Abstract
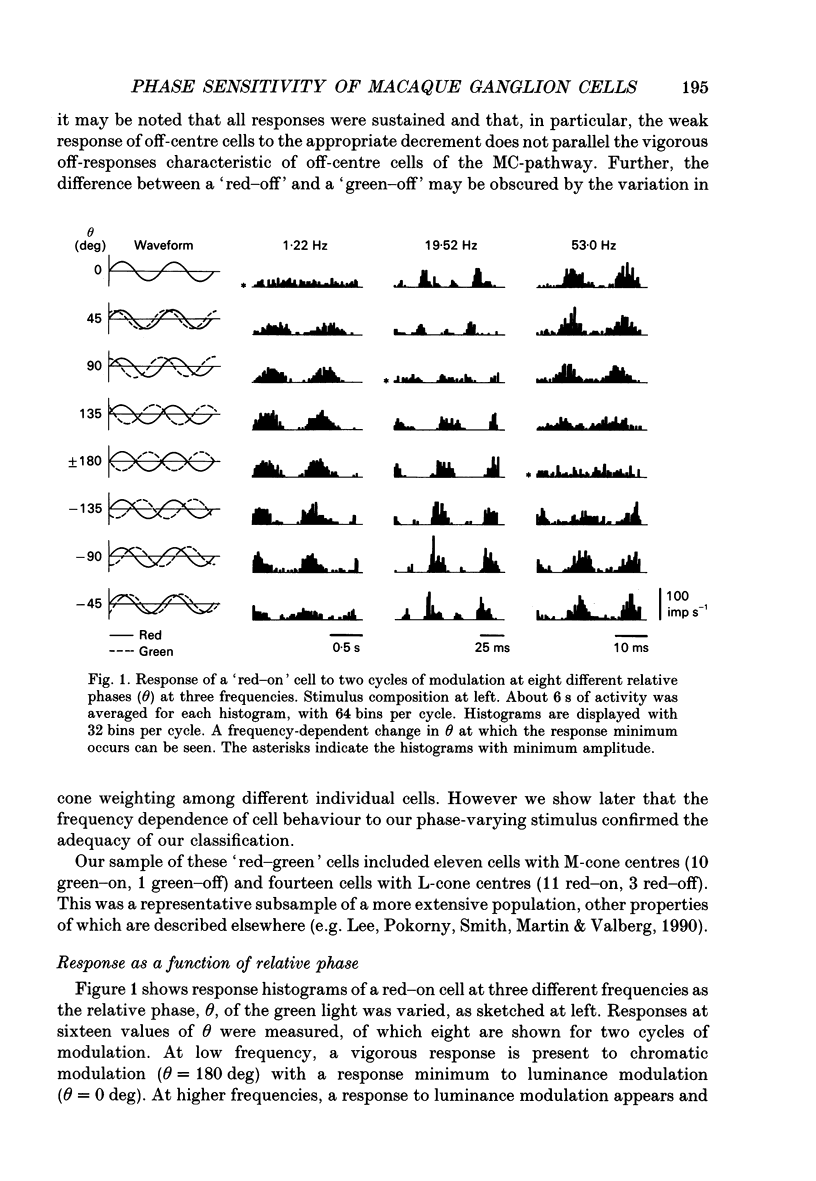
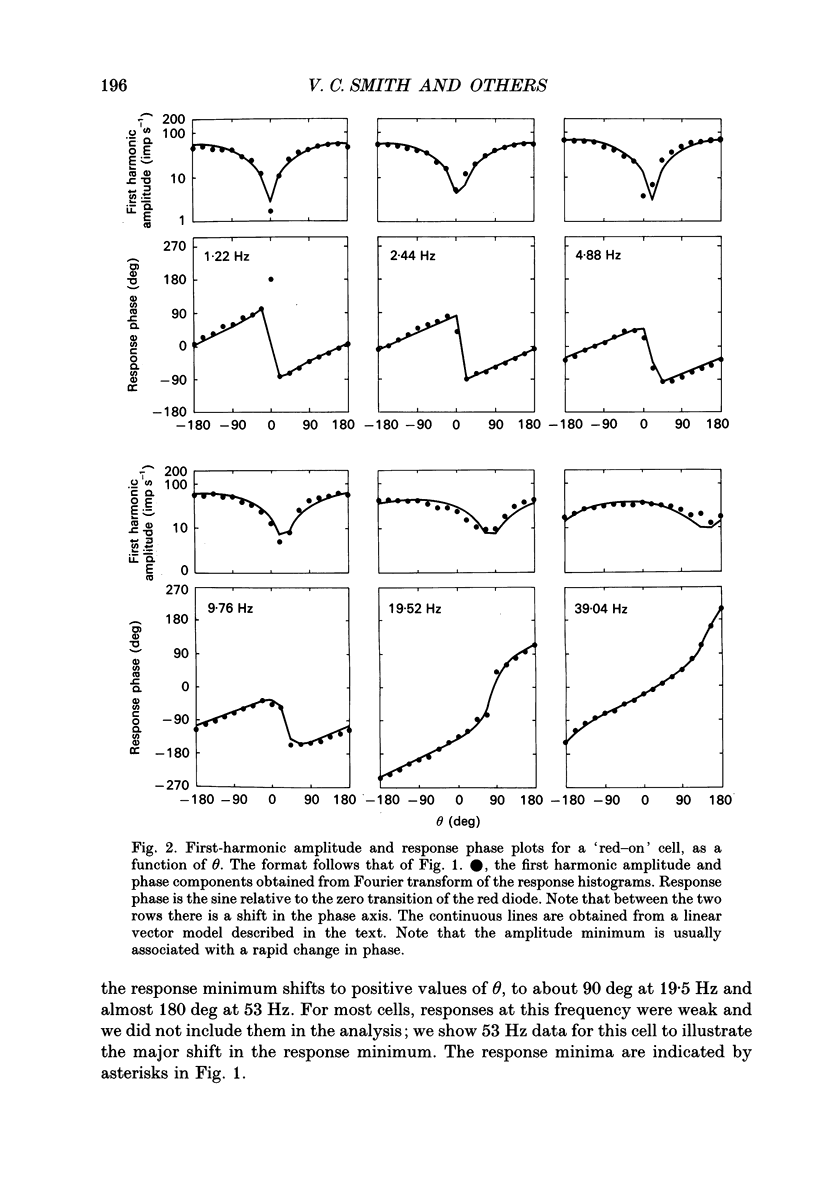
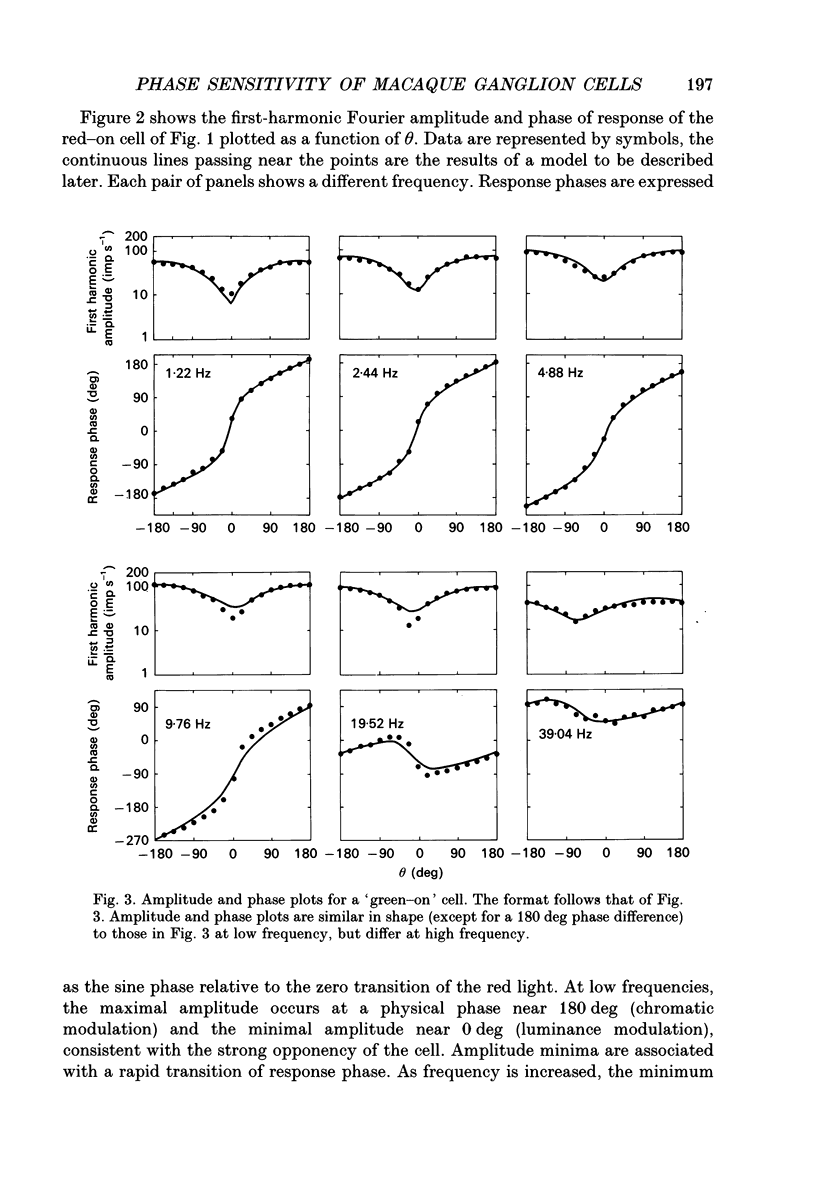
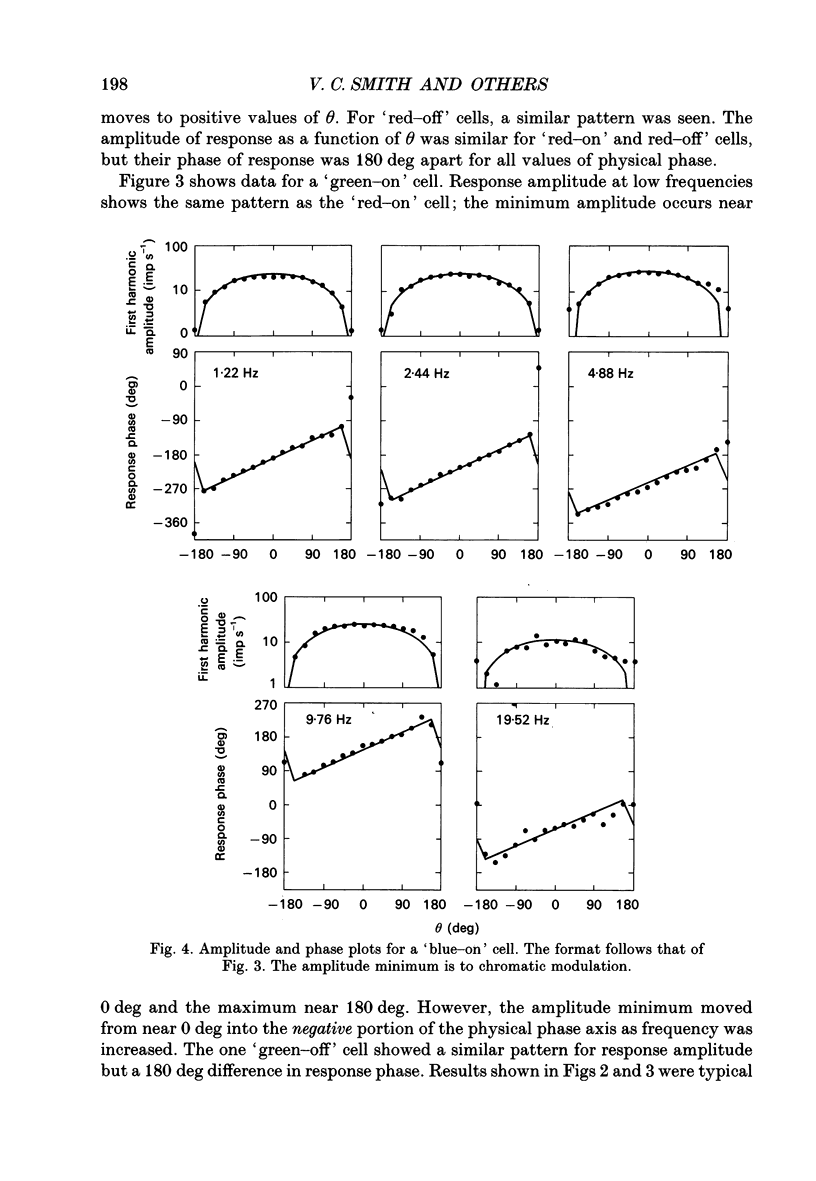
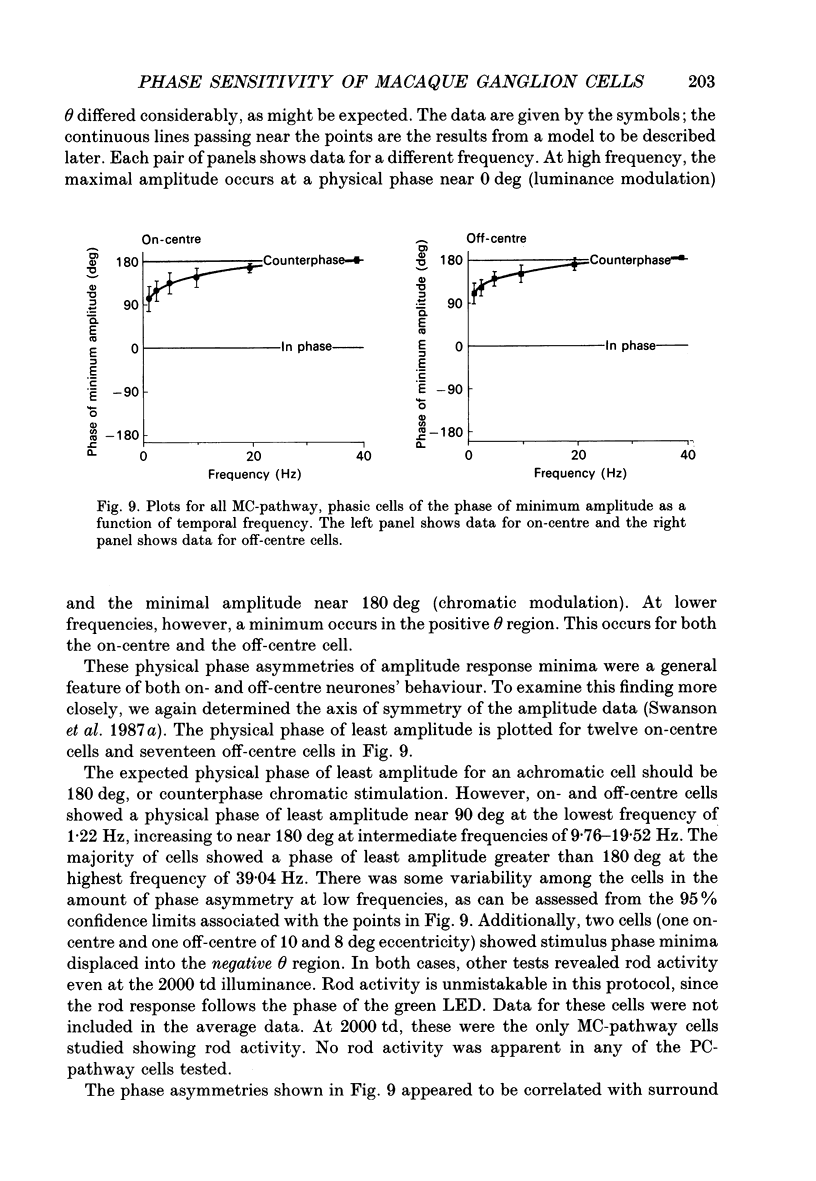
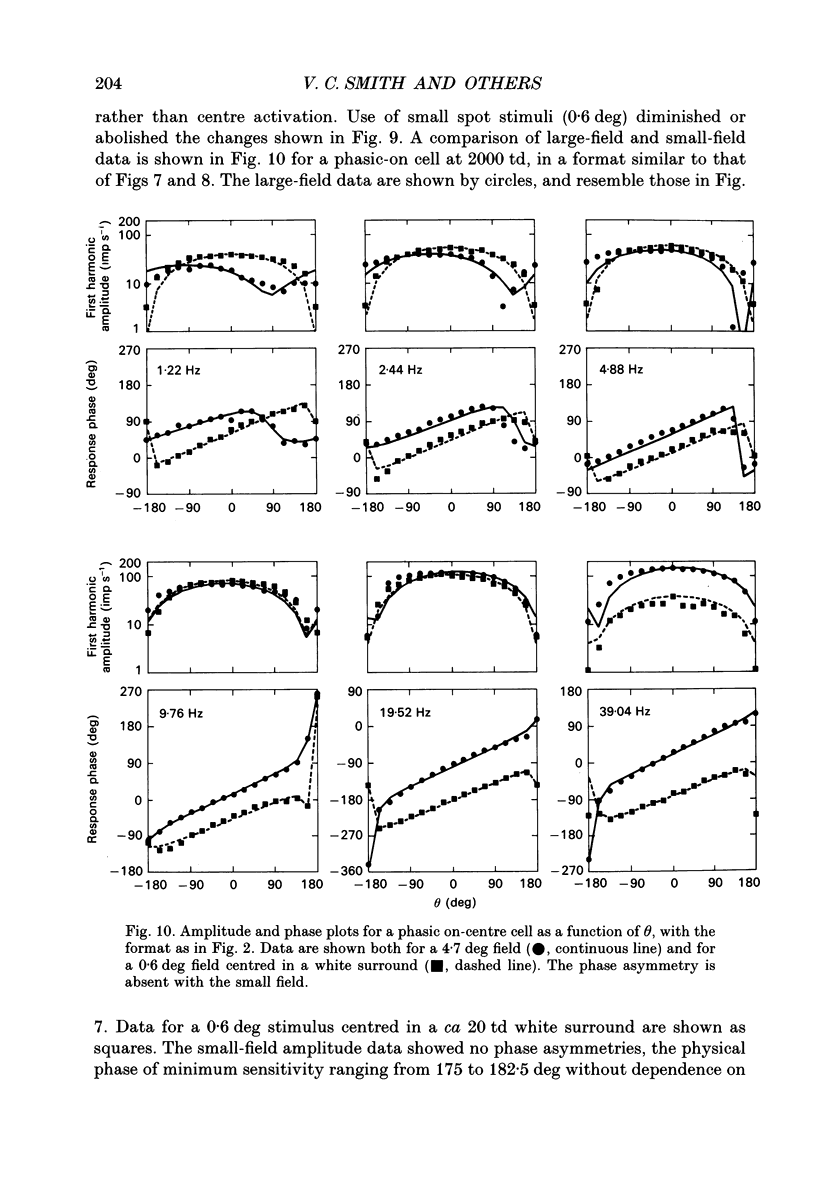
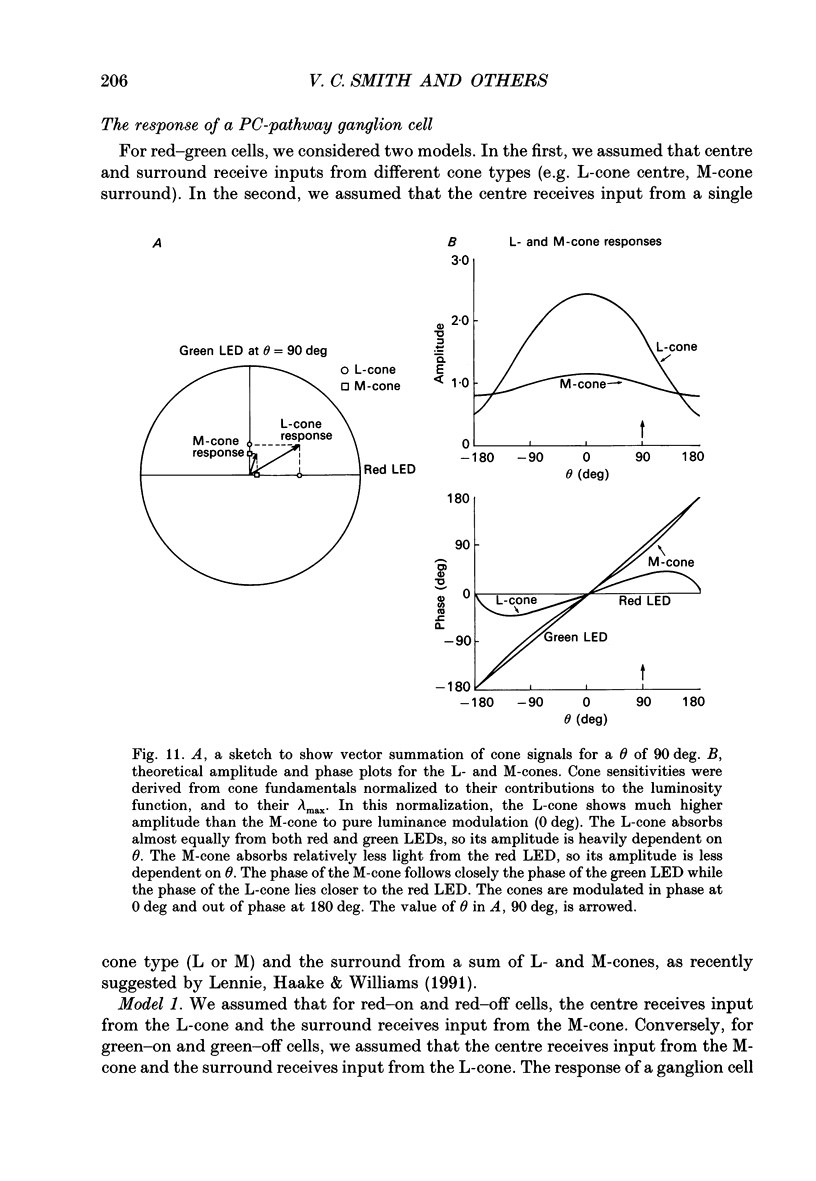
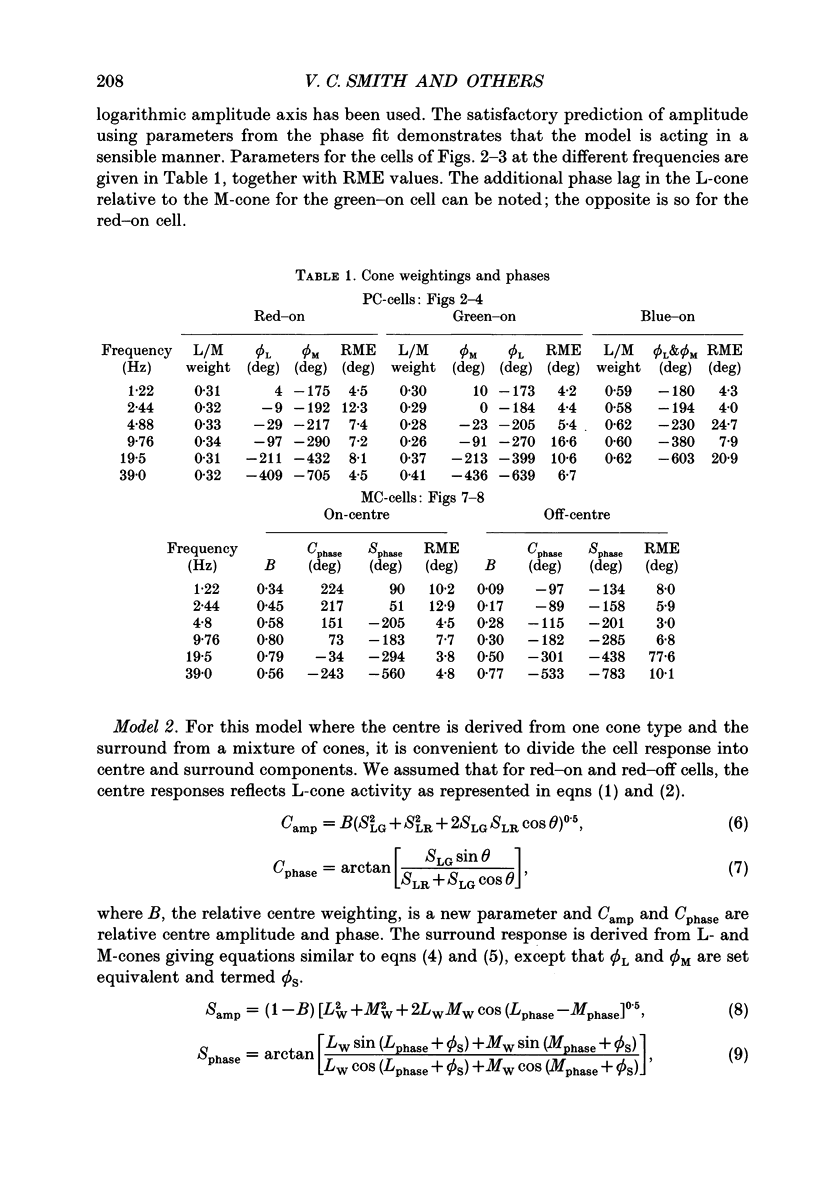
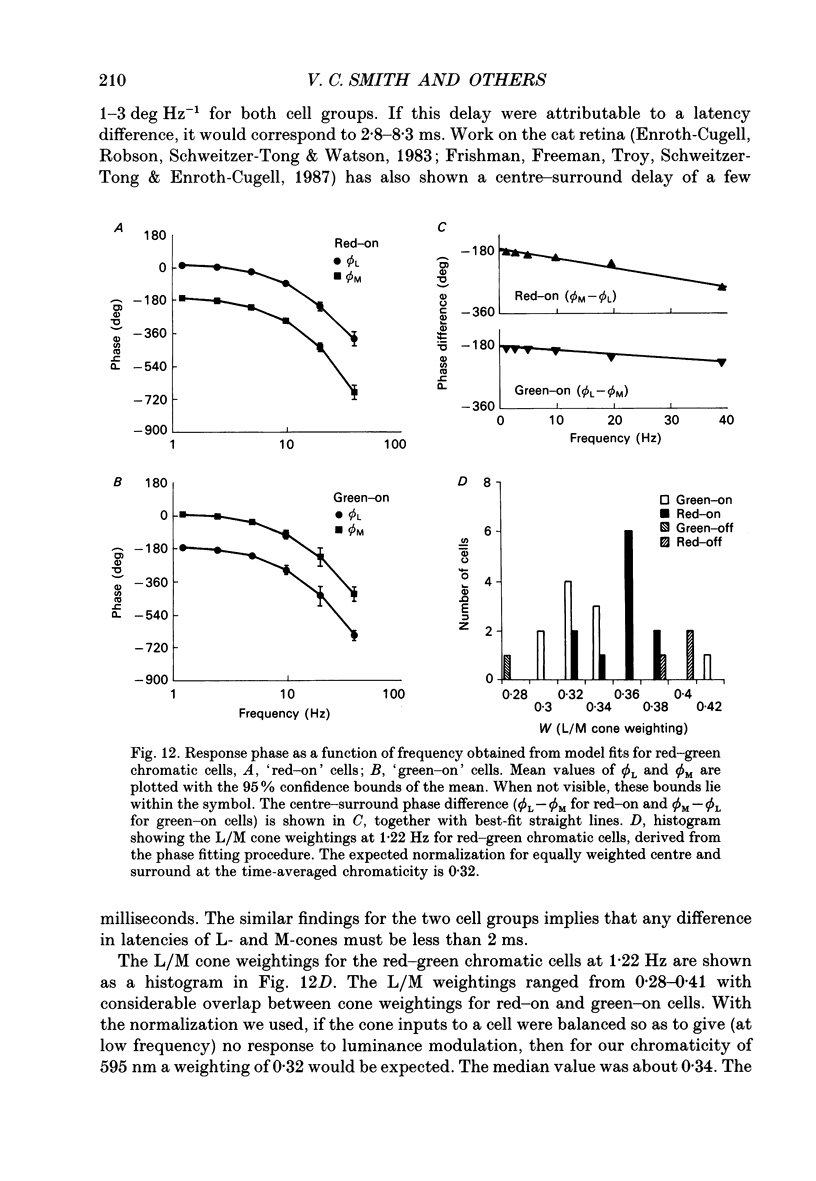
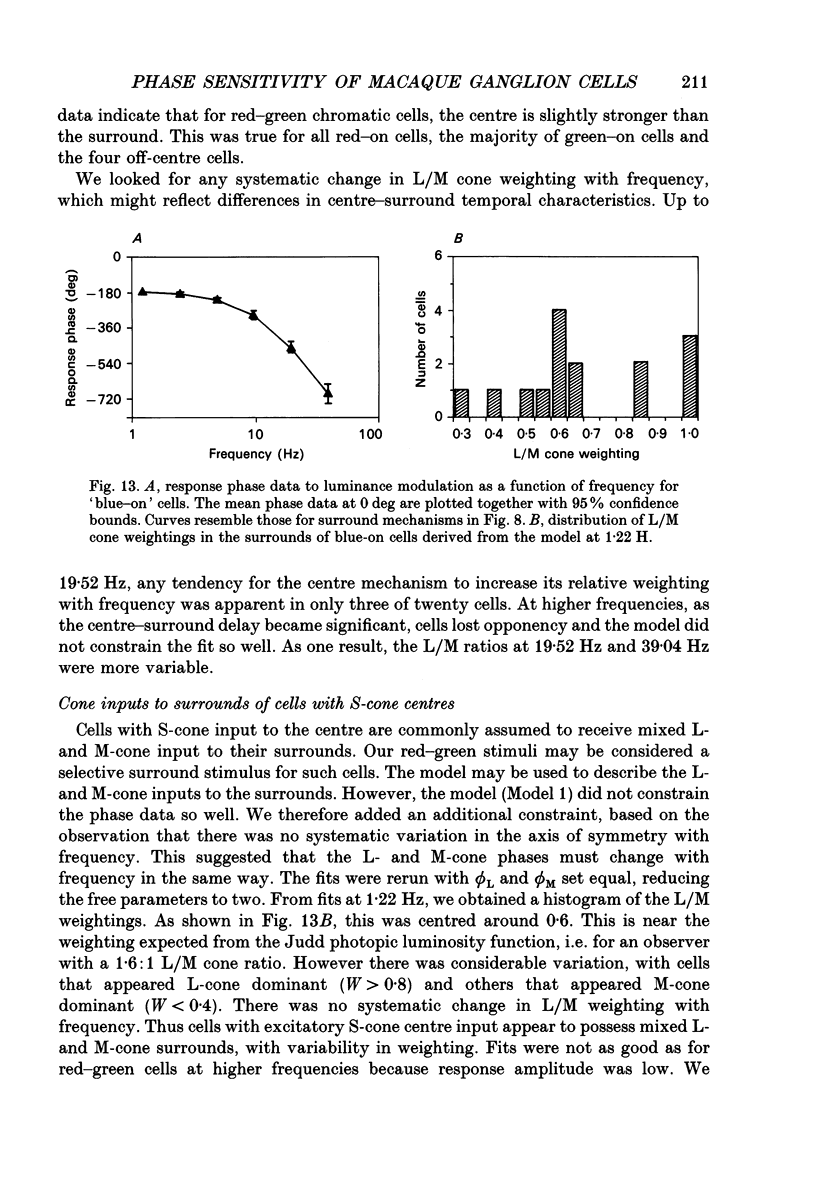
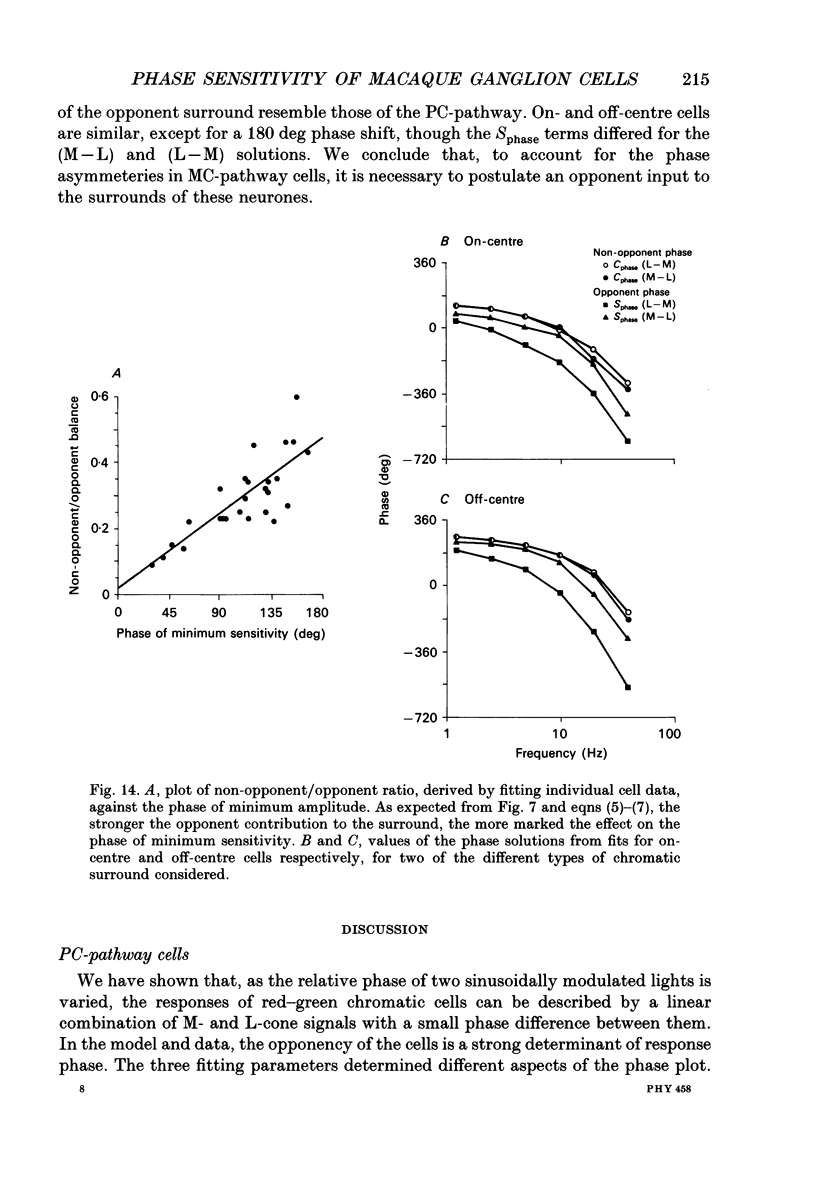
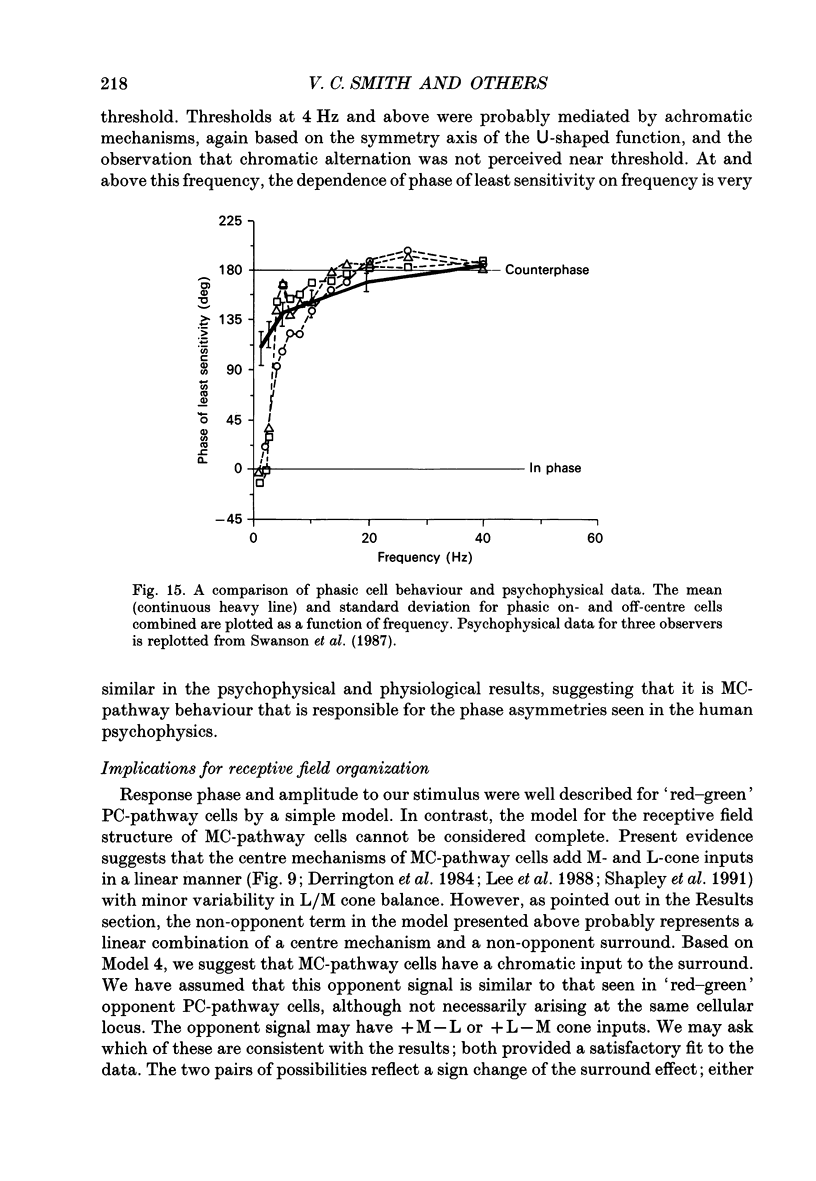
1. We measured the response of macaque ganglion cells to sinusoidally modulated red and green lights as the relative phase, theta, of the lights was varied. 2. At low frequencies, red-green ganglion cells of the parvocellular (PC-) pathway with opponent inputs from middle-wavelength sensitive (M-) and long-wavelength sensitive (L-) cones were minimally sensitive to luminance modulation (theta = 0 deg) and maximally sensitive to chromatic modulation (theta = 180 deg). With increasing frequency, the phase, theta, of minimal amplitude gradually changed, in opposite directions for cells with M- and L-cone centres. 