Abstract
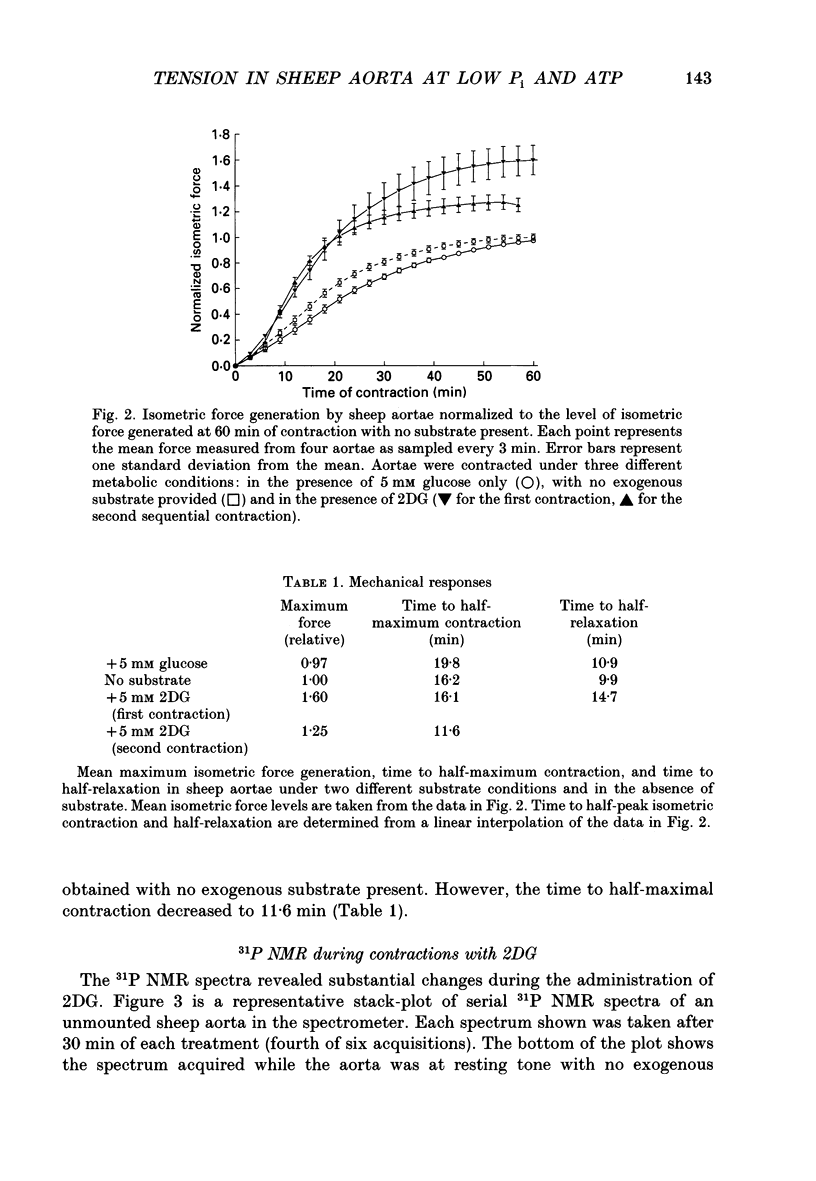
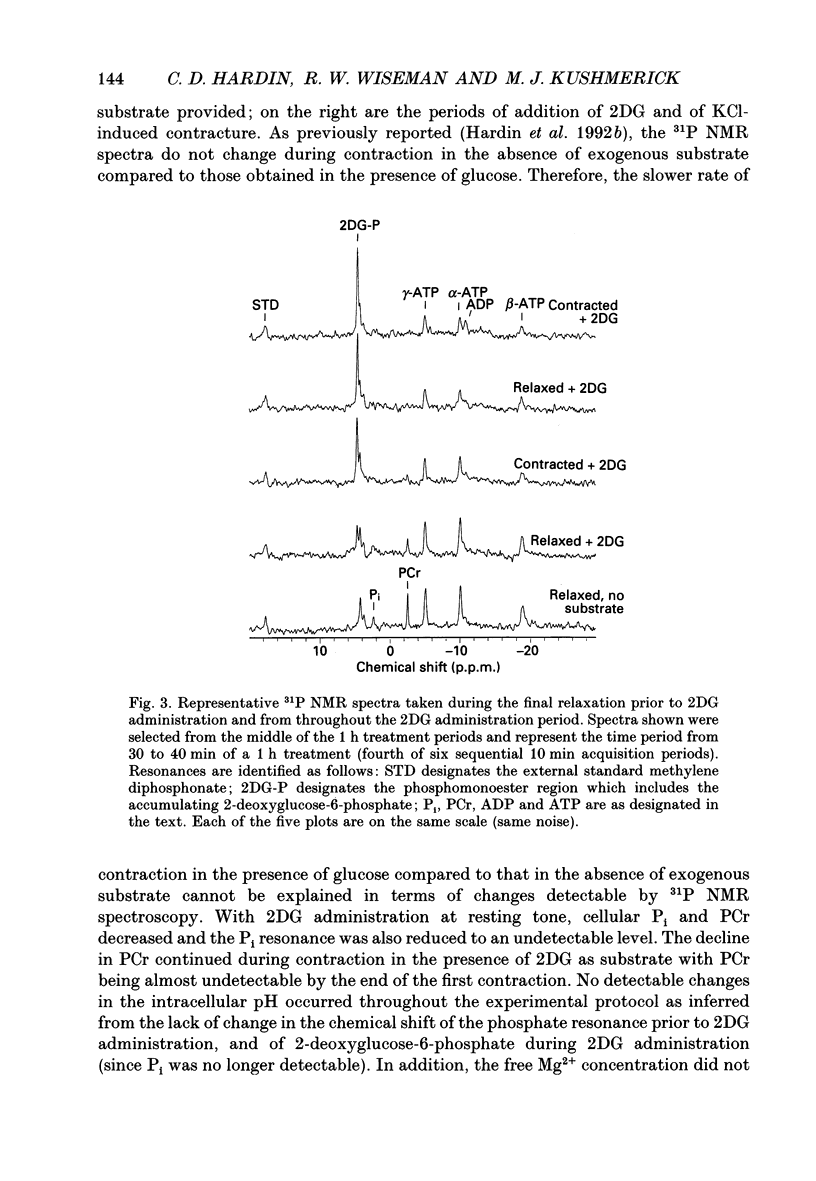
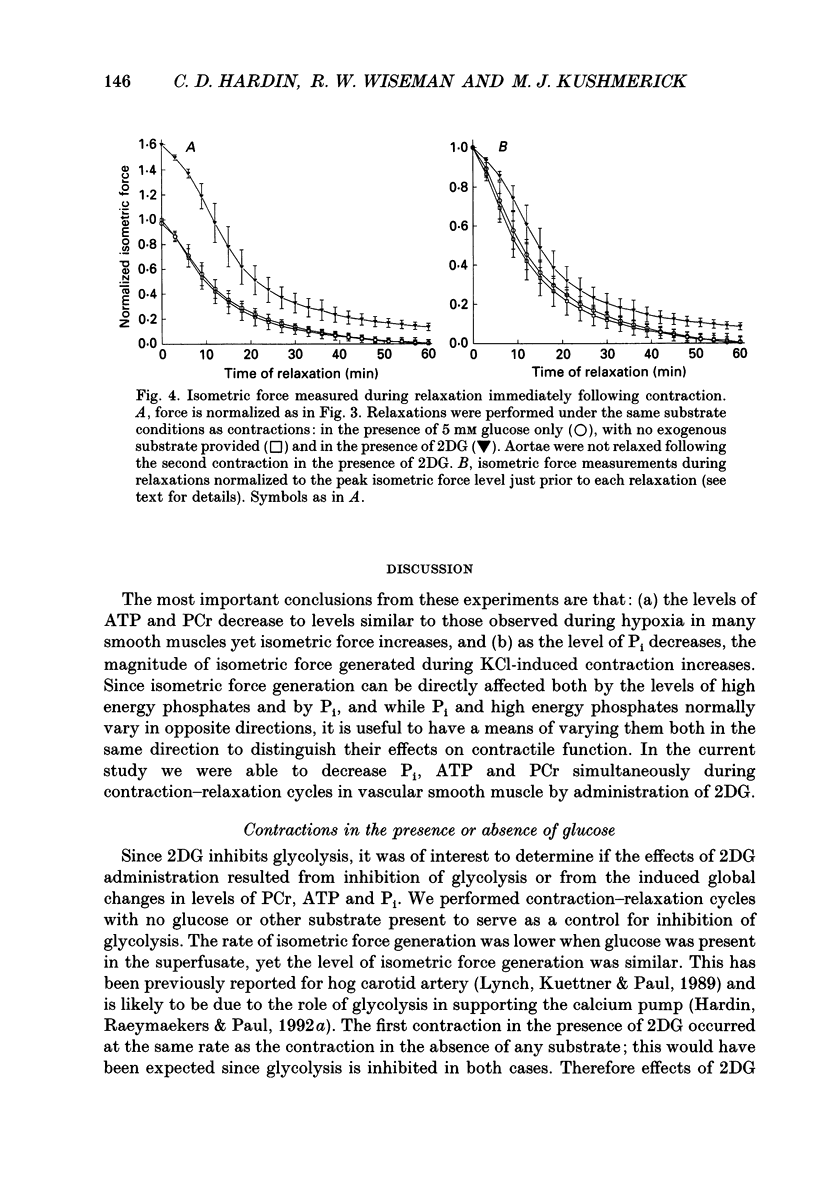
1. Tension responses of sheep aortae were investigated when different substrates were included in the superfusing medium. The magnitude of tension development was similar whether or not 5 mM glucose was present in the medium. However, the rate of tension development was greater in the absence of glucose. 2. When 5 mM 2-deoxyglucose (2DG) was present in the medium, the magnitude of tension generation was 1.6 times that in the absence of exogenous substrate. A second sequential contraction with 2DG generated tension 1.25 times that in the absence of exogenous substrate. The rate of tension development during the first contraction in the presence of 2DG was similar to that in the absence of substrate. However, the second contraction in the presence of 2DG had a substantially increased rate of tension development. 3. 31P nuclear magnetic resonance (NMR) spectroscopy revealed that, at resting tone, in the presence of 2DG, inorganic phosphate (P(i)) and phosphocreatine (PCr) simultaneously decrease while 2-deoxyglucose-6-phosphate accumulates. During contraction-relaxation cycles, in the presence of 2DG, P(i) and PCr become undetectable while ATP declines to approximately 50% of control values as determined by NMR. During the second contraction in the presence of 2DG, the area of the ADP resonance was similar to that of the alpha-ATP resonance. 4. The increase in the magnitude of tension generation, during 2DG administration, correlated with a decrease in P(i) levels. The rate of relaxation from a contraction, in the presence of 2DG, was slower than in the presence of glucose or in the absence of exogenous substrate. These results are consistent with the role of P(i) in the release of the proposed 'latch-bridge' state of maintained contraction at low energy demand. 