Abstract
Introduction
Endotracheal tubes (ETT) are used in patients who require ventilatory support. Colonization of ETTs by microorganisms is associated with developing ventilator-associated pneumonia (VAP). Thus, this meta-analysis aims to compare conventional endotracheal tubes with those made using materials designed to prevent colonization.
Methods
This analysis was conducted according to the PRISMA guidelines. During May 2024, we searched multiple databases (PubMed, Cochrane and Embase) for randomized controlled trials (RCT) comparing the incidence of ventilator-associated pneumonia between conventional and coated tubes. Studies with patients with less than 48 h of intubation were excluded. Our primary endpoint was the incidence of VAP in patients intubated with either conventional or coated endotracheal tubes. Statistical analysis was performed using Review Manager Software, and a The Mantel-Haenszel test was performed using a random effects model, and risk ratios (RR) were calculated for binary outcomes. Subgroup analyses were conducted using a fixed effects model when heterogeneity was low. Risk assessment was carried out using the Risk of Bias 2 tool.
Results
Our search identified 6 RCTs eligible in our inclusion criteria, enrolling 2680 patients, with 1361 (50,78 %) undergoing intubation using a polymer-coated tube. The statistical data indicated that coated endotracheal tubes perform better in preventing pneumonia than conventional tubes (RR 0.57 Cl 95 % 0.45–0.90; p < 0.001; I2 0 %). Additionally, conventional tubes were also associated with higher bacterial colonization (47.02 CI 95 % 26.88–68.18; p < 0.01; I2 81 %) compared to coated tubes.
Conclusions
These findings indicate that utilizing a silver-coated endotracheal tube for intubation is more efficacious than conventional tubes, presenting it as a strategy to combat ventilator-associated pneumonia.
1. Introduction
Ventilator-associated pneumonia (VAP), a type of nosocomial pneumonia, is the most common infectious complication among patients admitted to the intensive care unit (ICU), occurring after at least 48 h of mechanical ventilation. It emerges as a clinically challenging and recurrent complication for individuals on mechanical ventilation, representing a significant concern for healthcare professionals [1]. VAP is characterized by pulmonary infection resulting from the introduction of an endotracheal tube for respiratory support, this condition not only prolongs the patient's stay in the intensive care unit but also increases the costs associated with treatment [2]. Thus, the seriousness of VAP is aggravated by the fact that many patients are already in critical condition, making them more susceptible to respiratory infections. In this context, the importance of researching and implementing effective prevention strategies stands out as an undeniable imperative (see Fig. 3, Fig. 10, Fig. 11, Fig. 12).
Fig. 3.
Sensitivity assessment of studies with silver tube.
Fig. 10.
Sensitivity assessment of studies.
Fig. 11.
ICU Stay duration with polyurethane tube.
Fig. 12.
Sensitivity assessment of studies with polyurethane tubes.
The search for innovative approaches, such as rigorous respiratory hygiene protocols, constant monitoring of endotracheal cuff pressure, and prudent use of antibiotics, becomes crucial to mitigate the impact of VAP and improve clinical outcomes. Ultimately, these efforts aim to enhance the quality of life for mechanically ventilated patients [3]. Addressing this challenge requires interdisciplinary collaborations and technological advancements to develop more effective preventive strategies, reshaping the current approach towards a comprehensive and adaptable care model [4]. Moreover, beside the bundle of prevention of VAP, the evolution includes the utilization of coated endotracheal tubes, representing a promising solution to reduce mechanical ventilation complications. These tubes incorporate special materials like silver and polyurethane, potentially impacting the prevention of respiratory infections such as VAP [3].
The purpose of this meta-analysis is to conduct a comparative analysis between traditional endotracheal tubes, metal-coated tubes such as silver, and polyurethane-cuffed tubes, highlighting the implications of these distinctions in terms of clinical outcomes. The central focus of this investigation is to explore recent technological advances in these coatings and understand their potential impact on significantly reducing the incidence of Ventilator-Associated Pneumonia (VAP). The research sets out to closely examine how these coated tubes can positively influence the reduction of biofilm formation, bacterial colonization and, consequently, the mitigation of lung infection resulting from the use of mechanical ventilation. By integrating polymers with antimicrobial and anti-adhesive properties, this meta-analysis aims to contribute to the ongoing search for effective solutions to prevent complications associated with mechanical ventilation. The integration of polymers with antimicrobial and anti-adherent properties into endotracheal tubes represents a promising step in the search for materials that can mitigate the risks associated with mechanical ventilation [[5], [14], [15]].
