Abstract
Objectives: The significant correlation between acute myocardial infarction and subsequent hepatorenal dysfunction could result in a higher mortality rate in patients. The study aimed to evaluate the effect and mechanisms of coenzyme-Q10 (Q10) administration on hepatorenal dysfunction in an isoprenaline (ISO)-induced myocardial infarction model in rats. Materials and Methods: Twenty male rats were assigned into four groups (n = 5). Groups 1-2 were administered intraperitoneally with normal saline, groups 3-4 were pretreated with Q10 (10 mg/kg, i.p.) for 28 days, and groups 2 and 4 received ISO (200 mg/kg, i.p.) on the last two days. Body, kidney, and liver weights, antioxidants and biochemical biomarkers, and histopathological investigation of the liver and kidney tissues were performed. Results: The administration of ISO significantly (P < 0.05) increased oxidative stress and altered the liver and renal function integrity and morphology. Pretreatment with Q10 demonstrated a protective effect against biochemical and histological alterations through significantly enhanced antioxidant actions, notably increasing the levels of superoxide dismutase, catalase, glutathione, and glutathione transferase; reduced liver enzymes (aspartate transaminase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase), decreased urea and creatinine concentrations and reduced the gravity of histomorphological changes in hepatic and renal tissues of ISO treated rats. Conclusion: Overall, our result suggests that Q10 confers hepatic and renal protection against ISO-induced hepatorenal dysfunction accompanying myocardial infarction through its antioxidant effects and amelioration of fibrotic changes.
Keywords: Isoprenaline, myocardial infarction, hepatorenal dysfunction, coenzyme-Q10, antioxidant
Introduction
Cardiovascular diseases such as myocardial infarction (MI) are a major public health concern that puts human health at risk. Synthetic catecholamines such as isoprenaline (ISO), a β-adrenergic agonist, promote extreme stress in cardiac cells, leading to necrosis that mimics MI in humans [1,2]. It has been recognized for many years that the heart, kidneys and liver are intrinsically interconnected [3]. The hepatorenal system describes the interaction between the liver and the kidneys in regulating various metabolic and physiological body functions [4]. In principle, the dysfunction of an organ may aggravate the malfunctioning of other organs under pathological conditions [5].
Hypothetically, blood congestion from an inefficient cardiac pump would result in blood pressure building up in the central vein connected to the kidney and consequently congesting the microvasculature of the kidney [6]. The effects of MI on kidney function and systemic circulation can be mediated through three main mechanisms: increased peripheral vascular resistance, modulation of the renin-angiotensin-aldosterone system, and direct impacts on renal function. For example, ISO inhibits renin secretion by blocking beta-adrenergic receptors, affecting aldosterone production and altering sodium, potassium, and water regulation in the kidneys [6]. This disruption raises blood pressure and renal blood flow, while reduced renin pathway activation is linked to renal impairment. Consequently, ISO-induced MI can result in elevated blood pressure and hepatic circulatory overload, triggering the release of markers indicative of renal impairment and portal hypertension, which further compounds organ damage [7,8]. Similarly, acute circulatory failure following MI is associated with cardiogenic liver damage [7]. Since the liver is a highly vascular organ, the detrimental consequences of MI on the liver are multifactorial, including reduced blood flow to the liver, elevated hepatic vein pressure, and reduced arterial saturation [7,8]. As a result, MI patients develop manifestations of liver dysfunctions [9]. Liver cells contain enzymes released into the blood during several pathological conditions [10]. Markers of hepatic dysfunction such as gamma-glutamyl transferase, aspartate transaminase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine transaminase are also employed as indicators of cardiovascular risk [11].
Another critical predictor of mortality in MI patients is renal function, and consequently, the importance of renal function impairment for cardiovascular prognosis after MI has been investigated [12,13]. Heart pump failure leading to volume retention by the kidneys, previously believed to be the primary pathophysiological basis of cardiorenal syndrome, is now considered only a portion of a very complex phenomenon. Several bodily functions may aid this pathologic constellation in a coordinated sequence of events [12]. As stated, oxidative stress is a significant etiopathological element in ISO-induced myocardial necrosis. It is typically linked to an increase in the generation of reactive oxygen and nitrogen species (ROS/RNS), which is crucial in cardiac physiology and pathology [14-16]. High levels of oxidative stress incite necrosis or apoptosis in cells, resulting in tissue damage. Notably, free radical-induced myocardial injury is often associated with elevated ROS levels and inadequate antioxidant defense mechanisms [17]. Reportedly, oxidative stress is also a known pathological factor in initiating and progressing various hepatorenal diseases [2,18-20].