5. The increase in isometric tension generation during contraction in the presence of 2DG appears to be related to the decreased levels of P(i). In the presence of 2DG, the reduction of PCr and of ATP occur to a similar extent to that during hypoxia, yet no inhibition of force takes place. The low levels of ATP and PCr reported with 2DG administration in these studies do not energetically limit the contractile apparatus.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner A., Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. J Physiol. 1985 Mar;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagelmann M., Güth K. Effect of inorganic phosphate on the Ca2+ sensitivity in skinned Taenia coli smooth muscle fibers. Comparison of tension, ATPase activity, and phosphorylation of the regulatory myosin light chains. Biophys J. 1987 Mar;51(3):457–463. doi: 10.1016/S0006-3495(87)83367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. D., Raeymaekers L., Paul R. J. Comparison of endogenous and exogenous sources of ATP in fueling Ca2+ uptake in smooth muscle plasma membrane vesicles. J Gen Physiol. 1992 Jan;99(1):21–40. doi: 10.1085/jgp.99.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. D., Wiseman R. W., Kushmerick M. J. Vascular oxidative metabolism under different metabolic conditions. Biochim Biophys Acta. 1992 Jan 13;1133(2):133–141. doi: 10.1016/0167-4889(92)90060-o. [DOI] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. M., Kuettner C. P., Paul R. J. Glycogen metabolism during tension generation and maintenance in vascular smooth muscle. Am J Physiol. 1989 Oct;257(4 Pt 1):C736–C742. doi: 10.1152/ajpcell.1989.257.4.C736. [DOI] [PubMed] [Google Scholar]