This study will delve into the physical, mechanical, and microbiological characteristics of these devices, aiming to provide a comprehensive overview of the potential advantages offered by polymer-coated endotracheal tubes. The research will address aspects such as resistance, flexibility, and the ability to inhibit bacterial proliferation, thus contributing to the construction of a solid knowledge base that can revolutionize clinical practices and ultimately substantially improve the clinical outcomes of patients undergoing mechanical ventilation. Additionally, the results of recent clinical studies investigating the efficacy of these tubes in clinical practice will be examined, contributing to a robust evidence base to guide the choice of preventive strategies in hospital settings.
2. Methods
This systematic review and meta-analysis was conducted following the recommendations of Cochrane and the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [7]. The protocol for this study was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) under protocol number ID CRD42023426562. We systematically searched for studies published in PubMed, Embase, and Cochrane Library from the inception of the databases up to May 05, 2024.
2.1. Search strategy
We conducted a systematic search in the PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases up to May 5, 2024, using terms such as “Endotracheal” OR “Tracheal” AND “Polymer” OR “Coated” OR “Coating” AND “Pneumonia” AND “Mechanical ventilation.” The complete search strategy can be found in Supplementary Appendix A. We did not apply language filters or restrictions in our search, and grey literature was not included. Additionally, we used the snowball technique to identify additional eligible studies by reviewing the references of previous publications [6]. Three authors (OB, LG, and TB) independently conducted the literature search, following predefined criteria, and any conflicts were resolved by consensus among the authors. Any additional study data, such as extracted data, included studies, and data used for all analyses, are available in the drive at the following link: Google Drive Link.
We restricted the inclusion only the outcome met the following eligibility criteria: (1) VAP; (2) Mortality; (3) ICU length of stay; (4) Bacterial colonization; and (5) Comparison of VAP in tubes coated with silver and polyurethane. We considered secondary analyses of included randomized clinical trials (RCTs) as a single index study. We excluded: (1) Observational studies; (2) Studies with patients intubated for less than 24 h; (3) Animal studies; (4) Studies without a control group; (5) Unpublished studies or only abstracts presented at conferences. Title/abstract and full-text screening were performed in duplicate by two independent reviewers (LG and OB). Discrepancies were resolved through consensus, including a third author (TB).
2.2. Study selection and data extraction
The definition of the research question followed the PICOTT criteria, and studies were included in the systematic review if they met the following eligibility requirements: (1) involvement of adults (>18 years-old) under mechanical ventilation; (2) comparison between coated and conventional endotracheal tubes; (3) assessment of any outcome of interest; (4) randomized clinical trials with parallel or crossover designs; and (5) minimum duration of intubation for 48 h. Studies with overlapping patient populations, from overlapping institutions, patients or recruitment periods, and clinical trials with no results were excluded.
Two authors (OB and LG) carried out independent extraction of outcome data using a standardized document, with disagreements resolved by consensus. In addition, three independent authors (OB, LG, TB) extracted additional baseline data, including study and patient characteristics (see Table 1). No participant-level data were requested.
Table 1.
Baseline qualitative characteristics of included studies.
| Study | Country | Population | Age (I/C)† | Female (%) | Intervention | Control | Primary Outcome |
|---|---|---|---|---|---|---|---|
| Lorente 2007 | Spain | Adult patients expected to require mechanical ventilation for more than 24 h (280 patients) | 60.6 (42.9–78.2)/60 (43.2–76.7) | 76 (27,1 %) | Tube with Polyurethane | Uncoated tube | VAP |
| Kollef 2008 | United States of America | Adult patients at risk of requiring mechanical ventilation with an endotracheal tube for 24 h or more (1509 patients) | 61.5 (60.5–62.4)/62.2 (61.2–63.1) | 815 (42,1 %) | Silver-Coated Endotracheal Tubes | Uncoated tube | VAP |
| Poelaert 2008 | Belgium | Adult patients scheduled for cardiac surgery (134 patients) | 68 (59–77)/67 (56–78) | 49 (36.5 %) | Endotracheal tubes with polyurethane cuff | Uncoated tube | VAP after cardiac surgery |
| Philippart 2015 | France | Adult hospitalized patients for acute respiratory failure who were anticipated to be mechanically ventilated for at least 48 h (292 patients) | 65.3 (55.2–75.6)/65.6 (53.0–77.2) | 117 (40 %) | Polyurethane tube | Uncoated tube | VAP |
| Ciprian 2022 | Romania | Adult patients admitted for drug poisoning-induced coma and expected to require orotracheal intubation for >48 h (180 patients) | 40.08 (26.8–54.8)/38.9 (27.9–50.0) | 64 (35,5 %) | noble metal–alloy ETT |
Uncoated tube | VAP and the duration of mechanical ventilation |
| Damas 2022 | Belgium | Adult hospitalized patients who may require mechanical ventilation for more than 24 h (323 patients) | 67.9 (56.4–74.7)/67.3 (58–74.5) | 144 (44.5 %) | metal coating of endotracheal tubes |
Uncoated tube | VAP |
†mean or median; I: intervention; C: control.