Currently, no specific drugs exist for these complex conditions. Treatment involves supportive care, including intravenous fluid rehydration, antibiotics for infections, vasoconstrictors to enhance kidney blood flow, hemodialysis, and organ transplant [6,8]. However, over time, various antioxidants and their supplementations have exhibited significant efficacy in ameliorating several debilitating diseases [21-23]. Coenzyme-Q10 (Q10) is a naturally occurring antioxidant synthesized in living organisms [24]. The “Q” signifies the quinone group, and “10” refers to the sum of isoprenyl units in its tail end (1,4-benzoquinone). It is present primarily in mitochondria-abundant, high energy-demanding organs such as the liver, kidney, and heart [24]. Additionally, it is found in all biological membranes. It is a vital component of the mitochondrial electron transport chain necessary for adenosine triphosphate (ATP) generation and control of redox reaction [25]. Remarkably, Q10 preserves cell function against oxidative stress due to its observed free radical scavenging activity, aiding the maintenance of the mitochondrial membrane potential and preventing macromolecule oxidation and DNA damage [25]. The antioxidant potency of Q10 has been evidenced against oxidative damage to the liver and kidneys prompted by oxytetracycline [26], paracetamol [27], piroxicam [25], mercuric chloride [28], carbofuran [29] and ischemia reperfusion-induced apoptosis and oxidative stress [30]. Therefore, considering the antioxidant potential of Q10 via suppressing ROS and promoting cellular antioxidant capacity at the mitochondria and cytoplasmic levels, we anticipated that its administration might mitigate oxidative stress and also enhance tissue remodelling of the frequent secondary impact of heart failure, which is liver and renal dysfunction. The biochemical and histopathological assessments of the liver and the kidneys in MI and the protective effects of Q10 on hepatorenal dysfunction caused by MI have not been investigated. Thus, this study evaluated the protective effects of Q10 pretreatment on liver and renal injury after isoprenaline-induced MI in rats.
Materials and methods
Drugs and chemicals
Isoprenaline and Q10 were obtained from Sigma Aldrich, USA. Chemicals included thiobarbituric acid (TBA) (Sigma Aldrich, USA), 5,5’-dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent), reduced glutathione (GSH) (J.I. Baker, USA) and trichloracetic acid (TCA) (J.I. Baker, USA). DCI Diagnostics (Budapest, Hungary) provided the ALT, AST, ALP, urea and creatinine assay kits. The sodium hydroxide came from Merck in Germany. All other substances and reagents were of analytical grades. The doses of isoprenaline [31] and Q10 [32] were chosen based on data from previous reports.
Animals
Twenty (20) male Wistar rats weighing 120-200 g were used. Animals were acquired from the central animal facility of the Faculty of Basic Medical Sciences, College of Medicine, Delta State University, Abraka. Two weeks before the start of the experiment, the rats were housed and acclimatized to the environmental conditions (25 ± 2°C; 35 to 60%) in a 12-hour light-dark cycle. They were given standard rat pellets and water ad libitum. The care and handling of experimental animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines set by the ethical committee of the Faculty of Basic Medical Sciences, Delta State University, Abraka (ethical approval no: RBC/FBMC/DELSU/23/175).
Experimental design
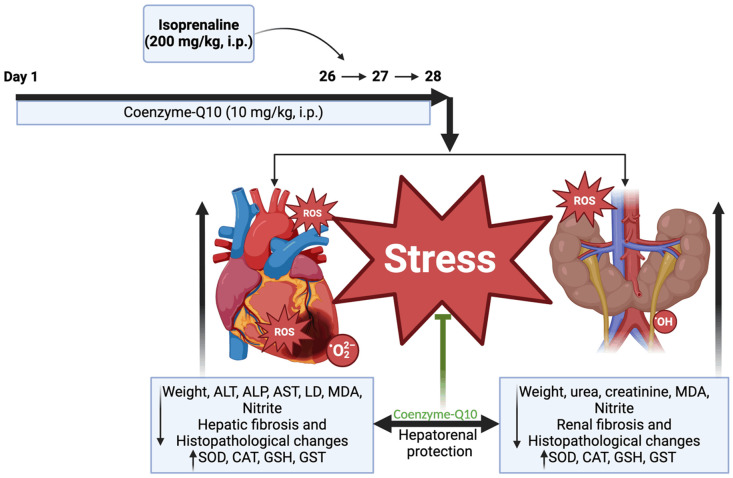
Twenty (20) male Wistar rats were randomly allocated into four treatment groups of 5 rats each as follows: Group 1 (normal control) rats were given distilled water for 28 days and normal saline (i.p.) on days 26 and 27. Group 2 (negative control) rats were given distilled water for 28 days and ISO (200 mg/kg, i.p.) on days 26 and 27. Group 3 rats were given Q10 (10 mg/kg, i.p.). Group 4 rats were given Q10 (10 mg/kg, i.p.) for 28 days and ISO (200 mg/kg, i.p.) on days 26 and 27 consecutively at intervals of 24 hours.
At the end of the experiment, the rats were weighed and then euthanized under ketamine anesthesia (70 mg/kg i.m.), and blood samples were collected in plain tubes using cervical dislocation after a night of fasting with access only to water. Blood samples were collected from each rat via the retro-orbital sinus, and the blood was allowed to clot before centrifugation (15 min at 10,000 rpm at 4°C). After that, the sera were separated for the biochemical assays. The liver and kidneys were also harvested. Some were set up for antioxidant assays, while others were fixed in 10% buffered formalin for histopathological studies.
Serum biochemical and tissue homogenate antioxidant analysis
To confirm liver and kidney complications, biomarkers of liver injury such as ALT, AST, ALP, and LDH, and the concentrations of creatinine and urea for renal function were estimated using a Randox test kit according to Reitman and Frankel [33] protocol.
