Abstract
Background
Trunk reconstruction following sarcoma excision involves significant defects. Pedicled and free latissimus dorsi myocutaneous flap (LDMF) reconstruction is commonly employed for thoracic defects; however, skin paddle design is limited to 10-12 cm to achieve primary donor closure. Paucity of data exists regarding the utility of V-Y advancement of LDMF, previously described for moderately sized thoracic defects. Efficacy of the technique's application, axial reach, maximum skin paddle, and overall success or complications have not been defined in the literature.
Methods
A retrospective review of records was conducted from 2015 to 2021 at our center to identify patients who underwent large, islanded V-Y LDMF reconstructions. Results were assessed by reviewing photographs and clinical notes. PubMed search of articles using the search terms “Latissimus Dorsi Flap Reconstruction” AND “V-Y” was conducted, abstracts were screened, and relevant articles were selected for review by the 2 author (NL/YS). We summarized the findings in our review and discussion.
Results
Eleven patients underwent V-Y LDMF reconstruction with large skin paddles designed to close the donor site and oncological defect. Flaps were mobilized in superior (n = 7), anterior (n = 2), inferior (n = 1) and medial (n = 1) directions. Satisfactory coverage and patient recovery with few complications was achieved. Average length of hospital stay was 4.7 days and secondary surgery was not required.
Discussion and conclusion
V-Y advancement of LDMF is reliable up to 24 cm axially with a skin paddle width of 24 cm based superiorly, inferiorly, or anteriorly. The technique is safe and effective in comorbid patients following high-dose radiotherapy, without prolonged general anesthesia or microsurgery. It represents a versatile locoregional reconstructive option for large trunk defects.
Keywords: Latissimus Dorsi Myocutaneous Flap, V-Y Flap, Trunk Reconstruction, Sarcoma Reconstruction
Introduction
The latissimus dorsi myocutaneous flap (LDMF) was first described by Tansini in 1906 for the reconstruction of mastectomy defects. In 1978, Quillen and colleagues described the use of pedicled LDMF for covering head and neck defects.1 Subsequently, the LDMF has become a reliable option for the reconstruction of the head and neck, chest wall, and breast, particularly when free tissue transfer is contraindicated.
Better understanding of the vascular anatomy allowed further applications of the LDMF. The LD is a type-five muscle under the Mathes and Nahai classification and affords reconstructive surgeons the opportunity to use it as a “turn-over” flap to reach the contralateral back defects based on the multiple minor pedicles or thoraco-lumber perforators. The microsurgical era expanded applications as free flap, and more recently perforator-based fasciocutaneous flap based on thoracodorsal artery perforators (TDAP), are being used for resurfacing local, regional, or distant defects. The LDMF provides robust bulk for coverage of larger defects and obliteration of dead space for which smaller flaps will not suffice. It is particularly useful for reconstructing resection defects of large locally aggressive soft tissue neoplasms, such as sarcoma. This is especially relevant as 33% of sarcomas occur within the trunk.2
Despite the extensive use of the LDMF as a pedicled and free flap, there is a paucity of literature on locally based LDMF reconstruction without skeletonization of the thoracodorsal pedicle. Few case series have outlined the use of V-Y advancement and closure of the LDMF for anterior and posterior chest wall reconstruction.3, 4, 5 Moreover, precise defect sizes and anatomical locations reliably reconstructed using this method remain undefined.
Materials and Methods
We retrospectively interrogated electronic theatre records from 2015 to 2021 at our center to identify the senior author's patients who underwent LDMF reconstruction for oncological resection defects to identify those who had V-Y advancement skin closure. Post-operative results were assessed by reviewing the photographs and clinical notes. PubMed search using the search terms “Latissimus Dorsi Flap Reconstruction” AND “V-Y” was conducted to screen abstracts and select relevant articles that were reviewed by the 2 authors (NL/YS). We summarized the findings in our review and discussion below.