2.3. Outcome measures and quality assessment
The main outcomes considered were ventilator-associated pneumonia (VAP), length of stay, bacterial colonization and mortality. Each included study was assessed by at least two independent investigators (OB, RM and TB) using the Cochrane Risk of Bias Assessment Tool (RoB-2) for Randomized Clinical Trials [20].
2.4. Statistical analysis
Binary adverse outcomes were summarized using the Mantel-Haenszel test, presenting risk ratio (RR) and 95 % confidence interval (CI) as a measure of effect size. Continuous outcomes were compared using weighted and standardized mean differences. Sources of heterogeneity were sought when I2 was greater than 50 %. In cases of low heterogeneity (I2 < 25 %), a fixed effects model was applied. Subgroup analyses were carried out to investigate the causes of heterogeneity. In addition, a comparative random effect analysis was conducted to assess the impact of coated tubes on length of stay, mortality and VAP prevention. In addition, a comparative random effect analysis was conducted to assess the impact of coated tubes on length of stay, mortality, and prevention of VAP. Publication bias was assessed for VAP by creating a funnel plot and Egger's test, where a p-value greater than 0.05 would indicate the presence of publication bias. Statistical analyses were performed using Review Manager 5.4.1 software (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and R (version 4.2.2) software (6).
2.5. Diagnostic criteria PAV
A review was conducted on the diagnosis of Ventilator-Associated Pneumonia (VAP) across all studies included in this article. The diagnosis was confirmed through imaging and had to meet at least two of the following criteria: temperature greater than 38 °C or less than 35 °C; leukocyte count greater than 10,000/μL or less than 4,000/μL; presence of purulent secretions in the airways; significant quantitative culture of respiratory secretions obtained from tracheal aspirates (>10⁶ CFU/mL); presence of crackles or dullness; and a reduction greater than 10 % in arterial partial pressure of oxygen [[1], [2], [3], [4]]. One of the studies noted that the definition and diagnosis were conducted according to the CDC criteria published in January 2021.
3. Results
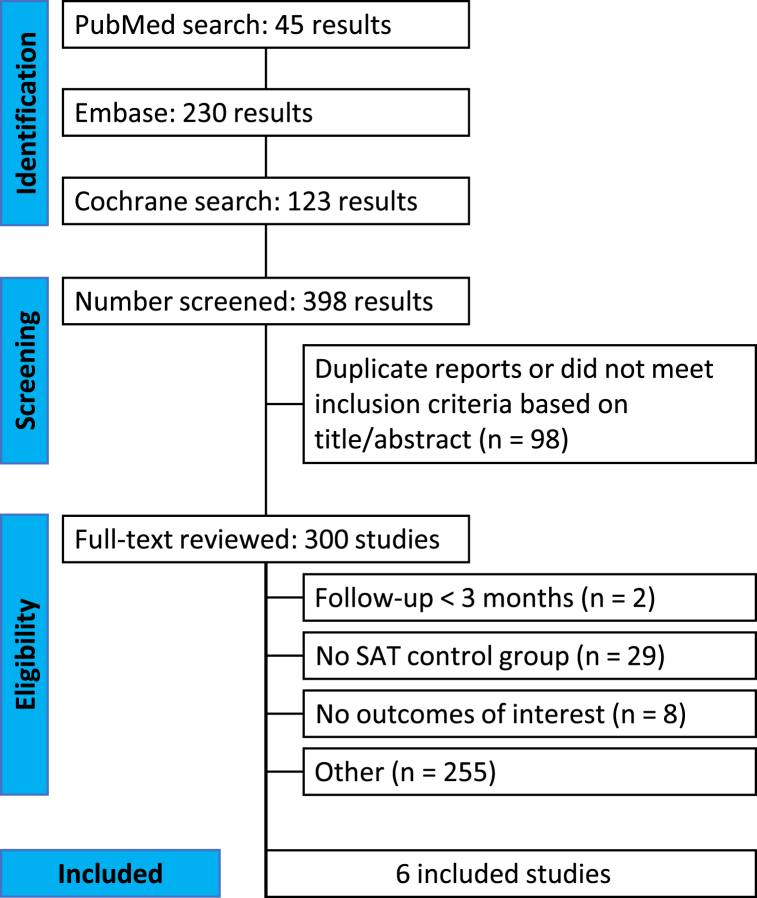
Our research identified a total of 398 studies, of which 6 RCTs, including 2680 randomized participants, met the study eligibility criteria (Fig. 1) [7]. Among the 6 identified studies, all primarily assessed the outcome of Ventilator-Associated Pneumonia (VAP) post-intubation for 48 h or more (Kollef, Ciprian, Damas, Kollef, Poelaert, and Lorente), and evaluated other outcomes, such as mortality, ICU length of stay, bacterial colonization on tubes, and comparison of VAP between silver and polyurethane tubes [[1], [2], [3], [4], [5], [6]] (see Fig. 2).