Harvested tissues were homogenized in a 10% w/v phosphate buffer (0.1 M, pH 7.4), centrifuged for 10 min at 4°C at 10,000 rpm, and the supernatants were immediately frozen and stored for oxidative parameters (at 10°C). Lipid peroxidation of the tissue samples was determined according to Ohkawa et al. [34] using a thiobarbituric acid reactive substance (TBARS) assay detection method of lipid oxidative products such as malonaldehyde (MDA). The nitric oxide (NO) was assessed through nitrite content assay of the homogenized tissue sample using the GriessIllosvoy reaction [35]. Reduced glutathione (GSH) and glutathione s-transferase (GST) concentrations were determined by the McCord and Fridovich protocol [36]. Superoxide dismutase was measured using the kinetic method with adrenaline as the reactive substrate [37]. At the same time, catalase activity was determined using hydrogen peroxide as a substrate following the protocol of Beers and Sizer [38].
Histopathological study
Histopathological examinations of the liver and kidney tissues were carried out according to previously reported protocol [39]. Following fixation in 10% neutral-buffered formalin, the organs were dehydrated in increasing ethanol concentrations, washed with xylene, and finally embedded in paraffin. The liver and kidney paraffin blocks were stained with hematoxylin and eosin in 2 µm sections. The stained slides were captured at 400× using light microscope magnifications, and the ensuing photomicrograph was evaluated for histoarchitectural changes.
Statistical analysis
All data were expressed as Mean ± Standard Error of Mean (SEM) and analyzed using one-way analysis of variance (ANOVA). The post hoc Tukey test for multiple group comparisons was used on GraphPad Prism 7.0 (GraphPad Software, San Diego, CA), and statistical significance was set at P < 0.05.
Results
Effect of Q10 on the body, relative kidney and liver weights of rats exposed to adrenergic stimulation by isoprenaline
As indicated in Figure 1A-C, the kidney weights showed no significant (P > 0.05) changes [F(3, 12) = 3.132, P = 0.0657, R2 = 0.4391] across treatment groups in comparison with the control group. Also, ISO exposure significantly reduced the liver weight [F(3, 12) = 3.881, P = 0.0376, R2 = 0.4925] when compared with the control. However, pretreatment with Q10 (10 mg/kg) slightly but not significantly increased the relative liver weight.
Figure 1.
Effects of coenzyme Q10 and taurine on body (A), kidney (B) and liver (C) body weights. The values were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Q10 alleviates liver damage and improves renal functions in isoprenaline-induced hepatorenal dysfunction in rats
The liver and renal functions were investigated as shown in Figures 2 and 3. Serum indices of ALT [F(3, 12) = 29.85, P < 0.0.0001, R2 = 0.8818], AST [F(3, 12) = 10.73, P = 0.0010, R2 = 0.7284], ALP [F(3, 12) = 88.52, P < 0.0001, R2 = 0.9568], LDH [F(3, 12) = 12.39, P = 0.0006, R2 = 0.7560], urea [F(3, 12) = 8.892, P = 0.0012, R2 = 0.6897], and creatinine [F(3, 12) = 10.41, P = 0.0022, R2 = 0.7225] were significantly increased in ISO-treated animals when compared with control groups. However, pretreatment with Q10 (10 mg/kg) significantly (P < 0.05) reduced the elevated liver enzymes (Figure 2A-D) and renal markers (Figure 3A, 3B) when compared with the ISO-treated groups.
Figure 2.
Q10 prevents hepatic damages in ISO-treated rats: (A) alanine aminotransferase (ALT), (B) aspartate aminotransferase (AST), (C) alkaline phosphatase (ALP) and (D) lactate dehydrogenase (LDH) levels. The values were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Figure 3.
Q10 prevents renal damages in ISO-treated rats: (A) urea, and (B) creatinine levels. The values were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Q10 impedes lipid peroxidation and improves endogenous antioxidant activity in the liver of rats exposed to isoprenaline
Lipid peroxidation (MDA), nitrergic and oxidative stress assessment in the liver tissues following acute adrenergic stimulation in rats are illustrated in Figure 4, respectively. Interestingly, ISO exposure negatively impacted the hepatocellular oxidative state through drastic increment in the MDA [F(3, 12) = 8.866, P = 0.0023, R2 = 0.6891] and nitrite [F(3, 12) = 20.23, P < 0.0001, R2 = 0.8346] levels, with an outstanding depletion in CAT [F(3, 12) = 18.49, P < 0.0001, R2 = 0.8221], SOD [F(3, 12) = 35.41, P < 0.0001, R2 = 0.8985] and GST [F(3, 12) = 7.355, P = 0.0047, R2 = 0.6477] activities as well as GSH [F(3, 12) = 125.1, P < 0.0001, R2 = 0.9690] concentration in the liver tissues when compared to the Control. However, oral pretreatment with Q10 (10 mg/kg) notably amended the hepatic oxidative harm prompted by ISO exposure, revealing a refinement of the oxidative status when compared, as presented in Figure 4.
Figure 4.
