillin-resistance-conferring plasmid pKK223-3 (as a control/reference) or the b562 CWSCK plasmid were also grown anaerobically on minimal medium containing formate and nitrate, after which periplasmic extracts were prepared. Under these conditions, the native Nrf and Ccm systems of E. coli are expressed and active, but there was no evidence for significant maturation of b562 CWSCK judged by the spectral maxima in comparison with those for the control (pKK223-3) samples. In each case, the spectra of endogenous soluble c-type cytochromes (mainly NrfA [30]) were readily apparent, but c-type cytochromes based on cytochrome b562 (such as Ccm-matured b562 CWSCH) have distinct (red-shifted) absorption peaks from those of the natural E. coli c-type cytochromes. A very small population of protein with mass corresponding to that of cytochrome b562 was, however, observed on haem-stained SDS/polyacrylamide gels of periplasmic extracts from large (5 litre) cultures of E. coli expressing cytochrome b562 CWSCK; the apocytochrome was shown to be plentiful by Coomassie staining. An attempt was made to purify the holocytochrome b562 CWSCK by anion-exchange chromatography, but it was present in only minimal amounts and co-eluted with NrfA, which was by a long way the dominant cytochrome present. Thus we cannot state categorically that no properly matured holocytochrome b562 CWSCK was produced by the E. coli Ccm/Nrf systems, but any such protein was present at a very low level. It is also entirely possible that the holocytochrome b562 CWSCK observed on gels may have been improperly matured, as was seen when the protein was expressed under aerobic conditions (see data above).
Conclusions
A great virtue of the cytochrome b562-based experimental system used in the present study is the production of large quantities of stable apoprotein for each variant whether or not any holoprotein was also formed. Therefore, for each b562 variant we have investigated, it has been shown that the protein (i) was expressed (detected by SDS/PAGE), (ii) contained the correct mutations and was intact/undigested in the periplasm (by ESI–MS), and (iii) could bind haem in vivo and/or in vitro (spectroscopically, e.g. Figure 1). CXXCH variants of b562 form stable and spectroscopically distinguishable holocytochromes in the presence and absence of the Ccm apparatus (where ‘XX’=WS (the present study) or NA [7]). Thus we can say clearly that the failure of the Ccm system to mature cytochromes with CXXCR, CXXCM or CXXCK haem-binding motifs was due to the properties of the maturation system and not due to a lack of expression of the apocytochrome substrate, proteolysis of the apocytochrome or instability/proteolysis of the holocytochrome.
In vivo, the most likely reason for the failure of the Ccm system to mature the CXXCK, CXXCR and CXXCM b562 variants is an inability to recognize the modified haem attachment site. Crow et al. [31] have recently suggested that reduced ResA, a protein of the distinct (non-Ccm) cytochrome c biogenesis system of Bacillus subtilis, may contain a specific recognition site for the histidine of the CXXCH motif of apocytochromes c. They argue that “such a ‘histidine clamp’ mechanism would confer specificity to the recognition of apo-cytochrome c without compromising the capacity to recognize several different sequences”. A similar site, specifically requiring the histidine, might be present in one (or more) of the Ccm proteins. Additionally or alternatively, the CXXCH histidine may play a crucial role in the kinetics of haem attachment; it might, for example, serve as the necessary proton donor to the haem vinyl group during thioether bond formation.
Clearly, the critical specificity determinant for apocytochrome substrates of the E. coli Ccm system is the combination of the two cysteine residues and the histidine residue of the CXXCH haem-binding motif. Both cysteine residues have been shown to be required [9,14,32] along with the histidine residue (the present study). However, the system will mature both natural and variant c-type cytochromes with three or four residues between the cysteine residues, rather than the usual two [10,33,34]. A distal ligand to the haem iron is not required for Ccm-mediated haem attachment [17,35]. Very recently, it has been shown that the Ccm system can covalently attach haem to a peptide of only 12 amino acids, with a CXXCH motif and a C-terminal His6 tag [12]. The occurrence in a multihaem cytochrome of two CXXCH motifs separated by as few as five other residues [13] also implies recognition of little more than the CXXCH motif by the Ccm system.
In contrast, the biogenesis (covalent haem attachment) proteins of the Nrf system seemingly detect more in NrfA than simply the CWSCK active-site haem-binding motif, e.g. other motifs or structural features in the latter protein might be recognized. The Nrf haem attachment proteins may function only after attachment of haems to some or all of the CXXCH motifs of NrfA by CcmA-H. It is also possible that the Nrf biogenesis proteins were inhibited by our attempt to mature a CWSCK-containing cytochrome that would also (presumably) have a distal methionine ligand, in contrast with the CWXCK-bound haem of NrfA, which is ultimately penta-co-ordinate. A further possibility is that the Nrf apparatus is saturated by the production of the NrfA required for respiration under anaerobic growth conditions and thus does not have sufficient capacity to mature our overexpressed (exogenous) cytochrome. It appears likely that b562 CWSCK could, in principle, form a stable, correctly matured c-type cytochrome because its CWSCH homologue is matured in large amounts by the Ccm apparatus (Figure 1D). The molecular basis for the attachment of haem to the CWXCK motif of NrfA nitrite reductase evidently warrants further study.