Fig. 1.
PRISMA flow diagram of study screening and selection.
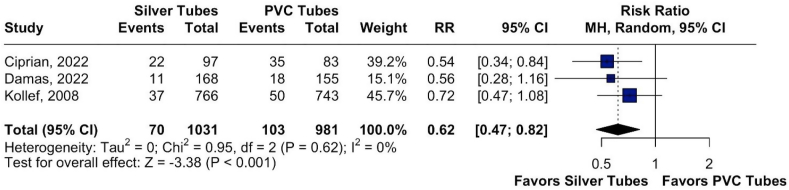
Fig. 2.
Assessment of pneumonia development in intubated patients using silver-coated versus PVC endotracheal tubes.
The characteristics of the studies that contributed data to this meta-analysis are presented in Table 1. The trials were conducted in five countries across two continents.
3.1. PRISMA study selection flowchart
255 articles were excluded for other reasons: intubation duration less than 48 h; poorly defined study population; non-randomized studies; conference abstracts.
3.2. VAP in silver-coated tubes and PVC tubes
The statistical data indicated that silver-coated endotracheal tubes outperform conventional PVC tubes in preventing pneumonia. The results were reported as Risk Ratio (RR 0.62, 95 % CI 0.47–0.82; p < 0.001; I2 0 %). The calculated Risk Ratio (RR) was 0.62, with a 95 % confidence interval ranging from 0.47 to 0.82. This means that patients using silver-coated endotracheal tubes have approximately a 38 % lower chance of developing pneumonia compared to those using PVC tubes. Additionally, the p-value was less than 0.001, indicating a statistically significant difference between the groups. It is important to note that no heterogeneity was observed among the included studies, as indicated by the I2 value of 0 %, suggesting that the variation in results was primarily due to chance, with no significant differences between the studies.
A sensitivity analysis was then performed to check for heterogeneity in VAP development across the studies. It was observed that none of the studies (Ciprian, Kollef, and Damas) showed high heterogeneity, with a Risk Ratio of 0.62 [0.47; 0.82] and an I2 of 0 %. This indicates minimal variation between the results, suggesting consistency among the studies analyzed.
3.3. VAP in polyurethane tubes and PVC tubes
The statistical data indicated no significant difference in pneumonia prevention between polyurethane-coated endotracheal tubes and conventional PVC tubes. The results were reported as Risk Ratio (RR 0.67, 95 % CI 0.30–1.54; p = 0.348; I2 82 %).
The calculated Risk Ratio (RR) was 0.67, with a 95 % confidence interval ranging from 0.30 to 1.54, showing that there is no statistically significant difference between the groups, as indicated by the p-value of 0.348. Additionally, the I2 value of 82 % suggests a high level of heterogeneity, meaning that there was substantial variation between the results of the included studies, likely due to real differences between them rather than random chance.
A sensitivity analysis was performed to evaluate the impact of individual studies on the overall assessment of VAP development in polyurethane tubes. The following results were obtained:
Lorente:RR 0.92 [0.33; 2.57], I2 84 %
Philippart: RR 0.46 [0.30; 0.71], I2 9 %
Poeleart: RR 0.75 [0.17; 3.23], I2 90 %
Overall: RR 0.67 [0.30; 1.54], I2 82 %
The analysis revealed substantial heterogeneity among the studies, particularly for Lorente and Poeleart, which had high I2 values of 84 % and 90 %, respectively (see Fig. 4). In contrast, Philippart showed minimal heterogeneity (I2 9 %). The overall Risk Ratio of 0.67, with a 95 % confidence interval of [0.30; 1.54], suggests that while there is no statistically significant difference in pneumonia development in polyurethane tubes, the high levels of heterogeneity indicate variability among the study outcomes. This underscores the importance of examining each study's methodologies and results to understand the reasons behind the observed differences (Fig. 5).
Fig. 4.
Assessment of pneumonia development in intubated patients using polyurethane tubes versus PVC endotracheal tubes.
Fig. 5.
Sensitivity assessment of studies with polyurethane tube.
3.3.1. Mortality, 30-day
The Risk Ratio (RR) for mortality was found to be 1.04, with a 95 % confidence interval ranging from 0.89 to 1.22. Although the difference in mortality between the two groups did not reach statistical significance (p = 0.59), it is worth noting that the point estimate of the RR suggests a slight increase in mortality among patients using conventional tubes: Risk Ratio (RR 1.04 Cl 95 % 0.89–1.22; p = 0.59; I213 %) (Fig. 6). The mortality outcome was collected in only 4 studies. Therefore, it was not possible to perform a statistical analysis of mortality between silver-coated tubes and conventional tubes, as well as between polyurethane and conventional tubes.