Q10 prevents ISO-induced hepatorenal oxidative and nitrergic stress in rats in (A) Reduced GSH, (B) MDA, (C) Nitrite, (D) CAT, (E) SOD, (F) GST. The values were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Q10 inhibits lipid peroxidation and improves endogenous antioxidant activity in the kidneys of rats exposed to isoprenaline
Lipid peroxidation (MDA), nitrergic and oxidative stress assessment in the kidney tissues following acute adrenergic stimulation in rats are illustrated in Figure 5, respectively. Interestingly, ISO exposure negatively impacted the renal oxidative state through drastic increment in the MDA [F(3, 12) = 18.65, P < 0.0001, R2 = 0.8234] and nitrite [F(3, 12) = 13.51, P = 0.0004, R2 = 0.7715] levels, with an outstanding depletion in CAT [F(3, 12) = 137.4, P < 0.0001, R2 = 0.9717], SOD [F(3, 12) = 16.94, P = 0.0001, R2 = 0.8090] and GST [F(3, 12) = 14.20, P = 0.0003, R2 = 0.7802] activities as well as GSH [F(3, 12) = 115.5, P < 0.0001, R2 = 0.9665] concentration in the liver tissues when compared to the Control. However, oral pretreatment with Q10 (10 mg/kg) notably amended the renal oxidative harm prompted by ISO exposure, revealing a refinement of the oxidative status when compared.
Figure 5.
Q10 prevents ISO-induced hepatorenal oxidative and nitrergic stress in rats in (A) Reduced GSH, (B) MDA, (C) Nitrite, (D) CAT, (E) SOD, (F) GST. The values were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Q10 abates liver and kidney fibrosis induced by isoprenaline in rats
Liver and kidney fibrosis was determined by mason trichrome stain of histological tissues as shown in Figure 6, and the results revealed a significant (P < 0.05) increase in deposition of collagen and extracellular matrix proteins forming fibrotic plague in isoprenaline-treated rat liver and kidney tissues. However, treatment with Q10 (10 mg/kg) subsided hepatorenal fibrosis significantly by reducing collagen deposition (Figure 6).
Figure 6.
Effects of coenzyme Q10 on hepatorenal fibrosis. The densitometric scores were expressed as Mean ± SEM; n = 5. Significance was considered at *P < 0.05 vs. Control group; #P < 0.05 vs. Isoprenaline group. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10.
Q10 administration protects against acute adrenergic stimulation-induced liver and kidney histoarchitectural alterations in male Wistar rats
As denoted in Figure 7, rats exposed to ISO had histoarchitectural alterations as mild atrophy of hepatic cords and single necrosis of hepatocytes was observed. However, treatment with Q10 improved the histoarchitecture of hepatocytes. The kidney also showed glomerular and tubular atrophy in ISO-exposed animals and random tubular epithelial coagulation necrosis. Treatment with Q10 restored the kidney tissue to its normal histoarchitecture.
Figure 7.
Coenzyme Q10 defends against histopathological damage to the liver and kidney in rats exposed to isoprenaline. CNTRL = Control, ISO = Isoprenaline, Q10 = Coenzyme Q10 (H&E; ×200 magnification). The arrows indicate a significant lesion; there was glomerular degeneration and interstitial inflammation in the kidney tissue in the isoprenaline-treated group, while Q10 and Q10+ISO-treated groups appeared normal. The liver tissues were also observed to be normal in all treated groups.
Discussion
A close pathophysiological relationship exists between the liver, kidney and heart [40]. MI is a common fatal condition that is exacerbated during chronic states of oxidative and nitrergic stress and may promote hepatorenal disorder [41]. Our study shows the protective effects of Q10 on liver and renal tissues after MI. Q10 attenuated ISO-induced hepatic and renal imbalances, reduced free radical formation and improved tissue repair by reducing degenerative changes in male Wistar rats.
Myocardial necrosis induced by overstimulating β-adrenergic receptors increases hepatic transaminase activities due to hepatocellular damage caused by reduced blood supply to the liver [42]. As shown in this study, ISO significantly increased ALT, AST, ALP and LDH levels in our study. Q10 significantly reduced the serum levels of these enzymes, according to a previous report by Mahmoud et al. [30], where Q10 protected liver cells against ischemia reperfusion-induced apoptosis and oxidative stress. The elevation of hepatic transaminases and LDH values indicate their leakage from the hepatic membrane after hepatocellular damage [43,44]. Interestingly, the quantification of serum LDH, a cytoplasmic enzyme found in almost all tissues but at high concentrations in the muscle, liver, and kidney, is clinically significant and reflects tissue-specific pathological states of insufficient oxygen supply [45].
The heart is responsible for pumping oxygenated blood to all body parts, including the kidney, which eliminates waste products and excess water from the blood. The inadequate supply of oxygenated blood to the kidney alters its functionality, leading to impairment of renal function with the release of enzyme substrates [46]. This could be the mechanism of the elevated urea and creatinine levels in the ISO-treated rats (negative control). These levels were significantly decreased by Q10 administration, in agreement with the report of Ulla et al. [47], in which Q10 protected renal functionality in ISO-induced cardiac remodelling in aged rats.