Results
Case Series
Eleven patients underwent reconstruction of large thoracic defects via V-Y advancement of the LDMF over a 6-year period from 2015 to 2021. Defect location ranged from the anterior chest wall (n = 3), posterior chest wall (n = 5), superior shoulder (trapezius) region (n = 1), and lower paramedian lumbar region (n = 2). Most cases (n = 9) were referred to the plastic surgery team for reconstruction prior to wide oncological resection of soft tissue neoplasms. One case was referred for immediate reconstruction following radical excision of a locally advanced breast carcinoma by the breast surgery team. Moreover, 1 case was under the care of the plastic surgery team for malignant melanoma deposit to the left scapula region.
All but 1 patient underwent high-dose neoadjuvant radiotherapy to the reconstructed region with a mean radiotherapy dose of 50.2 Gy. All post-excision defects required muscle coverage, which could not be provided by a perforator-based flap. Ten patients underwent immediate and 1 underwent delayed reconstruction. Patient demographics and case characteristics are outlined in Table 1.
Table 1.
Demographic and clinical characteristics of patients undergoing variations of LD myocutaneous advancement flaps in this series.
| Age (years) | Defect location | Defect size (Transverse x Craniocaudal) | Pathology | Neoadjuvant RT | Reconstruction timing | Orientation of flap and dissection performed | Patient comorbidities | Complications |
|---|---|---|---|---|---|---|---|---|
| 68 | Left scapula post wide resection | 15 cm × 15 cm | Recurrent neurofibromatosis | Yes (50.4 Gy/25#) | Immediate | Superior advancement of V-Y LDMF. Superior and inferior thirds of VY released, LD incised distally. | Gastroesophageal reflux, laminectomy, and current smoker. | Distal tip necrosis and V-segment necrosis—both healed by secondary intent |
| 67 | Right anterior chest wall post radical mastectomy | 12 cm × 12 cm | Fungating right breast carcinoma | Yes (50.4 Gy/25#) | Immediate | Horizontal advancement of V-Y LDMF to anterior chest wall. The medial and lateral thirds of VY released, LD incised distally to mobilize flap anteriorly. | Venous thromboembolism, osteoporosis, and iron deficiency. | Nil |
| 43 | Left scapula post wide resection | 10 cm × 12 cm | Leiomyosarcoma | Yes (50.4 Gy/25#) | Immediate | Superomedial advancement. Superior and inferior thirds of VY released, LD incised distally. | Nil | Seroma—drained. Superficial wound breakdown—healed with dressings. |
| 75 | Left upper back post wide resection | 10 cm × 7 cm | Myxofibrosarcoma | Yes (50.4 Gy/25#) | Immediate | Superior advancement. Superior and inferior thirds of VY released, LD incised distally. | Myelodysplasia, hypertension, bronchiectasis, subarachnoid hemorrhage, and cerebral aneurysm. | Nil |
| 38 | Left scapula post wide resection | 18 × 24 cm | Malignant melanoma | No | Immediate | Superolateral advancement. Superior and inferior thirds of VY released, LD incised distally. Skin bridge maintained in the axillary fold. | Malignant melanoma, anxiety, and asthma | Nil |
| 62 | Anterior chest wall | 24 cm × 14 cm | Osteosarcoma | Yes (50.4 Gy/25#) | Immediate | Horizontal advancement of V-Y LDMF to anterior chest wall. The medial and lateral thirds of VY released, LD incised distally to mobilize flap anteriorly. | Hypertension and hypercholesterolemia | Nil |
| 59 | Paramedian superior back post wide resection | 15 cm × 13 cm | Malignant adnexal tumor | No | Delayed | Superomedial advancement. Superior and inferior thirds of VY released, LD incised distally. | Breast cancer | Nil |
| 56 | Right upper trapezius region post wide local resection | 25 cm × 21 cm | High grade malignant mesenchymoma | Yes (50.4 Gy/25#) | Immediate | Superior advancement. Superior and inferior thirds of VY released, LD incised distally to mobilize flap superiorly. Combined with trapezius flap to cover superior aspect of shoulder. | Atrial fibrillation and gastroesophageal reflux | Superficial tip necrosis—healed by secondary intent |
| 67 | Left anterior chest wall wide resection; underlying chest wall reconstruction (Goretex) | 18 cm × 15 cm | Fibrosarcoma of the left breast and anterior ribs | Yes (50.4 Gy/25#) | Immediate | Horizontal advancement of V-Y LDMF to anterior chest wall. The medial and lateral thirds of VY released, LD incised distally to mobilize flap anteriorly. | Hypothyroidism and hysterectomy | Nil |
| 69 | Right lumbar region post wide resection | 17 cm × 14 cm | Myxofibrosarcoma | Yes (50.4 Gy/25#) | Immediate | Inferior advancement. Superior and inferior thirds of VY released, LD incised distally and the flap was mobilized inferiorly. | Nil | Seroma, managed conservatively; superficial necrosis |
| 52 | Right paraspinal region post wide local resection | 21 cm × 11 cm | Myxofibrosarcoma | Yes (50.4 Gy/25#) | Immediate | Horizontal advancement of V-Y LDMF medially to the paramedian spine. Medial and lateral thirds of V-Y released; LD incised proximally to mobilize the flap. | Nil | Nil |
LD, latissimus dorsi.