Fig. 6.
Assessment of mortality in intubated patients with coated tubes and conventional tubes.
3.3.2. Bacterial colonization
Four studies indicated that bacterial colonization was analyzed quantitatively (Kollef, Poleart, Ciprian, Lorent), while one study reported that bacterial colonization was assessed qualitatively (Damas). Quantitative analysis provides a more precise measurement of bacterial load, allowing researchers to establish clear correlations between colonization levels and clinical outcomes, such as the development of ventilator-associated pneumonia (VAP). This method helps understand the severity of the infection and tailor appropriate treatment strategies.
On the other hand, qualitative analysis offers valuable information about the presence or absence of specific pathogens, which can be crucial for identifying potential infectious agents and guiding empirical therapy. While it may lack the detail provided by quantitative methods, qualitative assessments can be effective in certain clinical contexts, especially when rapid decisions are necessary. Together, these methodologies highlight different aspects of bacterial colonization, contributing to a comprehensive understanding of infection dynamics in patients undergoing mechanical ventilation.
The significance of this finding lies in its potential implications for patient outcomes. Bacterial colonization on endotracheal tubes can lead to VAP. Coated tubes, designed to reduce bacterial adherence and colonization, are hypothesized to lower the risk of VAP compared to conventional tubes. Therefore, Fig. 7 represents bacterial colonization in coated and non-coated tubes through Events per observation: events per 100 observations, IV, Random (47.02 CI 95 % 26.88–68.18; p < 0.01; I2 81 %) (Fig. 7).
Fig. 7.
Bacterial culture assessment in coated and conventional tubes.
3.3.3. Silver and polyurethane in VAP comparison
In addition, the coatings used in the studies in this meta-analysis, such as silver and polyurethane, were compared to see which material has the best response in combating VAP. The analysis revealed that silver-coated tubes had a lower average number of events, with 10.01 events per 100 observations, while polyurethane-coated tubes had an average of 14.81 events per 100 observations (Fig. 8) (see Fig. 9).
Fig. 8.
Silver and polyurethane in VAP comparison.
Fig. 9.
Total ventilator days in ICU intubated patients with silver tubes and conventional tubes.
3.3.4. Total ventilator days in ICU with silver tubes
The analysis of ICU length of stay for patients using silver tubes revealed intriguing results. The relative risk (RR) was calculated at −0.99, suggesting a slight trend toward reduced length of stay compared to those who did not use these tubes. However, the wide confidence interval (CI) ranging from −2.94 to 0.97 indicates significant uncertainty in the obtained estimates. This interval, which spans both negative and positive values, suggests that the observed difference may not be clinically relevant, raising questions about the effectiveness of silver tubes in decreasing length of stay.
Furthermore, the high heterogeneity identified, with an I2 value of 97 %, suggests considerable variability among the analyzed studies. This heterogeneity may be attributed to differences in patient demographics, clinical conditions, employed methods, or contextual factors influencing clinical outcomes. Such variations complicate data interpretation and highlight the need for caution when extrapolating conclusions regarding the efficacy of silver tubes.
The p-value of 0.32, exceeding the conventional significance level of 0.05, reinforces the lack of robust statistical evidence to assert that the use of silver tubes results in a significant reduction in ICU length of stay. Thus, while the results indicate a favorable trend, the absence of statistical significance, coupled with high heterogeneity, underscores the need for further investigations that can elucidate the real impact of silver tubes on clinical outcomes. Additional research with more homogeneous samples and rigorous methodologies is essential to clarify the role of silver tubes concerning ICU length of stay and to identify potential factors that may influence this relationship.
The sensitivity analysis revealed notable variations among the studies included, particularly highlighting the data from Kollef and Damas, which reported mean differences (MD) of −1.50 (CI [−4.47; 1.47], I2 = 98 %) and −1.40 (CI [−4.46; 1.48], I2 = 98 %), respectively. Both studies exhibited high heterogeneity, indicating significant variability in their results. In contrast, Tincu's study presented an MD of 0 (CI [−0.14; 0.14]) with no heterogeneity (I2 = 0 %), suggesting a consistent effect in this particular analysis. These discrepancies underline the importance of evaluating individual study methodologies to ascertain the reasons behind such variations. The high heterogeneity in Kollef and Damas's results may indicate differing patient populations or treatment protocols, warranting further investigation into their methodologies to clarify these findings.