From our study, ISO administration equally promoted degenerative lesions and oxidative damage to hepatic tissue. The multifunctional liver plays essential roles in biosynthesis, secretion, metabolism, excretion, and detoxification. These processes generate large quantities of ROS/RNS, making the liver vulnerable to oxidative injury [48] due to the exhaustion of endogenous antioxidant molecules. ROS-induced protein damage reduces the activity and synthesis of antioxidant enzymes [24]. Subsequently, tissue damage is elicited by several mechanisms, such as promoting lipid peroxidation (LPO), DNA damage, protein nitration, mitochondrial perturbation, and apoptotic cell death [24]. This eventually disrupts the liver’s physiological functions, causing direct or indirect damage to other body organ systems. The current study demonstrated considerable oxidative damage, as indicated by a prominent decline in GSH concentrations and CAT activities in ISO rats. Also, it is widely known that significant quantities of OH are produced from H2O2 via Fenton’s reaction when CAT is depleted. OH is the most harmful radical among the ROS because it causes membrane damage by directly breaking down membrane lipid content, initiating LPO and producing MDA [24]. This process was validated in the present study by the substantial increase in MDA in the liver and kidney, confirming membrane damage. This possibly contributed to the permeation and elevation of liver enzymes detected in the serum.
Similarly, kidney histopathological examination affirms the occurrence of LPO in the renal cell membrane, as shown by the disintegrated brush border, which contributed to tubular impairment as indicated by the marked increase in serum urea and creatinine levels. Intriguingly, Q10 essentially exists as a component of the electron transport chain that generates energy. It conveys electrons of complexes I and II to complex III. It is considered a unique mitochondrial antioxidant that limits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression, a great source of O2 -, and prevents excessive NO production, protects against LPO, protein oxidation, and DNA damage [17,49]. On this basis, pretreatment with Q10 conferred marked protection against ISO-induced oxidative injury, as demonstrated by considerable improvement in the biochemical parameters and histopathology. These changes might be due to marked enhancement of GSH levels and CAT activities, as well as decreased LPO due to the ROS-quenching activity of Q10. Our findings concur with those obtained by Abdeen et al. [24], which showed the beneficial antioxidant potential of Q10 in piroxicam-induced oxidative tissue injury. Notably, hepatic cells have a rich repertoire of enzymatic defense mechanisms to combat active free radicals. SOD and CAT play critical roles in protecting against ROS. SOD, the first line of defense against superoxide radicals, is produced in the mitochondria and endoplasmic reticulum during cellular respiration and metabolism. It converts O2 - and other superoxide radicals to H2O2 [50]. Remarkably, SOD levels in our study remained unchanged across all groups.
Furthermore, CAT levels were not impaired in rats after ISO treatment. The ability of Q10 to resist oxidative stress is due to its highly lipotropic nature, which readily traverses the lipid bilayer membrane and diffuses into the intracellular compartment. Q10 conferred a significant degree of protection to the liver tissue. It reduced the severity of ISO-induced histopathological alterations in this study, as revealed by only mild necrosis and degeneration in the liver parenchyma. The continuous accumulation of lipid peroxides and peroxynitrite by reactions of nitroxidation and nitrosation modify biomolecules by inducing changes in their structure, function, and electron leakage in the respiratory chain [51]. The membrane potential derangement generates a mitochondrial dysfunction and interrupts ATP-dependent glutathione synthesis. A disruption of ATP synthesis intensifies cellular apoptosis both in the liver and kidney tissues [52]. With the progression of a chronic hepatorenal failure, an elevated generation of reactive species, including MDA, exceeds the liver capacity for synthesizing the necessary amounts of reduced glutathione and glutathione dependent enzymes such glutathione S-transferase (GST), glutathione peroxidase (GPx) and glutathione reductase, responsible for detoxifying toxic endogenous and exogenous compounds. Glutathione neutralizes MDA, peroxynitrites and other oxidative cellular molecules and, indirectly, as a coenzyme of GST and GPx, detoxifies toxic compounds. GST converts MDA to a water-soluble product excreted by the kidneys [53].
The kidney histology of the negative control group showed mild multifocal vacuole degeneration of the renal tubular epithelial lining cells, indicating slight renal damage. The liver histology showed mononuclear cell infiltration and fibrous tissue deposition. The harmful effects of ISO were reduced, as evidenced by a significant adjustment of all hepatorenal biomarker levels. Data from our study further suggests that the synchronous use of Q10 has robust antioxidant advantages against ISO-induced adrenergic stimulation. The reduction of oxidative stress markers and fibrosis in liver and kidney tissue in this study was also observed in line with the improved functional and histological parameters.
In conclusion, the findings from the present study showed that ISO, an MI-inducing agent, disrupted the liver’s protein metabolism function and altered renal function integrity, as indicated by the histopathological study and markedly elevated serum urea and creatinine levels. This negatively interfered with the kidney’s filtration capacity, resulting in renal and hepatic dysfunctions. Furthermore, the results of the current study revealed that Q10 has a protective effect on hepatorenal dysfunction and improves antioxidant activity induced by isoprenaline (Figure 8).
Figure 8.
Hypothetical outcomes.
Acknowledgements
The authors are grateful to the technical staff of the Department of Pharmacology, Faculty of Basic Medical Sciences, Delta State University, for their technical assistance during the study.