In each case, V-Y advancement of the LDMF were designed to incorporate the largest possible LD muscle component to ensure a robust blood supply and were raised from tissue adjacent to each of the large thoracic defects depending on the axis of advancement (Figure 1). Direction of advancement in this series included superior, inferior, anterior, or medial movements. In all cases but 1, the LD muscle was released distal to the skin island. The flap whereby the LDMF was released proximally, when moving the V-Y flap medially, to facilitate its mobilization in this axis. Flaps were then mobilized in a triangular format incorporating advancement in a linear manner based on the pre-planned axis and subsequently closed in a V-Y fashion. Almost all flaps were islanded, except in 1 case whereby an anterior skin bridge was retained to preserve the axillary fold and improve venous drainage (Figure 2). Figure 1 illustrates flap planning associated with the cases discussed based on defect location.
Figure 1.
Planning schematic for the LD myocutaneous V-Y advancement flap depending on the thoracic defect location. From left to right: superior movement of the V-Y flap to incorporate as much of the LD muscle as possible and incising distally to mobilize the flap. Moving anteriorly, the flap is also incised distally and the end thirds of the flaps are raised and islanded on the LD centrally. Moving inferiorly, the flap is also incised distally with the skin island centered over the LD to incorporate as much of the LD muscle as possible. When mobilizing the flap medially (far right), the LD is incised proximally, unlike the others, to facilitate its movement toward the paraspinal region.
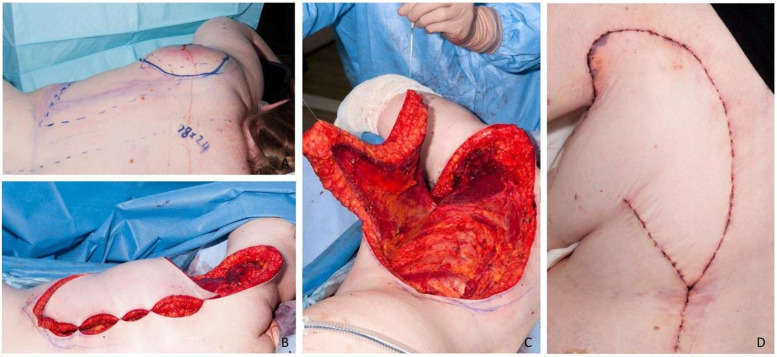
Figure 2.
Image A demonstrates the preoperative markings for V-Y advancement LDMF to the upper left scapula region. Image B illustrates the original defect size, measuring 18 cm × 24 cm (craniocaudal by transverse) following wide excision of invasive melanoma; while image C depicts the distance required for superior flap mobilization into the defect. Image D illustrates the final post-operative outcome at 1-week post-operative, demonstrating a small amount of distal tip necrosis. Notably, an anterior skin island was left intact in this case.
Defect size with primary V-Y closure ranged from 10 cm × 7 cm to 18 cm × 24 cm (transverse by craniocaudal). One patient required additional split thickness grafting to the inset LD muscle on the anterior chest, and another patient underwent combined LD and trapezius myocutaneous flaps to cover a significant superior shoulder defect by extending anteriorly post wide resection of sarcoma (Figure 3). This defect was too anterior to close using the V-Y LDMF. Linear flap advancement varied up to 24 cm with a maximum paddle width of 25 cm at the base of the triangle. As illustrated in Figure 2, 1 case incorporated LDMF rotation at approximately 20° from its linear axis, to better reconstruct the defect by preserving an axillary skin bridge. Meanwhile, a single Z-plasty was incorporated medially to de-tension the LDMF inset and lengthen the scar in 1 case (Figure 4).
