Abstract
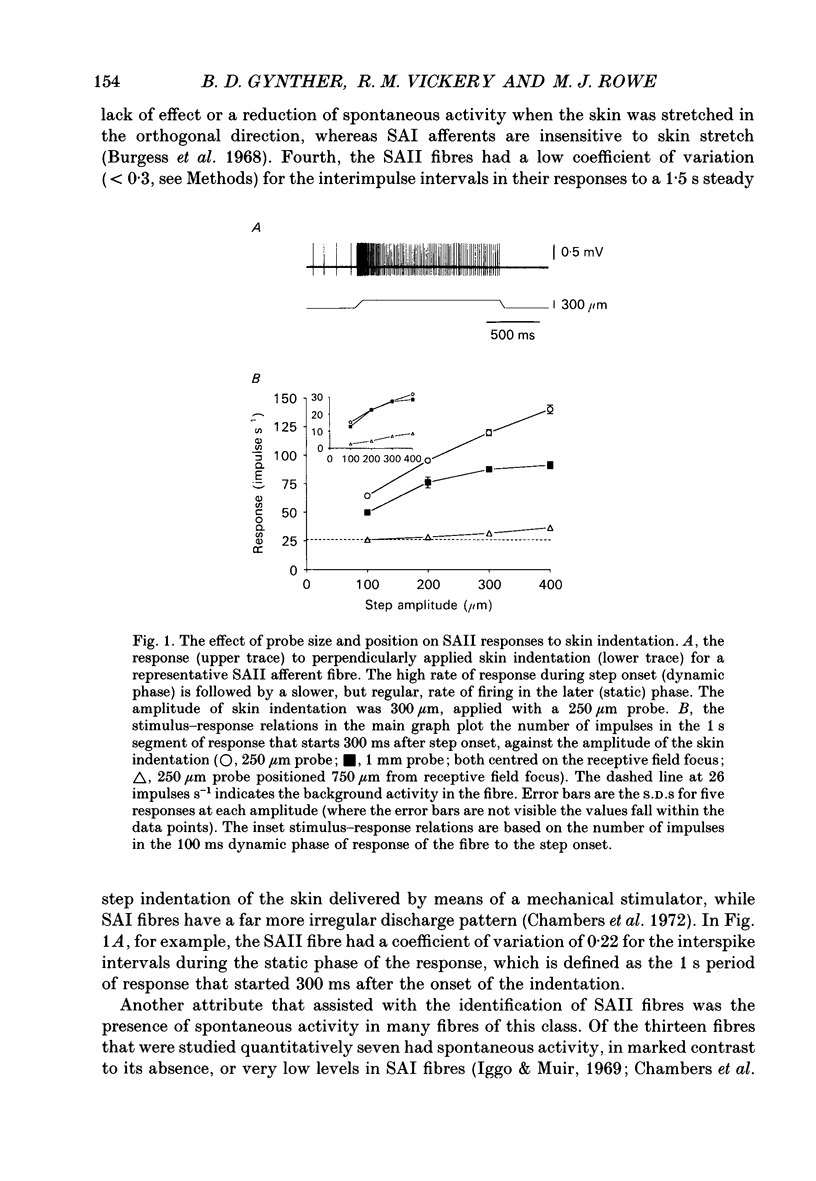
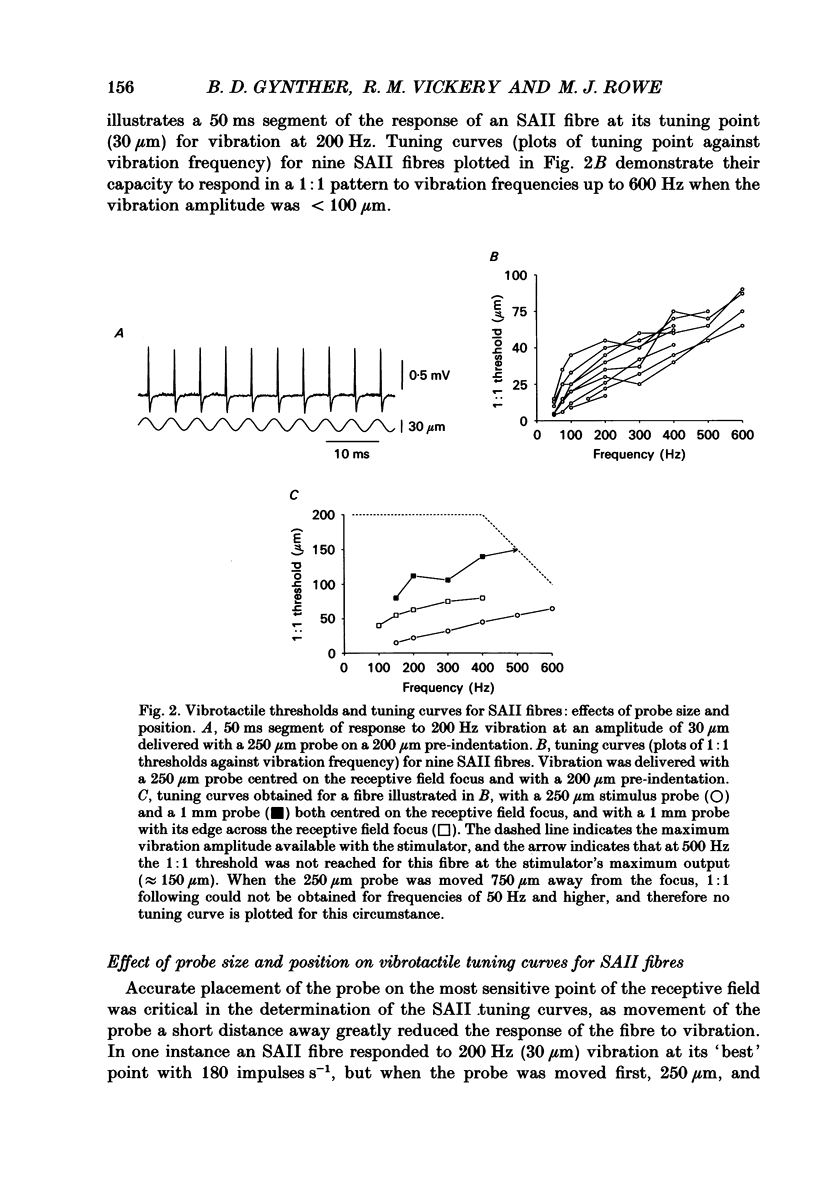
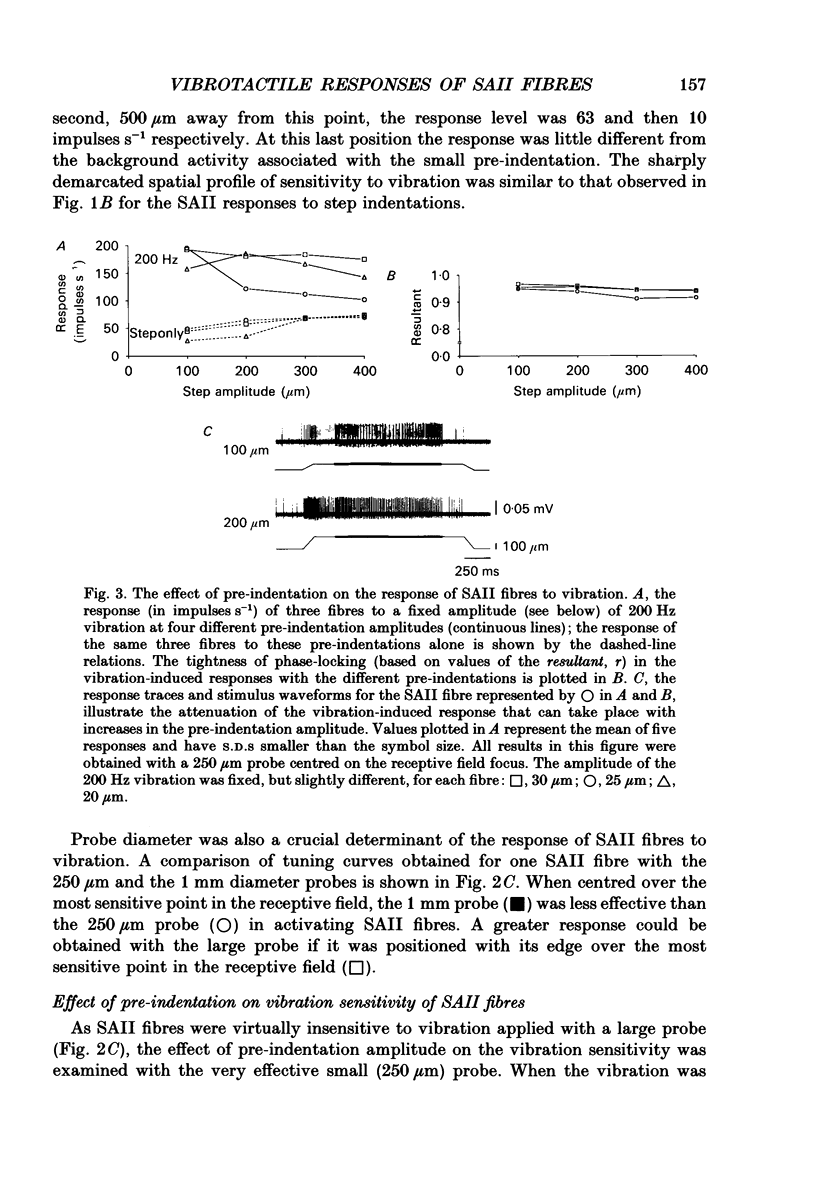
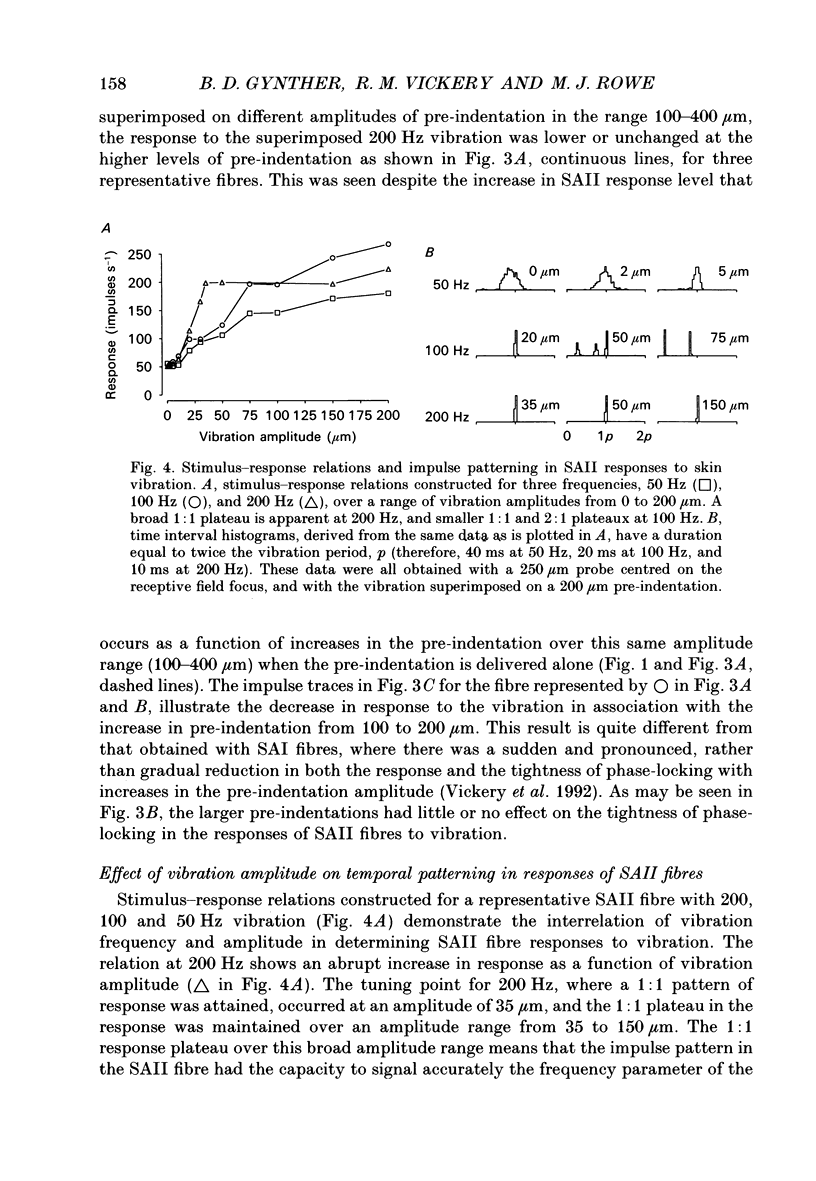
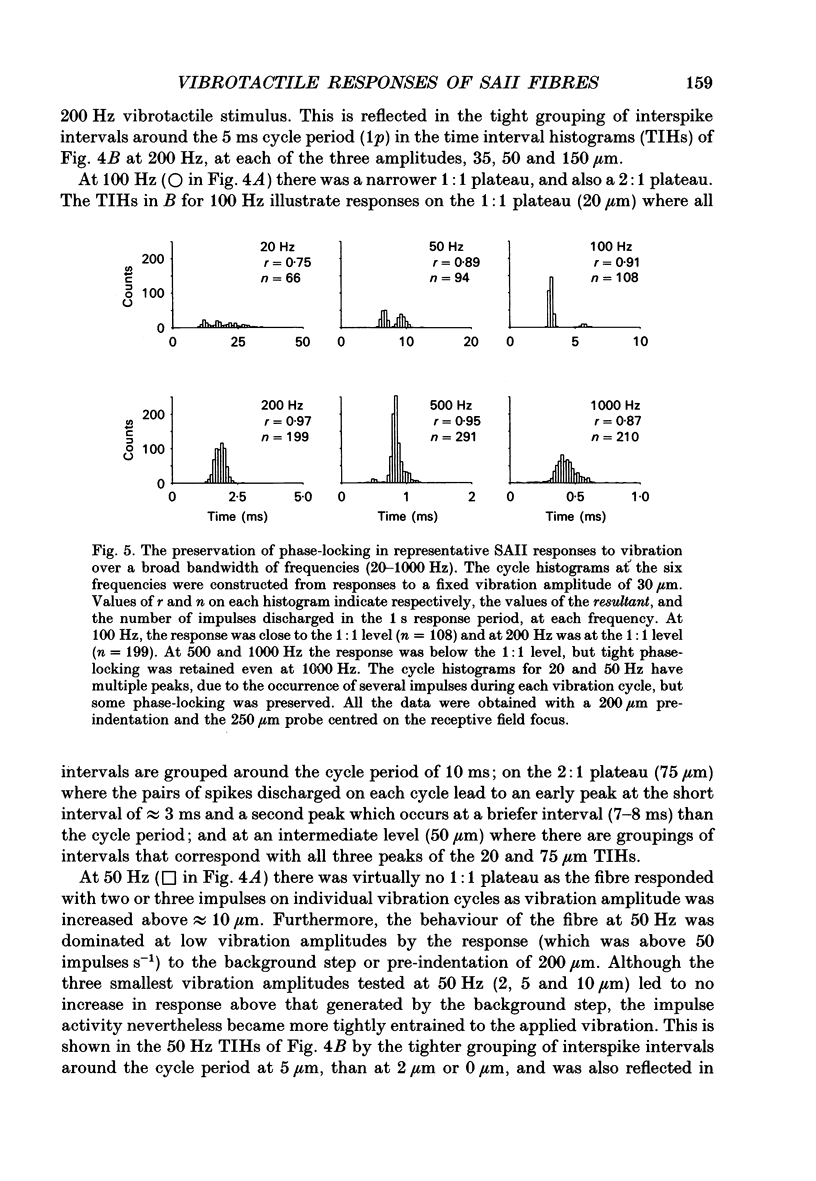
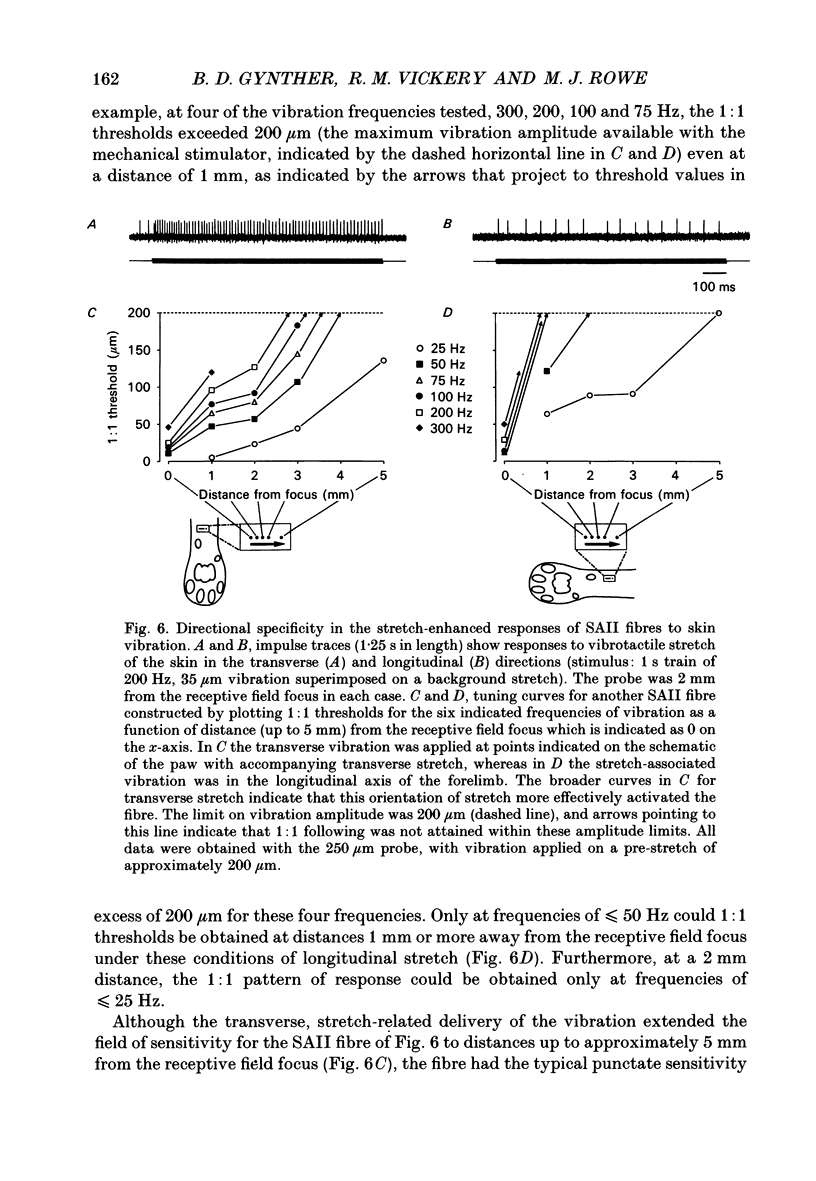
1. Slowly adapting type II (SAII) afferent fibres that supply the forelimb were isolated from the medial cutaneous nerve of anaesthetized cats and examined for their capacity to signal information about vibrotactile events in the hairy skin. 2. The SAII fibres had a single spot-like receptive field focus where they were highly sensitive to steady indentation and vibration applied with probes normal to the skin surface. However, their sensitivity was affected profoundly by the size of the stimulus probe, its position in relation to the receptive field focus and, to a lesser extent, the magnitude of any pre-indentation on which vibration was superimposed. Small stimulus probes (e.g. 250 microns diameter) were much more effective than larger (> or = 1-2 mm) ones, and small shifts in the position of the perpendicularly applied probe away from the receptive field focus led to a marked decline in responsiveness. 3. With appropriate choice of stimulus parameters for vibratory stimuli applied at the receptive field focus, the SAII fibres could respond at low threshold (< 100 microns), with a tightly phase-locked, regular 1:1 impulse