3.3.5. Total ventilator days in ICU with poliuretany tubes
The comparative analysis of polyurethane tubes revealed promising results regarding ICU length of stay, outperforming silver tubes. The observed mean difference (MD) was −0.40, with a confidence interval ranging from −0.96 to 0.17, suggesting a trend toward reduced patient duration in the ICU. With an extremely low heterogeneity (I2 = 0 %), the data indicate robust consistency in results across the analyzed studies. Although the p-value (0.17) did not achieve statistical significance, the unique properties of polyurethane, such as its biocompatibility, flexibility, and resistance to microbial colonization, may have contributed to these favorable findings. These characteristics highlight the potential of polyurethane tubes as an effective alternative in clinical practice, emphasizing the need for further investigations to elucidate their impact on patient outcomes in intensive care settings.
3.3.6. Potential negative aspects associated with endotracheal tube coatings (ETT)
Firstly, the higher cost of these ETTs compared to conventional tubes may limit their use in resource-restricted environments, contributing to inequalities in patient care. In many developing countries, the availability of advanced technologies is scarce [[19], [21]], which can lead to unequal access to effective treatments. Additionally, research on ETT coatings often takes place in developed countries, where there is a monopoly on innovations and scientific publications, creating a disparity in the dissemination of knowledge and healthcare practices globally.
Another relevant aspect is that the efficacy of the coatings can vary significantly, depending on the methodology applied in the development of the tubes and the materials used. Some materials may be more effective against certain pathogens, while others may not yield the same results. Furthermore, it is crucial to assess the possibility that certain coatings could contribute to the development of bacterial resistance, which may compromise safety and long-term efficacy.
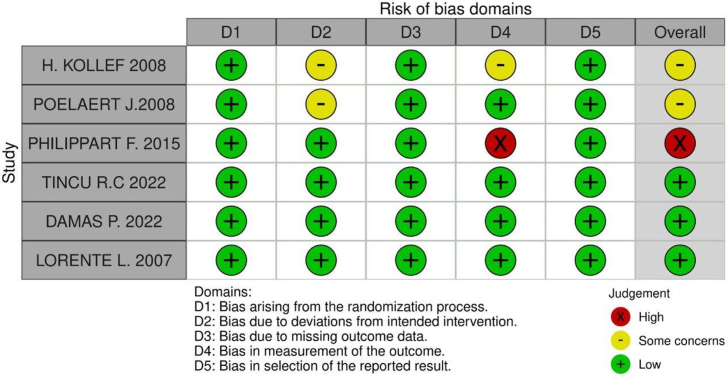
3.3.7. Risk of bias assessment
In Domain 2, both the Kollef and Poelaert studies were rated as having “some concerns.” Kollef's study received this rating because both patients and caregivers were aware of the intervention, which may have influenced behaviors and care, although no major protocol deviations occurred. The use of intention-to-treat analysis was appropriate, but the lack of blinding remains a potential source of bias. Similarly, Poelaert's study was rated “some concerns” because participants and anesthesiologists knew which intervention was applied, potentially affecting behaviors and care. While the intervention was consistently applied, minor, unreported variations may have influenced the results, raising concerns about bias.
In Domain 3, the Kollef and Philippart studies were rated as “some concerns” and “high risk,” respectively, for outcome measurement bias. In Kollef's study, although rigorous microbiological methods were used, the lack of blinding and variability across centers could have impacted the results. However, the use of robust diagnostic methods mitigated the bias to some extent, leading to an intermediate level of concern.
In contrast, Philippart's study was rated “high risk” due to more critical factors. These included the absence of blinding, significant variability in data collection methods across centers, and limited sensitivity of the diagnostic tools used for ventilator-associated pneumonia (VAP). These issues severely compromised the accuracy and consistency of outcome measurements, particularly in assessing tracheal colonization and VAP diagnosis.
Risk of bias summary for randomized studies (RoB 2)
4. Discussion
In this systematic review and meta-analysis of 6 randomized controlled trials (RCTs) involving 2,680 patients, we compared the use of polymer-coated tubes, particularly silver-coated and polyurethane-coated tubes, for periods exceeding 48 h of intubation. Our main findings were: (1) a significant improvement in the reduction of VAP (ventilator-associated pneumonia) in patients intubated with silver-coated polymer tubes, (2) a significant reduction in bacterial colonization on the coated tubes, and (3) no significant difference in mortality risk or ICU length of stay between the compared groups.
To the best of our knowledge, our study is the most comprehensive meta-analysis on the use of coated tubes versus conventional tubes, assessing VAP development as the primary outcome in intubations longer than 48 h. Our analysis integrated data from 6 studies with 2,680 participants, a population nearly double the size of a previous meta-analysis [8]. Furthermore, this is the first analysis with studies lasting more than 48 h. Our findings increase confidence in the beneficial effects of coated tubes in combating VAP in patients requiring prolonged intubation, as the longer the device is used, the higher the risk of respiratory complications and the longer the treatment duration [9]. Considering that the average incidence of VAP in our population was 8.9 % (122 patients), as shown in Fig. 8, our findings present a conservative and safe strategy to mitigate the risk of pneumonia commonly associated with mechanical ventilation [4]. Additionally, a 4.9 % increase in VAP was correlated with the use of PVC tubes [[10], [11]], which is slightly higher than the rate found in our evaluation of coated tubes.