Acknowledgments
This work was funded by grants from the U.K. BBSRC to S.J.F. (C13343 and BB/C508118/1) and by the E.P. Abraham Trust of Oxford University. We thank P. Barker (Department of Chemistry, University of Cambridge) and O. Daltrop (Departments of Biochemistry and Chemistry, University of Oxford) for helpful discussions.
References
- 1.Thony-Meyer L. Haem-polypeptide interactions during cytochrome c maturation. Biochim. Biophys. Acta. 2000;1459:316–324. doi: 10.1016/s0005-2728(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 2.Pettigrew G. W., Moore G. R. Cytochromes c: Biological Aspects. New York: Springer-Verlag; 1987. [Google Scholar]
- 3.Stevens J. M., Daltrop O., Allen J. W. A., Ferguson S. J. c-type cytochrome formation: chemical and biological enigmas. Acc. Chem. Res. 2004;37:999–1007. doi: 10.1021/ar030266l. [DOI] [PubMed] [Google Scholar]
- 4.Itagaki E., Hager L. P. Studies on cytochrome b562 of Escherichia coli: purification and crystallisation of cytochrome b562. J. Biol. Chem. 1966;241:3687–3695. [PubMed] [Google Scholar]
- 5.Matthews F. S., Bethge P. H., Czerwinski E. W. The structure of cytochrome b562 from Escherichia coli at 2.5 Å resolution. J. Biol. Chem. 1979;254:1699–1706. [PubMed] [Google Scholar]
- 6.Barker P. D., Nerou E. P., Freund S. M. V., Fearnley I. M. Conversion of cytochrome b562 to c-type cytochromes. Biochemistry. 1995;34:15191–15203. doi: 10.1021/bi00046a027. [DOI] [PubMed] [Google Scholar]
- 7.Allen J. W. A., Barker P. D., Ferguson S. J. A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation (Ccm) system. J. Biol. Chem. 2003;278:52075–52083. doi: 10.1074/jbc.M307196200. [DOI] [PubMed] [Google Scholar]
- 8.Allen J. W. A., Daltrop O., Stevens J. M., Ferguson S. J. c-type cytochromes: diverse structures and biogenesis systems pose evolutionary problems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:255–266. doi: 10.1098/rstb.2002.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen J. W. A., Ginger M. L., Ferguson S. J. Maturation of the unusual single cysteine (XXXCH) mitochondrial c-type cytochromes found in trypanosomatids must occur through a novel biogenesis pathway. Biochem. J. 2004;383:537–542. doi: 10.1042/BJ20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker P. D., Ferguson S. J. Still a puzzle: why is haem covalently attached in c-type cytochromes? Structure Fold. Des. 1999;7:R281–R290. doi: 10.1016/s0969-2126(00)88334-3. [DOI] [PubMed] [Google Scholar]
- 11.Sanders C., Lill H. Expression of prokaryotic and eukaryotic cytochromes c in Escherichia coli. Biochim. Biophys. Acta. 2000;1459:131–138. doi: 10.1016/s0005-2728(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 12.Braun M., Thöny-Meyer Biosynthesis of artificial microperoxidases by exploiting the secretion and cytochrome c maturation apparatuses of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12830–12835. doi: 10.1073/pnas.0402435101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M. D., Sambongi Y., Ferguson S. J. Contrasting routes of c-type cytochrome assembly in mitochondria, chloroplasts and bacteria. Trends Biochem. Sci. 1998;23:103–108. doi: 10.1016/s0968-0004(98)01173-6. [DOI] [PubMed] [Google Scholar]
- 14.Allen J. W. A., Tomlinson E. J., Hong L., Ferguson S. J. The Escherichia coli cytochrome c maturation (Ccm) system does not detectably attach heme to single cysteine variants of an apocytochrome c. J. Biol. Chem. 2002;277:33559–33563. doi: 10.1074/jbc.M204963200. [DOI] [PubMed] [Google Scholar]
- 15.Einsle O., Messerschmidt A., Stach P., Bourenkov G. P., Bartunik H. D., Huber R., Kroneck P. M. Structure of cytochrome c nitrite reductase. Nature (London) 1999;400:476–480. doi: 10.1038/22802. [DOI] [PubMed] [Google Scholar]
- 16.Eaves D. J., Grove J., Staudenmann W., James P., Poole R. K., White S. A., Griffiths I., Cole J. A. Involvement of products of the nrfEFG genes in the covalent attachment of haem c to a novel cysteine-lysine motif in the cytochrome c552 nitrite reductase from Escherichia coli. Mol. Microbiol. 1998;28:205–216. doi: 10.1046/j.1365-2958.1998.00792.x. [DOI] [PubMed] [Google Scholar]
- 17.Allen J. W. A., Ferguson S. J. Variation of the axial haem ligands and haem-binding motif as a probe of the Escherichia coli c-type cytochrome maturation (Ccm) system. Biochem. J. 2003;375:721–728. doi: 10.1042/BJ20030752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker P. D., Freund S. M. Bis-methionine ligation to heme iron in mutants of cytochrome b562 (2) Biochemistry. 1996;35:13627–13635. doi: 10.1021/bi961128p. [DOI] [PubMed] [Google Scholar]