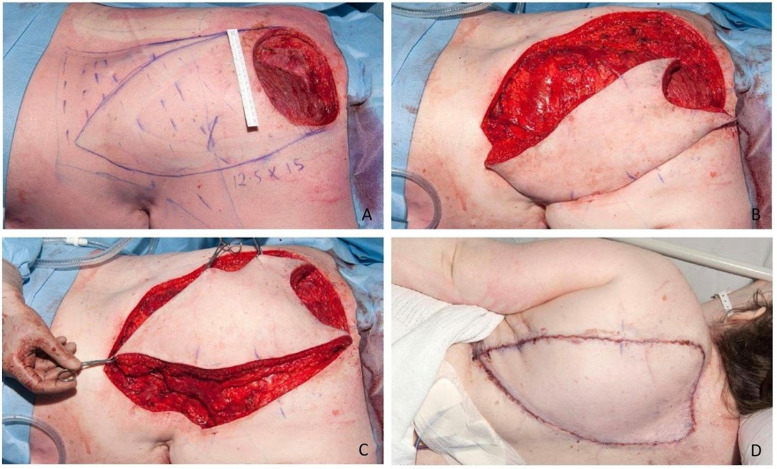
Figure 3.
Intra-operative photos demonstrating the original paramedian defect measuring 13 cm × 15 cm (craniocaudal by transverse) post-excision with marked planning for LDMF in a V-Y format (A). Images B and C demonstrate the incisions and mobilization of the flap superomedially toward the defect across the midline, respectively. Image D highlights the final on table outcome.
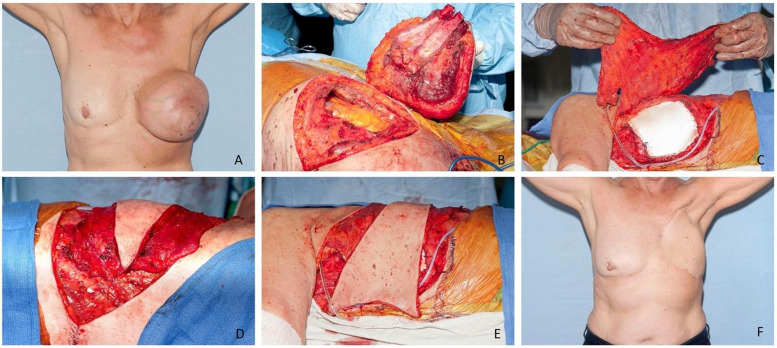
Figure 4.
Illustration of the transverse/anterior advancement of the LDMF to cover a full-thickness defect of the anterior chest wall following excision of a left breast and chest wall for locally advanced sarcoma. Image A is preoperative, images B and C demonstrate the Goretex chest wall reconstruction, and images D and E illustrate the movement of tissue from the posterior (back) to anterior (chest wall) regions, respectively. Image F demonstrates the post-operative results.
All patients demonstrated satisfactory post-operative outcomes, with adequate soft tissue coverage and subsequent overall recovery. The average length of hospital stay was 4.7 days. There was no grade 2 or higher level complications. Complications included minor infection (n = 1) and superficial necrosis (n = 3), the latter of which is shown in Figures 2d and 4d. All patients healed well with dressings in the outpatient setting and further surgery was not required. Two patients (n = 2) developed minor post-operative seromas that did not require further intervention. Tissue movements in all directions are illustrated in the figures including superior (Figure 2, Figure 3, Figure 4, Figure 5), anterior (Figure 6), and inferior (Figure 7) advancement.