Disclosure of conflict of interest
None.
References
- 1.Beyazcicek E, Beyazcicek O. Protective effects of Lacticaseibacillus rhamnosus on isoprenaline-induced myocardial infarction in rats. J Appl Microbiol. 2023;134:lxac008. doi: 10.1093/jambio/lxac008. [DOI] [PubMed] [Google Scholar]
- 2.Asiwe JN, Moke EG, Ben-Azu B, Onuelu JE, Nwabuoku US, Anachuna KK, Demaki WE, Chidebe EO, Oritsemuelebi B. Hepato-renal oxidative disturbances following acute β-adrenergic stimulation by isoprenaline in male Wistar rat: attenuative role of taurine, a β-amino acid. Nutrire. 2024;49:25. [Google Scholar]
- 3.Ojeda ML, Nogales F, del Carmen Gallego-López M, Carreras O. Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: a possible ameliorative approach with selenium supplementation. Life Sci. 2022;301:120618. doi: 10.1016/j.lfs.2022.120618. [DOI] [PubMed] [Google Scholar]
- 4.Virmani MA, Cirulli M. The role of l-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int J Mol Sci. 2022;23:2717. doi: 10.3390/ijms23052717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamadian M, Parsamanesh N, Chiti H, Sathyapalan T, Sahebkar A. Protective effects of curcumin on ischemia/reperfusion injury. Phytother Res. 2022;36:4299–4324. doi: 10.1002/ptr.7620. [DOI] [PubMed] [Google Scholar]
- 6.Tamayo-Gutierrez A, Ibrahim HN. The kidney in heart failure: the role of venous congestion. Methodist Debakey Cardiovasc J. 2022;18:4–10. doi: 10.14797/mdcvj.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail. 2019;7:87–97. doi: 10.1016/j.jchf.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Scalzo N, Canastar M, Lebovics E. Part 1: disease of the heart and liver: a relationship that cuts both ways. Cardiol Rev. 2022;30:111–122. doi: 10.1097/CRD.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 9.El Hadi H, Di Vincenzo A, Vettor R, Rossato M. Relationship between heart disease and liver disease: a two-way street. Cells. 2020;9:567. doi: 10.3390/cells9030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivas AN, Suresh D, Kaur S, Kumar DP. The promise of small particles: extracellular vesicles as biomarkers in liver pathology. J Physiol. 2023;601:4953–4971. doi: 10.1113/JP283074. [DOI] [PubMed] [Google Scholar]
- 11.Ho FK, Ferguson LD, Celis-Morales CA, Gray SR, Forrest E, Alazawi W, Gill JM, Katikireddi SV, Cleland JG, Welsh P, Pell JP, Sattar N. Association of gamma-glutamyltransferase levels with total mortality, liver-related and cardiovascular outcomes: a prospective cohort study in the UK Biobank. EClinicalMedicine. 2022;48:101435. doi: 10.1016/j.eclinm.2022.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Düsing P, Zietzer A, Goody PR, Hosen MR, Kurts C, Nickenig G, Jansen F. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. J Mol Med (Berl) 2021;99:335–348. doi: 10.1007/s00109-021-02037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asiwe JN, Ojetola AA, Ekene NE, Osirim E, Nnamudi AC, Oritsemuelebi B, Onuelu JE, Asiwe N, Eruotor HO, Inegbenehi S. Pleiotropic attenuating effect of Ginkgo biloba against isoprenaline-induced myocardial infarction via improving Bcl-2/mTOR/ERK1/2/Na+, K+-ATPase activities. Chin Herb Med. 2023;16:282–292. doi: 10.1016/j.chmed.2023.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Moke EG, Mordi JC, Umukoro EK. Effects of methanol leaf extract of Cuphea hyssopifolia Kunth on liver enzymes activity and antioxidant indices of paracetamol-induced hepatotoxicity in Wistar rats. Afr J Biomed Res. 2020;23:123–126. [Google Scholar]
- 16.Agbonifo-Chijiokwu E, Nwangwa KE, Oyovwi MO, Ben-Azu B, Naiho AO, Emojevwe V, Ohwin EP, Ehiwarior AP, Ojugbeli ET, Nwabuoku SU, Moke EG, Oghenetega BO. Underlying biochemical effects of intermittent fasting, exercise and honey on streptozotocin-induced liver damage in rats. J Diabetes Metab Disord. 2023;22:515–527. doi: 10.1007/s40200-022-01173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou T, Prather ER, Garrison DE, Zuo L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int J Mol Sci. 2018;19:417. doi: 10.3390/ijms19020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matyas C, Haskó G, Liaudet L, Trojnar E, Pacher P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat Rev Cardiol. 2021;18:117–135. doi: 10.1038/s41569-020-0433-5. [DOI] [PubMed] [Google Scholar]
- 19.Saka WA, Akhigbe RE, Abidoye AO, Dare OS, Adekunle AO. Suppression of uric acid generation and blockade of glutathione dysregulation by L-arginine ameliorates dichlorvos-induced oxidative hepatorenal damage in rats. Biomed Pharmacother. 2021;138:111443. doi: 10.1016/j.biopha.2021.111443. [DOI] [PubMed] [Google Scholar]