Our results suggest that silver-coated tubes may offer an advantage over polyurethane-coated tubes in preventing complications such as VAP in patients intubated for more than 48 h. This can be attributed to the antimicrobial properties of silver, which are known to inhibit the proliferation of a wide range of microorganisms. Silver can maintain its antimicrobial properties for prolonged periods, even in complex environments such as the respiratory tract, which can result in continuous protection against bacterial colonization and, consequently, a reduction in the risk of respiratory complications in intubated patients [1,3,4].
Furthermore, it is important to highlight that the studies by Kollef and Damas show significant changes in the analysis of total ventilator days, demonstrating more favorable results for conventional tubes compared to silver tubes. In contrast, the Tincu study indicates a better prognosis for silver tubes, suggesting a reduction in the number of days of tube utilization. This difference may be attributed to the fact that the study population in Tincu's research is younger compared to the groups in Kollef and Damas, which may have influenced the observed results regarding silver tubes.
The prevention of ventilator-associated pneumonia consists of a combination of various strategies, including early awakening, endotracheal tubes with subglottic secretion drainage, daily toothbrushing, among others. Nevertheless, our results introduce an additional measure that may contributed to reduce colonization of orotracheal tube and subsequent VAP [[16], [17]].
Although both analyses showed efficacy in reducing adverse events, the results suggest that silver-coated tubes may offer an advantage over polyurethane-coated tubes in preventing complications such as VAP in patients intubated for more than 48 h. This can be attributed to the antimicrobial properties of silver, which are known to inhibit the proliferation of a wide range of microorganisms. Silver can maintain its antimicrobial properties for prolonged periods, even in complex environments such as the respiratory tract, which can result in continuous protection against bacterial colonization and, consequently, a reduction in the risk of respiratory complications in intubated patients [1,3,4].
Our findings have practical implications, particularly concerning device selection. For example, certain coated devices demonstrate greater potential for VAP prevention, bacterial colonization, and length of hospital stay compared to conventional PVC devices. Furthermore, there is a growing number of researchers assessing the use of polymers in medical devices, seeking reduction in bacterial colonization, antibacterial activity, and inhibition of bacterial biofilm formation [[12], [13], [14]].
5. Conclusion
This systematic review and meta-analysis strengthen the existing literature by demonstrating that polymer-coated tubes, particularly silver-coated ones, are significantly less associated with ventilator-associated pneumonia (VAP) compared to both polyurethane-coated and conventional PVC endotracheal tubes. Furthermore, the findings indicate a notable reduction in bacterial colonization in coated tubes, which directly contributes to the decreased risk of VAP. These insights are valuable for the development of future long-term clinical trials aimed at evaluating even more effective strategies to minimize the impacts of endotracheal intubation. Ultimately, both clinicians and patients will need to weigh the potential benefits of coated tubes to determine the most appropriate approach, thus promoting better clinical outcomes, especially in cases of prolonged mechanical ventilation.
5.1. Limitations
Although this systematic review and meta-analysis provide important data on the comparative effectiveness of coated endotracheal tubes, particularly silver-coated ones, compared to conventional tubes in preventing ventilator-associated pneumonia, several limitations need to be acknowledged. First, it is important to note that this meta-analysis consists of only six randomized studies, which may compromise the robustness of the results. Additionally, one of the studies presented a high risk in its methodology, as assessed by Risk of Bias, which may affect the reliability of the data and the necessary information.
The inclusion of patient populations with varying clinical characteristics may also lead to variations in the observed outcomes, although this does not diminish the relevance of the findings. It is worth mentioning that, despite most studies employing rigorous criteria for outcome assessment, the variation in data collection methods may have influenced the consistency of the information.
CRediT authorship contribution statement
Oscar Inácio de Mendonça Bisneto: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Luanna Paula Garcez de Carvalho Feitoza: Formal analysis, Data curation, Conceptualization. Larissa Calixto Hespanhol: Software. Sávio Benvindo Ferreira: Methodology. Caroline Serafim Dagostin: Project administration, Investigation, Data curation. Roberto Augusto Mazetto Silva Vieira: Validation, Methodology. Lucas Alencar Queiroz: Investigation, Funding acquisition, Data curation. Lucas Chen: Supervision, Software, Resources, Project administration, Methodology. Taniela Marli Bes: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Funding acquisition, Formal analysis. Ho Yeh Li: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition.
Availability of data and material
Data are available upon reasonable request from the authors.