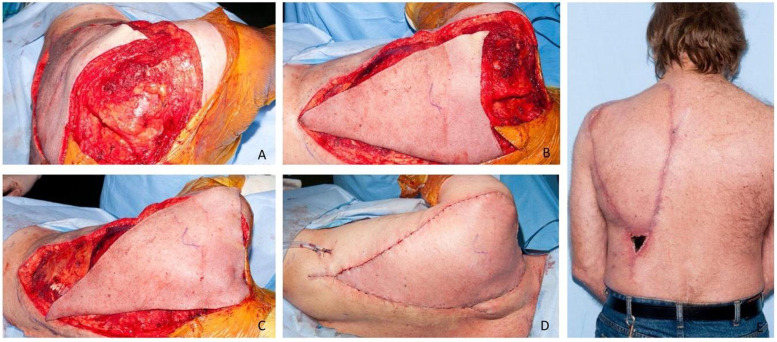
Figure 5.
Images demonstrating the original 15 cm × 15 cm defect with the LDMF incisions made in preparation for superior mobilization (A), the triangular islanded skin paddle demonstrating coverage of the defect when mobilized superiorly (B), and closure in a V-Y fashion. A single Z-plasty was incorporated medially to lengthen the scar, reduce inset tension, and mobilize the flap laterally. Image D shows the post-operative result at 2 weeks, highlighting the 2 areas of necrosis at the base of the V-segment and at the lateral distal tip. Both areas healed well via secondary intention.
Figure 6.
MRI demonstrating a large well circumscribed mass in the lower right flank (A), clinical photograph of the preoperative large right flank mass (B), and immediate post-operative outcome following inferior V-Y advancement of LD for coverage of a large 17 cm × 14 cm defect in the right lower lumber region (C).
Figure 7.
Images A and B demonstrate the large high grade malignant mesenchymoma to the right shoulder. Image C demonstrates the planning of the LDMF and trapezius flap as well as the 25×21 cm defect to the upper right shoulder extending anteriorly. Image D demonstrates the early post-operative result at 6 weeks.
Literature Review and Discussion
In its original format with an elliptical skin paddle design, the LDMF necessitated split thickness skin grafting to the donor site when larger defects were encountered (>10-12 cm width). Traditionally, the LDMF is raised with dissection of the thoracodorsal pedicle for mobilization and tunneling to the anterior chest. The LD receives a reliable type-five dominant (thoracodorsal) and segmental (intercostal and lumber perforators) blood supply.6 Our series illustrates how this dominant and segmental vasculature permits a broad area of tissue to be incorporated within the LDMF without complete dissection of the thoracodorsal pedicle or inclusion of all perforators.
Micali and Carramaschi3 first described a variant of the LDMF with a relatively broad skin paddle design using a V-Y primary donor site closure for anterior chest wall defects in 2001. Their series included 8 patients with significant parasternal defects following radical mastectomy for advanced breast cancer. As illustrated in Table 2, the defect size ranged from 15 cm × 15 cm to 19 cm × 21 cm (transverse x craniocaudal). Skin paddle width (triangular base) ranged from 12 cm to 17 cm incorporating a maximum flap length of 29.5 cm. Seven of the 8 defects were successfully covered with primary closure of the donor site, with 1 patient requiring additional mobilization of epigastric tissue in a random pattern design to facilitate closure. Distal flap tip necrosis occurred in 3 patients, 1 of whom also experienced partial thickness skin loss.
Table 2.
Defect size, location, excised pathology, and LDMF V-Y advancement flap design described in the literature.