- 19.Barker P. D., Nerou E. P., Cheesman M. R., Thomson A. J., de Oliveira P., Hill H. A. O. Bis-methionine ligation to heme iron in mutants of cytochrome b562 (1) Biochemistry. 1996;35:13618–13626. doi: 10.1021/bi961127x. [DOI] [PubMed] [Google Scholar]
- 20.Liliana Garcia L., Fredericks Z., Sorrell T. N., Pielak G. J. Site-directed mutagenesis of the histidine heme ligand on iso-1-cytochrome c of Saccharomyces cerevisiae. New J. Chem. 1992;16:629–632. [Google Scholar]
- 21.Fumo G., Spitzer J. S., Fetrow J. S. A method of directed random mutagenesis of the yeast chromosome shows that iso-1-cytochrome c heme ligand His18 is essential. Gene. 1995;164:33–39. doi: 10.1016/0378-1119(95)00457-h. [DOI] [PubMed] [Google Scholar]
- 22.Bernard D. G., Gabilly S. T., Dujardin G., Merchant S., Hamel P. P. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 2003;278:49732–49742. doi: 10.1074/jbc.M308881200. [DOI] [PubMed] [Google Scholar]
- 23.Arslan E., Schulz H., Zufferey R., Kunzler P., Thöny-Meyer L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 1998;251:744–747. doi: 10.1006/bbrc.1998.9549. [DOI] [PubMed] [Google Scholar]
- 24.Hussain H., Grove J., Griffiths L., Busby S., Cole J. A seven-gene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol. Microbiol. 1994;12:153–163. doi: 10.1111/j.1365-2958.1994.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 25.Iobbi-Nivoll C., Crooke H., Griffiths L., Grove J., Hussain H., Pommier J., Mejean V., Cole J. A. A reassessment of the range of c-type cytochromes synthesised by Escherichia coli K-12. FEMS Microbiol. Lett. 1994;119:89–94. doi: 10.1111/j.1574-6968.1994.tb06872.x. [DOI] [PubMed] [Google Scholar]
- 26.Sambongi Y., Ferguson S. J. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase-like protein. FEBS Lett. 1994;353:235–238. doi: 10.1016/0014-5793(94)01053-6. [DOI] [PubMed] [Google Scholar]
- 27.Goodhew C. F., Brown K. R., Pettigrew G. W. Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim. Biophys. Acta. 1986;852:288–294. [Google Scholar]
- 28.Bartsch R. G. Cytochromes: bacterial. Methods Enzymol. 1971;23:344–363. [Google Scholar]
- 29.Tomlinson E. J., Ferguson S. J. Conversion of a c type cytochrome to a b type that spontaneously forms in vitro from apo protein and heme: implications for c type cytochrome biogenesis and folding. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5156–5160. doi: 10.1073/pnas.090089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamford V. A., Angove H. C., Seward H. E., Thomson A. J., Cole J. A., Butt J. N., Hemmings A. M., Richardson D. J. Structure and spectroscopy of the periplasmic cytochrome c nitrite reductase from Escherichia coli. Biochemistry. 2002;41:2921–2931. doi: 10.1021/bi015765d. [DOI] [PubMed] [Google Scholar]
- 31.Crow A., Acheson R. M., Le Brun N. E., Oubrie A. Structural basis of redox-coupled protein substrate selection by the cytochrome c biosynthesis protein ResA. J. Biol. Chem. 2004;279:23654–23650. doi: 10.1074/jbc.M402823200. [DOI] [PubMed] [Google Scholar]
- 32.Schulz H., Hennecke H., Thöny-Meyer L. Prototype of a heme chaperone essential for cytochrome c maturation. Science. 1998;281:1197–1200. doi: 10.1126/science.281.5380.1197. [DOI] [PubMed] [Google Scholar]
- 33.Herbaud M.-L., Aubert C., Durand M.-C., Guerlesquin F., Thöny-Meyer L., Dolla A. Escherichia coli is able to produce heterologous tetraheme cytochrome c3 when the ccm genes are co-expressed. Biochim. Biophys. Acta. 2000;1481:18–24. doi: 10.1016/s0167-4838(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 34.Ríos-Velázquez C., Cox R. L., Donohue T. J. Characterization of Rhodobacter sphaeroides cytochrome c2 proteins with altered heme attachment sites. Arch. Biochem. Biophys. 2001;389:234–244. doi: 10.1006/abbi.2001.2330. [DOI] [PubMed] [Google Scholar]
- 35.McGuirl M. A., Lee J. C., Lyubovitsky J. G., Thanyakoop C., Richards J. H., Gray H. B., Winkler J. R. Cloning, heterologous expression, and characterization of recombinant class II cytochromes c from Rhodopseudomonas palustris. Biochim. Biophys. Acta. 2003;1619:23–28. doi: 10.1016/s0304-4165(02)00437-3. [DOI] [PubMed] [Google Scholar]