pattern (one impulse per vibration cycle) that accurately signalled the vibration frequency over a bandwidth that extended to 600 Hz. Furthermore, their responses remained phase-locked up to 1000 Hz. Phase-locking in SAII fibres was marginally tighter than that in SAI fibres and comparable to that of Pacinian corpuscle fibres. 4. The sensitivity of forelimb SAII fibres to tangential skin stretch was directionally selective; stretch across the forelimb was much more effective than along its long axis. Vibration associated with tangential skin stretch led to a marked spatial expansion of the field of vibration sensitivity. SAII fibres could therefore signal information about natural stimuli that contain elements of skin stretch and vibration, as may be encountered when the forelimb brushes against textured surfaces. Should the SAII fibres fail to contribute to the sensory experience of vibrotactile stimuli, the explanation may be related to limitations imposed centrally on the processing of their signals. Nevertheless, the present results demonstrate that, with appropriate stimulus conditions, the SAII afferent fibres have much greater vibrotactile sensitivity than has been suggested by past studies.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken S. C., Lal S. The functional properties and innervation density of type II mechanoreceptor units of the sural nerve of the rabbit. Brain Res. 1982 Mar;257(1):57–64. doi: 10.1016/0165-0173(82)90004-2. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Howe J. F., Lessler M. J., Whitehorn D. Cutaneous receptors supplied by myelinated fibers in the cat. II. Number of mechanoreceptors excited by a local stimulus. J Neurophysiol. 1974 Nov;37(6):1373–1386. doi: 10.1152/jn.1974.37.6.1373. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G. Functional properties of slowly adapting mechanoreceptors in cat footpad skin. Somatosens Res. 1985;2(3):249–261. doi: 10.3109/07367228509144567. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Hora M. O., Rowe M. J. Functional maturation of tactile sensory fibers in the kitten. J Neurophysiol. 1984 Jul;52(1):74–85. doi: 10.1152/jn.1984.52.1.74. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980 Oct;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987 May;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. High gain transmission of single impulses through dorsal column nuclei of the cat. Neurosci Lett. 1986 Apr 24;65(3):277–282. doi: 10.1016/0304-3940(86)90274-0. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Integrative processing of vibratory information in cat dorsal column nuclei neurones driven by identified sensory fibres. J Physiol. 