- 20.Asiwe JN, Kolawole TA, Ben-Azu B, Ajayi AM, Ojetola AA, Moke EG, Nwangwa EK. Up-regulation of B-cell lymphoma factor-2 expression, inhibition of oxidative stress and down-regulation of pro-inflammatory cytokines are involved in the protective effect of cabbage (Brassica oleracea) juice in lead-induced endothelial dysfunction in rats. J Trace Elem Med Biol. 2022;73:127014. doi: 10.1016/j.jtemb.2022.127014. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Azu B, Adebayo OG, Jarikre TA, Oyovwi MO, Edje KE, Omogbiya IA, Eduviere AT, Moke EG, Chijioke BS, Odili OS, Omondiabge OP, Oyovbaire A, Esuku DT, Ozah EO, Japhet K. Taurine, an essential β-amino acid insulates against ketamine-induced experimental psychosis by enhancement of cholinergic neurotransmission, inhibition of oxidative/nitrergic imbalances, and suppression of COX-2/iNOS immunoreactions in mice. Metab Brain Dis. 2022;37:2807–2826. doi: 10.1007/s11011-022-01075-5. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Azu B, Adebayo OG, Fokoua AR, Oritsemuelebi B, Chidebe EO, Nwogueze CB, Kumanwee L, Uyere GE, Emuakpeje MT. Antipsychotic effect of diosgenin in ketamine-induced murine model of schizophrenia: involvement of oxidative stress and cholinergic transmission. IBRO Neurosci Rep. 2024;16:86–97. doi: 10.1016/j.ibneur.2023.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Azu B, Moke EG, Chris-Ozoko LE, Jaiyeoba-Ojigho EJ, Adebayo OG, Ajayi AM, Oyovwi MO, Odjugo G, Omozojie VI, Ejomafuwe G, Onike N, Eneni AO, Ichipi-Ifukor CP, Achuba IF. Diosgenin alleviates alcohol-mediated escalation of social defeat stress and the neurobiological sequalae. Psychopharmacology (Berl) 2024;241:785–803. doi: 10.1007/s00213-023-06509-1. [DOI] [PubMed] [Google Scholar]
- 24.Abdeen A, Abdelkader A, Elgazzar D, Aboubakr M, Abdulah OA, Shoghy K, Abdel-Daim M, El-Serehy HA, Najda A, El-Mleeh A. Coenzyme Q10 supplementation mitigates piroxicam-induced oxidative injury and apoptotic pathways in the stomach, liver, and kidney. Biomed Pharmacother. 2020;130:110627. doi: 10.1016/j.biopha.2020.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirilli I, Damiani E, Dludla PV, Hargreaves I, Marcheggiani F, Millichap LE, Orlando P, Silvestri S, Tiano L. Role of coenzyme Q10 in health and disease: an update on the last 10 years (2010-2020) Antioxidants (Basel) 2021;10:1325. doi: 10.3390/antiox10081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oda SS, Waheeb RS, El-Maddawy ZK. Potential efficacy of coenzyme Q10 against oxytetracycline-induced hepatorenal and reproductive toxicity in male rats. J Appl Pharm Sci. 2018;8:098–107. [Google Scholar]
- 27.da Silva RHS, de Moura M, de Paula L, Arantes KC, da Silva M, de Amorim J, Miguel MP, Martins DB, de Melo E Silva D, Melo MM, Botelho AFM. Effects of coenzyme Q10 and N-acetylcysteine on experimental poisoning by paracetamol in Wistar rats. PLoS One. 2023;18:e0290268. doi: 10.1371/journal.pone.0290268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadan SS, El Zaiat FA, Habashy EA, Montaser MM, Hassan HE, Tharwat SS, El-Khadragy M, Abdel Moneim AE, Elshopakey GE, Akabawy AMA. Coenzyme Q10-loaded albumin nanoparticles protect against redox imbalance and inflammatory, apoptotic, and histopathological alterations in mercuric chloride-induced hepatorenal toxicity in rats. Biomedicines. 2023;11:3054. doi: 10.3390/biomedicines11113054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain M, Suchi TT, Samiha F, Islam MMM, Tully FA, Hasan J, Rahman MA, Shill MC, Bepari AK, Rahman GMS, Reza HM. Coenzyme Q10 ameliorates carbofuran induced hepatotoxicity and nephrotoxicity in Wister rats. Heliyon. 2023;9:e13727. doi: 10.1016/j.heliyon.2023.e13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoud AR, Ali FEM, Abd-Elhamid TH, Hassanein EHM. Coenzyme Q10 protects hepatocytes from ischemia reperfusion-induced apoptosis and oxidative stress via regulation of Bax/Bcl-2/PUMA and Nrf-2/FOXO-3/Sirt-1 signaling pathways. Tissue Cell. 2019;60:1–13. doi: 10.1016/j.tice.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Asiwe JN, Yovwin GD, Ekene NE, Ovuakporaye SI, Nnamudi AC, Nwangwa EK. Ginkgo biloba modulates ET-I/NO signalling in Lead Acetate induced rat model of endothelial dysfunction: involvement of oxido-inflammatory mediators. Int J Environ Health Res. 2024;34:979–990. doi: 10.1080/09603123.2023.2194612. [DOI] [PubMed] [Google Scholar]