- Lövgren B., Hellstrand P. Graded effects of oxygen and respiratory inhibitors on cell metabolism and spontaneous contractions in smooth muscle of the rat portal vein. Acta Physiol Scand. 1985 Apr;123(4):485–495. doi: 10.1111/j.1748-1716.1985.tb07614.x. [DOI] [PubMed] [Google Scholar]
- Nakayama S., Seo Y., Takai A., Tomita T., Watari H. Phosphorous compounds studied by 31P nuclear magnetic resonance spectroscopy in the taenia of guinea-pig caecum. J Physiol. 1988 Aug;402:565–578. doi: 10.1113/jphysiol.1988.sp017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. J. Functional compartmentalization of oxidative and glycolytic metabolism in vascular smooth muscle. Am J Physiol. 1983 May;244(5):C399–C409. doi: 10.1152/ajpcell.1983.244.5.C399. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Hardin C. D., Raeymaekers L., Wuytack F., Casteels R. Preferential support of Ca2+ uptake in smooth muscle plasma membrane vesicles by an endogenous glycolytic cascade. FASEB J. 1989 Sep;3(11):2298–2301. doi: 10.1096/fasebj.3.11.2528493. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Krisanda J. M., Lynch R. M. Vascular smooth muscle energetics. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S320–S327. doi: 10.1097/00005344-198406002-00006. [DOI] [PubMed] [Google Scholar]
- Schneider M., Sparrow M., Rüegg J. C. Inorganic phosphate promotes relaxation of chemically skinned smooth muscle of guinea-pig Taenia coli. Experientia. 1981;37(9):980–982. doi: 10.1007/BF01971791. [DOI] [PubMed] [Google Scholar]
- Scott D. P., Coburn R. F. Phosphocreatine and oxidative metabolism-contraction coupling in rabbit aorta. Am J Physiol. 1989 Aug;257(2 Pt 2):H597–H602. doi: 10.1152/ajpheart.1989.257.2.H597. [DOI] [PubMed] [Google Scholar]