3. At high frequencies (at and above 20 Hz), phasic cells of the magnocellular (MC-) pathway were maximally responsive when theta approximately 0 deg and minimally responsive when theta approximately 180 deg, as expected from an achromatic mechanism. At lower frequencies, the phase of minimal response shifted, for both on- and off-centre cells, to values of theta intermediate between 0 and 180 deg. This phase asymmetry was absent if the centre alone was stimulated with a small field. 4. For PC-pathway cells, it was possible to provide an account of response phase as a function of theta, using a model involving three parameters; phases of the L- and M-cone mechanisms and a L/M cone weighting term. For red-green cells, the phase parameters were monotonically related to temporal frequency and revealed a centre-surround phase difference. The phase difference was linear with a slope of 1-3 deg Hz-1. If this represents a latency difference, it would be 3-8 ms. Otherwise, temporal properties of the M- and L-cones appeared similar if not identical. By addition of a scaling term, the model could be extended to give an adequate account of the amplitude of responses. 5. We were able to activate selectively the surrounds of cells with short-wavelength (S-) cone input to their centres, and so were able to assess L/M cone weighting to the surround. M- and L-cone inputs added linearly for most cells. On average, the weighting corresponded to the Judd modification of the luminosity function although there was considerable inter-cell variability. 6. To account for results from MC-pathway cells, it was necessary to postulate a cone-opponent, chromatic input to their surrounds. We developed a receptive field model with linear summation of M- and L-cones to centre and surround, and with an additional M,L-cone opponent input to the surround.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



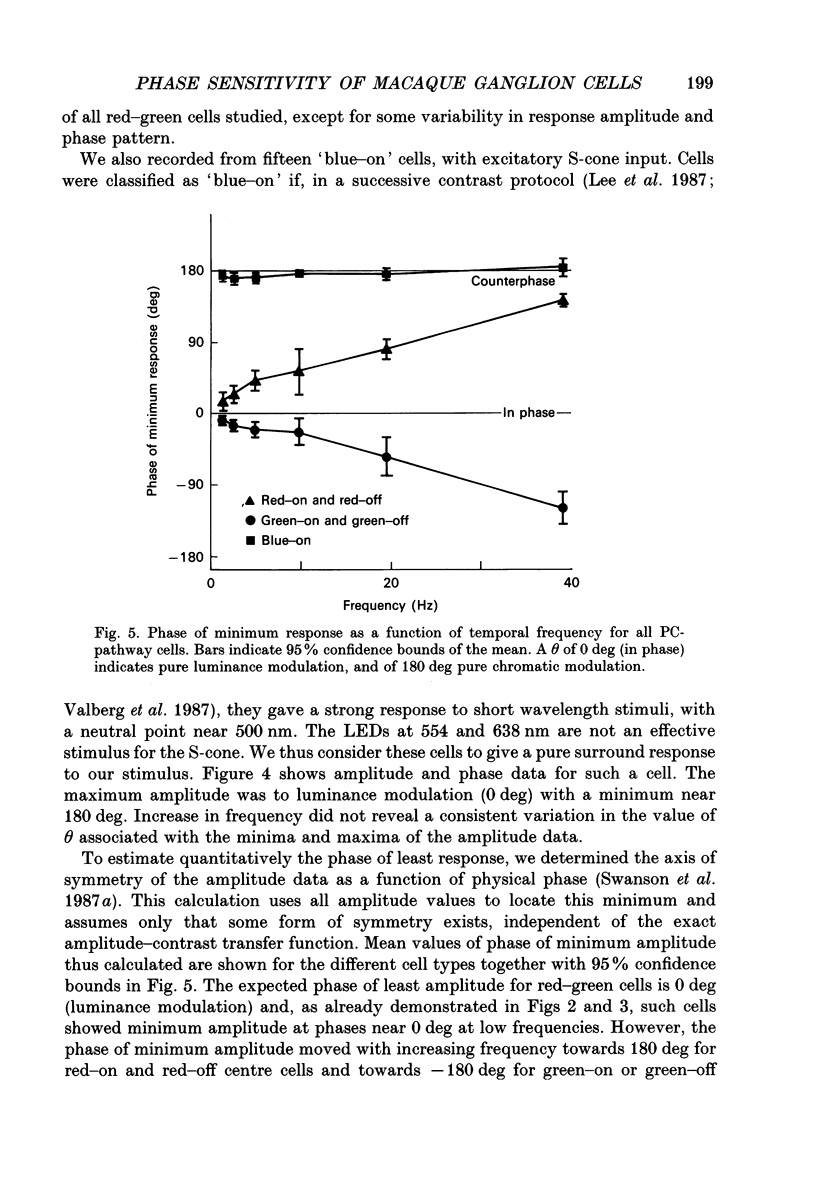
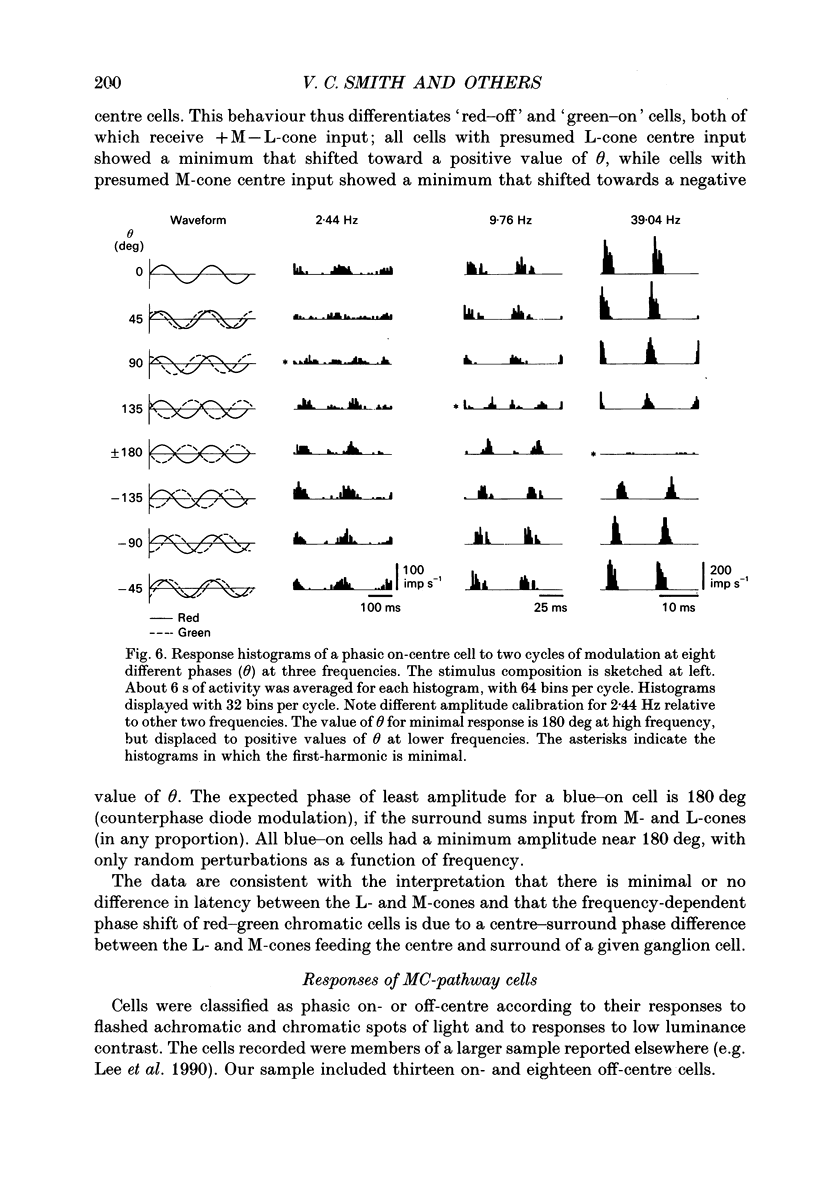
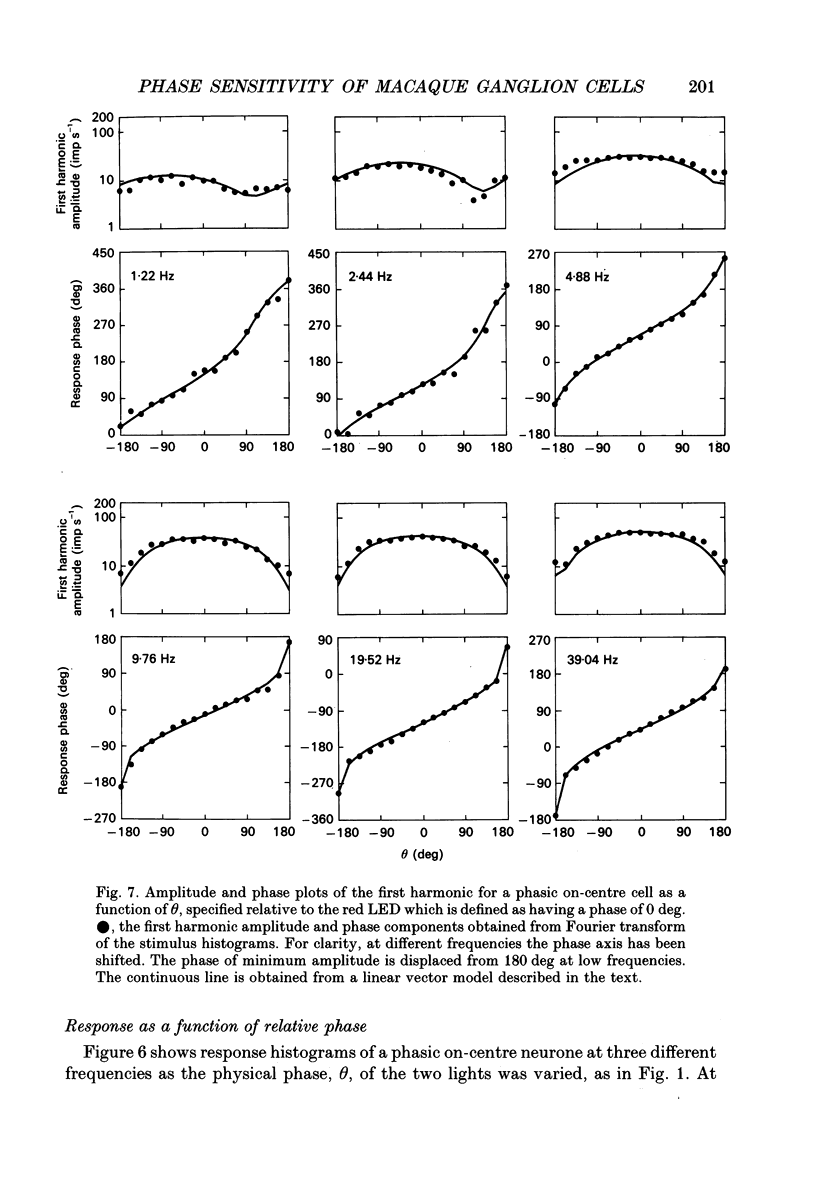
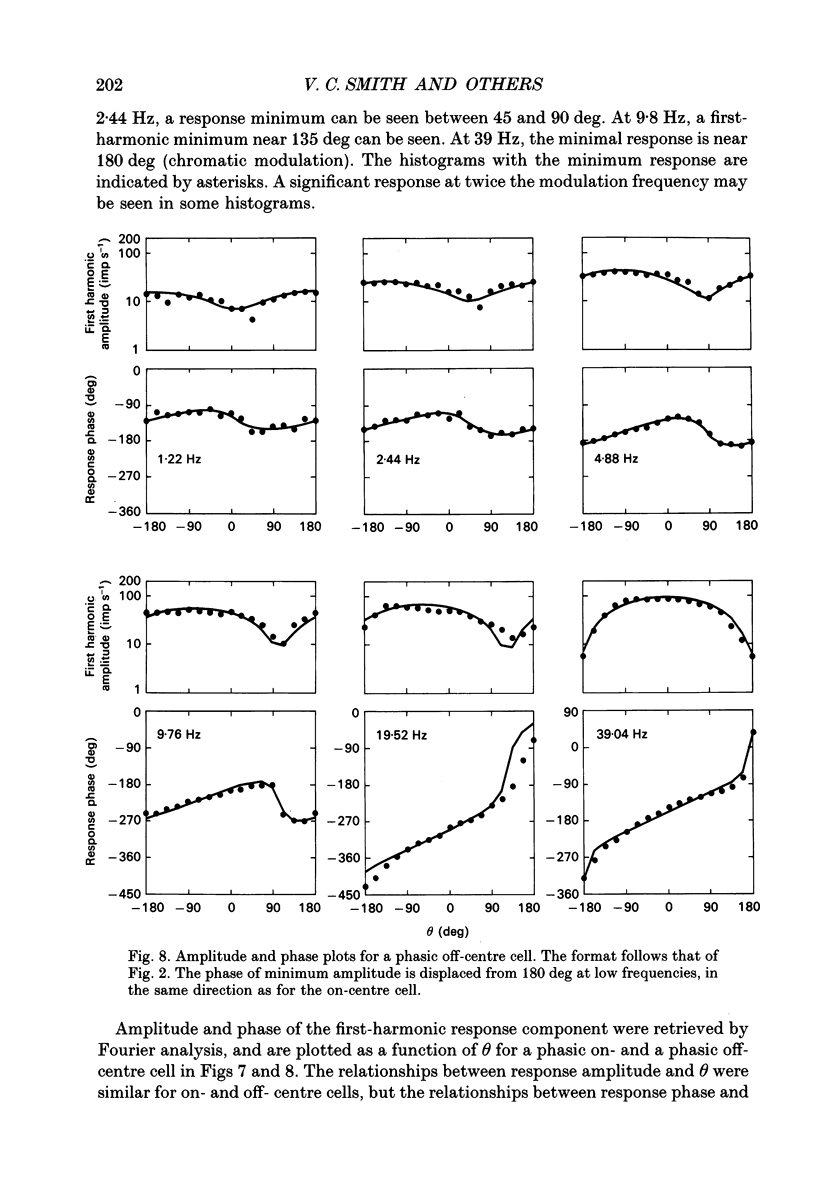











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nunn B. J., Schnapf J. L. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987 Sep;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J., Mollon J. D. Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. J Physiol. 