| Authors | Pts (n) | Defect location | Defect size (Trans x CC) | Pathology | Flap design described | Complications |
|---|---|---|---|---|---|---|
| Micali & Carramaschi | n = 8 | All defects to anterior chest wall (peri-sternal) post Halsted radical mastectomy | 17 cm × 17 cm | Advanced breast cancer | Anterolateral V-Y advancement and random advancement of the latissimus dorsi myocutaneous flap with primary closure of donor and other site(s) of tissue mobilization. | Nil |
| 14.5 cm × 11.5 cm + 14.5 cm × 16.5 cm (irregular) | Nil | |||||
| 18 cm × 17 cm | Nil | |||||
| 18 cm × 16.5 cm | 1 cm rear tip skin necrosis | |||||
| 15 cm × 15 cm | 1 cm × 4 cm distal necrosis | |||||
| 14 cm × 20 cm | Partial skin loss, 3 cm of necrosis distal tip | |||||
| 18 cm × 19 cm | Nil | |||||
| 19 cm × 21 cm | Nil | |||||
| Christen at al. | n = 3 | Posterior midline | 10 cm × 12 cm | Wound dehiscence post spinal fixation | Pedicled LD myocutaneous flap, oblique advancement, medial defect orientated cranially, and lateral side caudally. | No reported complications |
| Posterior midline | 8 cm × 10 cm | Wound dehiscence post T7 laminectomy and T6-T8 arthrodesis | ||||
| Posterior chest wall | 18 cm × 19 cm | Soft tissue sarcoma and wide resection with rib. | ||||
| Woo et al. | n = 12 | Anterior chest wall; muscle | 10.5 cm × 9 cm | Advanced or recurrent breast cancer; post radical mastectomy. | Straight extended V-Y advancement of the LDMF (n=9) and curvilinear V-Y advancement (n = 3). Two patients required STSG to LD muscle and one required STSG to serratus anterior (donor). One patient required secondary suturing. Eight patients underwent direct closure. | One patient experienced wound dehiscence, healed with dressings by secondary intent. |
| Anterior chest wall; muscle or ribs | 17 cm × 16 cm | |||||
| Anterior chest wall; muscle or ribs | 12.5 cm × 11.5 cm | |||||
| Anterior chest wall; muscle | 13.5 cm × 13.5 cm | |||||
| Anterior chest wall; muscle | 15 cm × 15 cm | |||||
| Anterior chest wall; muscle/ribs/lung | 20 cm × 20 cm | |||||
| Anterior chest wall; muscle/ribs | 14 cm × 12 cm | |||||
| Anterior chest wall; muscle | 9.5 cm × 7.5 cm | |||||
| Anterior chest wall; muscle | 15 cm × 15 cm | |||||
| Anterior chest wall; muscle | 13 cm × 12 cm | |||||
| Anterior chest wall; muscle | 16 cm × 14 cm | |||||
| Rocco et al. | n = 1 | Anterior chest wall | Unspecified | Recurrent chondrosarcoma | Transverse V-Y advancement | No complications |
LD, latissimus dorsi; STSG, Split thickness skin graft.
More recently, Christen et al.4 outlined V-Y advancement of the LDMF in 3 posterior chest wall defects in midline wound dehiscence following spinal surgery and following wide resection of a soft tissue sarcoma. In this limited series, defects ranged from 8 cm × 10 cm to 18 cm × 19 cm. The authors reported no complications with complete flap survival and no incidence of post-operative infection. Patient characteristics and flap features of these series are outlined in Table 2.
Woo et al.5 published a 12-patient series of anterior chest wall reconstruction following advanced or recurrent breast cancer resection using the LD myocutaneous V-Y flap. The defect sizes ranged from 10.5 cm × 9 cm to 20 cm × 20 cm and 3 patients had undergone preoperative radiotherapy. Three patients required split thickness skin grafting. One patient experienced wound dehiscence which healed with dressings.
A further case was described by Rocco et al.7 for the reconstruction of a large anterior chest wall defect following recurrent chondrosarcoma resection. The authors described anterior chest wall reconstruction of a substantial full thickness defect over a 26 cm × 34 cm Gore-Tex patch using a curvilinear V-Y advancement of LDMF without complications. However, the size of the final myocutaneous defect was not specified.
In modern practice, locoregional flaps are often used to the reconstruct small to medium sized defects, whereas free tissue transfer is undertaken in more complex procedures on patients fit for prolonged general anesthesia.6 The reconstructive surgeon often encounters patients requiring coverage of large (wide and deep) defects in the setting of significant patient comorbidities that prohibit a prolonged anesthetic use. Prior to Micali and Carramaschi's description of the V-Y advancement of the LDMF, larger pedicled flaps including the LDMF required donor site grafting. Apart from the poor cosmesis, donor site grafting is associated with poor graft take, in our experience, as the elevation of the LD muscle means that the graft is laid onto rib and intercostal fascia. Elevation of the muscle also increases the formation of serum fluid bathing the graft and interferes with adherence.