1987 May;386:311–331. doi: 10.1113/jphysiol.1987.sp016536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. W., Morley J. W. Sinusoidal movement of a grating across the monkey's fingerpad: representation of grating and movement features in afferent fiber responses. J Neurosci. 1987 Jul;7(7):2168–2180. doi: 10.1523/JNEUROSCI.07-07-02168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein J., Kavanagh P., Rowe M. J. Phase coherence in vibration-induced responses of tactile fibres associated with Pacinian corpuscle receptors in the cat. J Physiol. 1987 May;386:263–275. doi: 10.1113/jphysiol.1987.sp016533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T., Merzenich M. M. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10(3):251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H., Järvilehto T., Soininen K. Vibrotactile atonal interval correlated with activity in peripheral mechanoreceptive units innervating the human hand. Brain Res. 1985 May 6;333(2):311–324. doi: 10.1016/0006-8993(85)91585-9. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S., Landström U., Lundström R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res. 1982 Jul 22;244(1):17–25. doi: 10.1016/0006-8993(82)90899-x. [DOI] [PubMed] [Google Scholar]
- Johansson R. S. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol. 1978 Aug;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. O., Lamb G. D. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J Physiol. 1981 Jan;310:117–144. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietzny F., Hensel H. Response of rapidly and slowly adapting mechanoreceptors and vibratory sensitivity in human hairy skin. Pflugers Arch. 1977 Mar 11;368(1-2):39–44. doi: 10.1007/BF01063452. [DOI] [PubMed] [Google Scholar]
- Looft F. J. Response of cat cutaneous mechanoreceptors to punctate and grating stimuli. J Neurophysiol. 1986 Jul;56(1):208–220. doi: 10.1152/jn.1986.56.1.208. [DOI] [PubMed] [Google Scholar]
- Macefield G., Gandevia S. C., Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990 Oct;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D. I. Kinesthetic sensibility. Physiol Rev. 1978 Oct;58(4):763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Harrington T. H. The sense of flutter-vibration evoked by stimulation of the hairy skin of primates: comparison of human sensory capacity with the responses of mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res. 1969;9(3):236–260. doi: 10.1007/BF00234457. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Torebjörk E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983 Sep;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A., Hämäläinen H. Vibrotactile thresholds in non-Pacinian mechanoreceptive afferents: the importance of temporal parameters. Acta Physiol Scand. 1981 Dec;113(4):519–522. doi: 10.1111/j.1748-1716.1981.tb06931.x. [DOI] [PubMed] [Google Scholar]