1980 Jan;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Hopkins J. M., Sperling H. G. Cone connections of the horizontal cells of the rhesus monkey's retina. Proc R Soc Lond B Biol Sci. 1987 Jan 22;229(1257):345–379. doi: 10.1098/rspb.1987.0001. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Lee B. B., Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979 May 2;35(3):527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- Cushman W. B., Levinson J. Z. Phase shift in red and green counterphase flicker at high frequencies. J Opt Soc Am. 1983 Nov;73(11):1557–1561. doi: 10.1364/josa.73.001557. [DOI] [PubMed] [Google Scholar]
- DE LANGE DZN H. Research into the dynamic nature of the human fovea-cortex systems with intermittent and modulated light. II. Phase shift in brithtness and delay in color perception. J Opt Soc Am. 1958 Nov;48(11):784–789. doi: 10.1364/josa.48.000784. [DOI] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Krauskopf J., Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G., Schweitzer-Tong D. E., Watson A. B. Spatio-temporal interactions in cat retinal ganglion cells showing linear spatial summation. J Physiol. 1983 Aug;341:279–307. doi: 10.1113/jphysiol.1983.sp014806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman L. J., Freeman A. W., Troy J. B., Schweitzer-Tong D. E., Enroth-Cugell C. Spatiotemporal frequency responses of cat retinal ganglion cells. J Gen Physiol. 1987 Apr;89(4):599–628. doi: 10.1085/jgp.89.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen C. C., van Gisbergen J. A., Vendrik A. J. Reconstruction of cone-system contributions to responses of colour-opponent neurones in monkey lateral geniculate. Biol Cybern. 1982;44(3):211–221. doi: 10.1007/BF00344277. [DOI] [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Enchancement of luminance flicker by color-opponent mechanisms. Science. 1979 Aug 10;205(4406):587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Amplitude and phase of responses of macaque retinal ganglion cells to flickering stimuli. J Physiol. 1989 Jul;414:245–263. doi: 10.1113/jphysiol.1989.sp017686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989 Jul;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J Physiol. 