Recently, there has been an expansion in the use of perforator-based flaps such as the TDAP, which is a reliable option for the reconstruction of superficial defects when muscle bulk is not required and muscle-sparing with reduced donor site morbidity can be achieved. Nonetheless, we believe that in patients who routinely receive neoadjuvant radiotherapy and large resections requiring obliteration of significant dead space, fasciocutaneous single perforator flaps would not achieve equivalent outcomes.
From our experience and those of others, it is evident the LDMF can be raised to incorporate a broader skin paddle than conventionally attempted (up to approximately 24-25 cm). Direct closure of the donor site may be achieved by using V-Y advancement, with tissues advanced up to a 1:1 ratio (24 cm) without compromising flap perfusion. Although Micali and Christen did not outline the width to length ratio in their series, we found that the flap island should be in 1:1 to 2:1 ratio relative to the width of the defect and triangular base of the flap (i.e., equal length to width or higher, but not extending wider than 2 times the length of the linear axis of the flap). Our series demonstrates that the LDMF can be mobilized by incorporating dominant and segmental vasculature to reliably advance to reconstruct large and diverse regional thoracic defects with primary V-Y donor closure more easily and rapidly than free tissue transfer.
Conclusion
V-Y advancement of the LDMF is a versatile and robust reconstructive solution to significant thoracic defects ranging from the upper and lower back to the ipsilateral axilla and anterior chest wall in patients with poor preoperative prognostic characteristics. We demonstrate the flap's reliability in the context of high-dose neoadjuvant radiotherapy with no compromise on the reconstructive outcomes in terms of function and cosmesis. Our series confirms the diversity of applications, but also the reconstructive limit of this technique. We commend the LDMF V-Y advancement as an approach to mobilize a relatively large flap incorporating muscle, with fast operating times and direct closure of the donor site making it a useful reconstructive option.
Conflict of Interest Statement
No conflict of interest was reported by the authors.
Acknowledgments
Statement of Informed Consent
Informed consent has been obtained to utilize patient data included in this case report. All data and all images are deidentified.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Ethics approval was obtained from the Human Research and Ethics Committee, Sir Charles Gairdner Hospital.
Statement of Funding
No funding was received to conduct this study.
References
- 1.Quillen CG, Shearin JC, Jr, Georgiade NG. Use of the latissimus dorsi myocutaneous island flap for reconstruction in the head and neck area: case report. Plastic and Reconstructive Surgery. 1978;62(1):113–117. doi: 10.1097/00006534-197807000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Fabiano S, Contiero P, Barigelletti G, et al. Epidemiology of soft tissue sarcoma and bone sarcoma in Italy: analysis of data from 15 population-based cancer registries. Sarcoma. 2020;1 [Google Scholar]
- 3.Micali E, Carramaschi FR. Extended V-Y latissimus dorsi musculocutaneous flap for anterior chest wall reconstruction. Plastic and Reconstructive Surgery. 2001;107(6):1382–1390. doi: 10.1097/00006534-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Christen T, Koch N, Philandrianos C, et al. The V-Y latissimus dorsi musculocutaneous flap in the reconstruction of large posterior chest wall defects. Aesthetic Plastic Surgery. 2012;36(3):618–622. doi: 10.1007/s00266-011-9866-x. [DOI] [PubMed] [Google Scholar]
- 5.Woo E, Tan BK, Koong HN, Yeo A, Chan MY, Song C. Use of the extended V-Y latissimus dorsi myocutaneous flap for chest wall reconstruction in locally advanced breast cancer. The Annals of Thoracic Surgery. 2006;82(2):752–755. doi: 10.1016/j.athoracsur.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Mathes SJ, Nahai F. Churchill Livingstone; 1997. Reconstructive surgery: principles, anatomy & technique. [Google Scholar]
- 7.Rocco G, Scognamiglio F, Fazioli F, et al. V-Y latissimus dorsi flap for coverage of anterior chest wall defects after resection of recurrent chest wall chondrosarcoma. The Journal of Thoracic and Cardiovascular Surgery. 2009;138(5):1242–1243. doi: 10.1016/j.jtcvs.2008.08.025. [DOI] [PubMed] [Google Scholar]