Funding
There was no funding for this investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40793.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Damas P., Legrain C., Lambermont B., Dardenne N., Guntz J., Kisoka G., Demaret P., Rousseau A.F., Jadot L., Piret S., Noirot D., Bertrand A., Donneau A.F., Misset B. Prevention of ventilator-associated pneumonia by noble metal coating of endotracheal tubes: a multi-center, randomized, double-blind study. Ann. Intensive Care. 2022 Jan 4;12(1):1. doi: 10.1186/s13613-021-00961-y. PMID: 34981245; PMCID: PMC8723906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorente L., Lecuona M., Jiménez A., Mora M.L., Sierra A. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am. J. Respir. Crit. Care Med. 2007 Dec 1;176(11):1079–1083. doi: 10.1164/rccm.200705-761OC. Epub 2007 Sep 13. PMID: 17872488. [DOI] [PubMed] [Google Scholar]
- 3.Kollef M.H., Afessa B., Anzueto A., Veremakis C., Kerr K.M., Margolis B.D., Craven D.E., Roberts P.R., Arroliga A.C., Hubmayer R.D., Restrepo M.I., Auger W.R., Schinner R., NASCENT Investigation Group Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008 Aug 20;300(7):805–813. doi: 10.1001/jama.300.7.805. PMID: 18714060. [DOI] [PubMed] [Google Scholar]
- 4.Tincu R.C., Cobilanschi C., Tincu I.F., Macovei R.A. Efficacy of noble metal–alloy endotracheal tubes in ventilator-associated pneumonia prevention: a randomized clinical trial. Balkan Med. J. 2022;39(3):167–171. doi: 10.4274/balkanmedia.galenos.2021.2021-7-86. Epub 2022 Mar 25. PMID: 35332771; PMCID: PMC9136541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philippart F., Gaudry S., Quinquis L., Lau N., Ouanes I., Touati S., Nguyen J.C., Branger C., Faibis F., Mastouri M., Forceville X., Abroug F., Ricard J.D., Grabar S., Misset B., TOP-Cuff Study Group Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am. J. Respir. Crit. Care Med. 2015 Mar 15;191(6):637–645. doi: 10.1164/rccm.201408-1398OC. PMID: 25584431. [DOI] [PubMed] [Google Scholar]
- 6.Poelaert J., Depuydt P., De Wolf A., Van de Velde S., Herck I., Blot S. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: a pilot study. J. Thorac. Cardiovasc. Surg. 2008;135(4):771–776. doi: 10.1016/j.jtcvs.2007.08.052. PMID: 18374755. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rello J., Ollendorf DA.Oster G., et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 9.Cook D.J., Walter S.D., Cook R.J., et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann. Intern. Med. 1998;129(6):433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Eggimann P., Hugonnet S., Sax H., Tourneau S., Chevrolet J.C., Pittet D. Ventilator-associated pneumonia: caveats for benchmarking. Intensive Care Med. 2003;29(11):2086–2089. doi: 10.1007/s00134-003-1991-9. [DOI] [PubMed] [Google Scholar]
- 11.Cook D., Guyatt G., Marshall J., et al. Canadian Critical Care Trials Group A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N. Engl. J. Med. 1998;338(12):791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 13.Craven D.E. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006;130(1):251–260. doi: 10.1378/chest.130.1.251. [DOI] [PubMed] [Google Scholar]
- 14.Cook D. Ventilator associated pneumonia: perspectives on the burden of illness. Intensive Care Med. 2000;26(suppl 1):S31–S37. doi: 10.1007/s001340051116. [DOI] [PubMed] [Google Scholar]
- 15.Dodek P., Keenan S., Cook D., et al. Evidencebased clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann. Intern. Med. 2004;141(4):305–313. doi: 10.7326/0003-4819-141-4-200408170-00011. [DOI] [PubMed] [Google Scholar]
- 16.Tablan O.C., Anderson L.J., Besser R., Bridges C., Hajjeh R. Guidelines for preventing healthcare-associated pneumonia, 2003: recommendations of CDC and the healthcare infection control practices advisory committee. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 17.Babcock H.M., Zack J.E., Garrison T., et al. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Crit. Care Med. 2000;28(10):3547–3554. doi: 10.1378/chest.125.6.2224. [DOI] [PubMed] [Google Scholar]
- 19.Rello J., Lorente C., Bodi M., Diaz E., Ricart M. KollefMH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? a survey based on the opinions of an international panel of intensivists. Chest. 2002;122(2):656–661. doi: 10.1378/chest.122.2.656. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. Published 2019 Aug 28. [DOI] [PubMed] [Google Scholar]
- 21.Marcut Lavinia, et al. Antimicrobial solutions for endotracheal tubes in prevention of ventilator-associated pneumonia. Materials. 2023;16(14):5034. doi: 10.3390/ma16145034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request from the authors.