- 32.Oyovwi MO, Ben-Azu B, Edesiri TP, Victor E, Rotu RA, Ozegbe QEB, Nwangwa EK, Atuadu V, Adebayo OG. Kolaviron abates busulfan-induced episodic memory deficit and testicular dysfunction in rats: the implications for neuroendopathobiological changes during chemotherapy. Biomed Pharmacother. 2021;142:112022. doi: 10.1016/j.biopha.2021.112022. [DOI] [PubMed] [Google Scholar]
- 33.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Tracey WR, Tse J, Carter G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther. 1995;272:1011–1015. [PubMed] [Google Scholar]
- 36.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 37.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–51. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 38.Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 39.Quaresma AB, d’Acampora AJ, Tramonte R, Farias DC, Joly FS. Histological study of the liver and biochemistry of the blood of Wistar rats following ligature of right hepatic duct. Acta Cir Bras. 2007;22:68–78. doi: 10.1590/s0102-86502007000100013. [DOI] [PubMed] [Google Scholar]
- 40.Ciccarelli M, Dawson D, Falcao-Pires I, Giacca M, Hamdani N, Heymans S, Hooghiemstra A, Leeuwis A, Hermkens D, Tocchetti CG, van der Velden J, Zacchigna S, Thum T. Reciprocal organ interactions during heart failure: a position paper from the ESC Working Group on Myocardial Function. Cardiovasc Res. 2021;117:2416–2433. doi: 10.1093/cvr/cvab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Nguyen HH, Hwang SY, Lee SS. Oxidative mechanisms and cardiovascular abnormalities of cirrhosis and portal hypertension. Int J Mol Sci. 2023;24:16805. doi: 10.3390/ijms242316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70:2204–2215. doi: 10.1002/hep.30824. [DOI] [PubMed] [Google Scholar]
- 43.Hamza RZ, EL-Megharbel SM, Altalhi T, Gobouri AA, Alrogi AA. Hypolipidemic and hepatoprotective synergistic effects of selenium nanoparticles and vitamin. E against acrylamide-induced hepatic alterations in male albino mice. Appl Organomet Chem. 2020;34:e5458. [Google Scholar]
- 44.Moke EG, Omogbai EKI, Osagie-Eweka SDE, Uchendu AP, Omogbiya AI, Ben-Azu B, Eduviere AT, Edje KE, Umukoro EK, Anachuna KK, Asiwe JN, Ahante E, Oghoghovwe IJ. Co-administration of metformin and/or glibenclamide with losartan reverse NG-nitro-L-arginine-methyl ester-streptozotocin-induced hypertensive diabetes and haemodynamic sequelae in rats. Microvasc Res. 2023;147:104497. doi: 10.1016/j.mvr.2023.104497. [DOI] [PubMed] [Google Scholar]
- 45.Smith GS, Walter GL, Walker RM. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. Academic Press; 2013. Clinical pathology in non-clinical toxicology testing; pp. 565–594. [Google Scholar]
- 46.Ajah O, Omodamiro OD, Odo CE, Onyedikachi UB, Egbuonu ACC. Renal function outcome in isoprenaline induced myocardial Infarction in albino rats and protective effect of methanol leaves extract of Jatropha tanjorensis. Anim Res Int. 2021;18:4065–4072. [Google Scholar]
- 47.Ulla A, Mohamed MK, Sikder B, Rahman AT, Sumi FA, Hossain M, Reza HM, Rahman GMS, Alam MA. Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol Toxicol. 2017;18:29. doi: 10.1186/s40360-017-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Che Z, Zhou Z, Li SQ, Gao L, Xiao J, Wong NK. ROS/RNS as molecular signatures of chronic liver diseases. Trends Mol Med. 2023;29:951–967. doi: 10.1016/j.molmed.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Asiwe JN, Kolawole TA, Anachuna KK, Ebuwa EI, Nwogueze BC, Eruotor H, Igbokwe V. Cabbage juice protect against lead-induced liver and kidney damage in male Wistar rat. Biomarkers. 2022;27:151–158. doi: 10.1080/1354750X.2021.2022210. [DOI] [PubMed] [Google Scholar]
- 50.Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97:2499–2574. doi: 10.1007/s00204-023-03562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piacenza L, Zeida A, Trujillo M, Radi R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol Rev. 2022;102:1881–1906. doi: 10.1152/physrev.00005.2022. [DOI] [PubMed] [Google Scholar]
- 52.Lindblom R, Higgins G, Coughlan M, de Haan JB. Targeting mitochondria and reactive oxygen species-driven pathogenesis in diabetic nephropathy. Rev Diabet Stud. 2015;12:134–156. doi: 10.1900/RDS.2015.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens KA, Jania LA, Snouwaert JN, Nguyen M, Moy SS, Tikunov AP, Macdonald JM, Koller BH. Beyond detoxification: pleiotropic functions of multiple glutathione S-transferase isoforms protect mice against a toxic electrophile. PLoS One. 2019;14:e0225449. doi: 10.1371/journal.pone.0225449. [DOI] [PMC free article] [PubMed] [Google Scholar]