- Phillips J. R., Johnson K. O. Tactile spatial resolution. III. A continuum mechanics model of skin predicting mechanoreceptor responses to bars, edges, and gratings. J Neurophysiol. 1981 Dec;46(6):1204–1225. doi: 10.1152/jn.1981.46.6.1204. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Olsson K. A., Westberg K. G., Clark F. J. Microstimulation of single tactile afferents from the human hand. Sensory attributes related to unit type and properties of receptive fields. Brain. 1984 Sep;107(Pt 3):727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Vickery R. M., Gynther B. D., Rowe M. J. Vibrotactile sensitivity of slowly adapting type I sensory fibres associated with touch domes in cat hairy skin. J Physiol. 1992;453:609–626. doi: 10.1113/jphysiol.1992.sp019247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck C. J., Jr Comparisons of punctate, edge and surface stimulation of peripheral, slowly-adapting, cutaneous, afferent units of cats. Brain Res. 1979 Oct 12;175(1):155–159. doi: 10.1016/0006-8993(79)90524-9. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- Whitehorn D., Howe J. F., Lessler M. J., Burgess P. R. Cutaneous receptors supplied by myelinated fibers in the cat. I. Number of receptors innervated by a single nerve. J Neurophysiol. 1974 Nov;37(6):1361–1372. doi: 10.1152/jn.1974.37.6.1361. [DOI] [PubMed] [Google Scholar]