1988 Oct;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Pokorny J., Smith V. C., Martin P. R., Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A. 1990 Dec;7(12):2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Valberg A., Tigwell D. A., Tryti J. An account of responses of spectrally opponent neurons in macaque lateral geniculate nucleus to successive contrast. Proc R Soc Lond B Biol Sci. 1987 Apr 22;230(1260):293–314. doi: 10.1098/rspb.1987.0021. [DOI] [PubMed] [Google Scholar]
- Lindsey D. T., Pokorny J., Smith V. C. Phase-dependent sensitivity to heterochromatic flicker. J Opt Soc Am A. 1986 Jul;3(7):921–927. doi: 10.1364/josaa.3.000921. [DOI] [PubMed] [Google Scholar]
- Marrocco R. T., De Valois R. L. Locus of spectral neutral point in monkey oppenent cells depends on stimulus luminance relative to background. Brain Res. 1977 Jan 7;119(2):465–470. doi: 10.1016/0006-8993(77)90326-2. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., Nunn B. J., Meister M., Baylor D. A. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990 Aug;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975 Feb;15(2):161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Pokorny J., Smith V. C. Effects of chromatic adaptation on phase-dependent sensitivity to heterochromatic flicker. J Opt Soc Am A. 1988 Nov;5(11):1976–1982. doi: 10.1364/josaa.5.001976. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Pokorny J., Smith V. C. Effects of temporal frequency on phase-dependent sensitivity to heterochromatic flicker. J Opt Soc Am A. 1987 Dec;4(12):2266–2273. doi: 10.1364/josaa.4.002266. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Ueno T., Smith V. C., Pokorny J. Temporal modulation sensitivity and pulse-detection thresholds for chromatic and luminance perturbations. J Opt Soc Am A. 1987 Oct;4(10):1992–2005. doi: 10.1364/josaa.4.001992. [DOI] [PubMed] [Google Scholar]
- Valberg A., Lee B. B., Tryti J. Simulation of responses of spectrally-opponent neurones in the macaque lateral geniculate nucleus to chromatic and achromatic light stimuli. Vision Res. 1987;27(6):867–882. doi: 10.1016/0042-6989(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Virsu V., Lee B. B. Light adaptation in cells of macaque lateral geniculate nucleus and its relation to human light adaptation. J Neurophysiol. 1983 Oct;50(4):864–878. doi: 10.1152/jn.1983.50.4.864. [DOI] [PubMed] [Google Scholar]
- WALRAVEN P. L., LEEBEEK H. J. PHASE SHIFT OF ALTERNATING COLOURED STIMULI. Doc Ophthalmol. 1964;18:56–71. doi: 10.1007/BF00160563. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Rodieck R. W. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989 Nov 15;289(3):434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- Wesner M. F., Pokorny J., Shevell S. K., Smith V. C. Foveal cone detection statistics in color-normals and dichromats. Vision Res. 1991;31(6):1021–1037. doi: 10.1016/0042-6989(91)90207-l. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M., Schein S. J. Protan-like spectral sensitivity of foveal Y ganglion cells of the retina of macaque monkeys. J Physiol. 1980 Feb;299:385–396. doi: 10.1113/jphysiol.1980.sp013131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg T. J., Spekreijse H. Interaction between rod and cone signals studied with temporal sine wave stimulation. J Opt Soc Am. 1977 Sep;67(9):1210–1217. doi: 10.1364/josa.67.001210. [DOI] [PubMed] [Google Scholar]
- von Grünau M. W. Lateral interactions and rod intrusion in color flicker. Vision Res. 1977;17(8):911–915. doi: 10.1016/0042-6989(77)90065-7. [DOI] [PubMed] [Google Scholar]



