Abstract
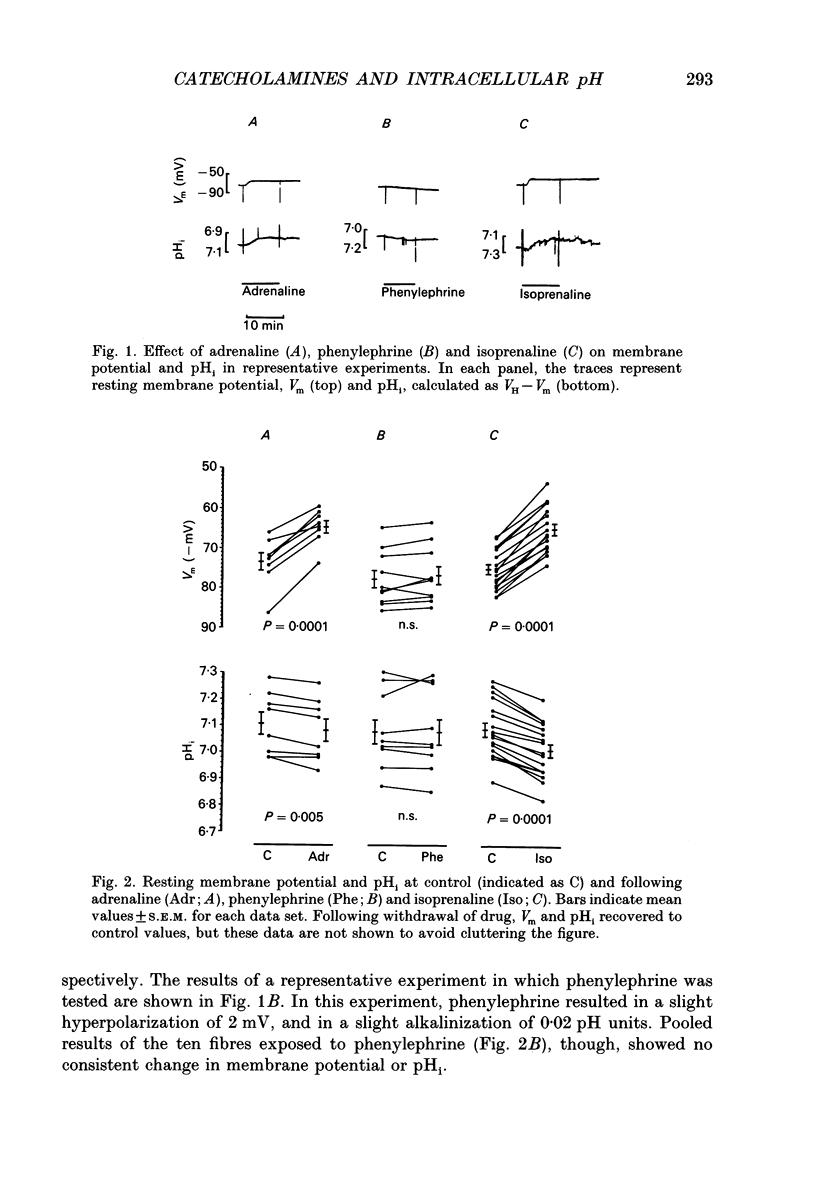
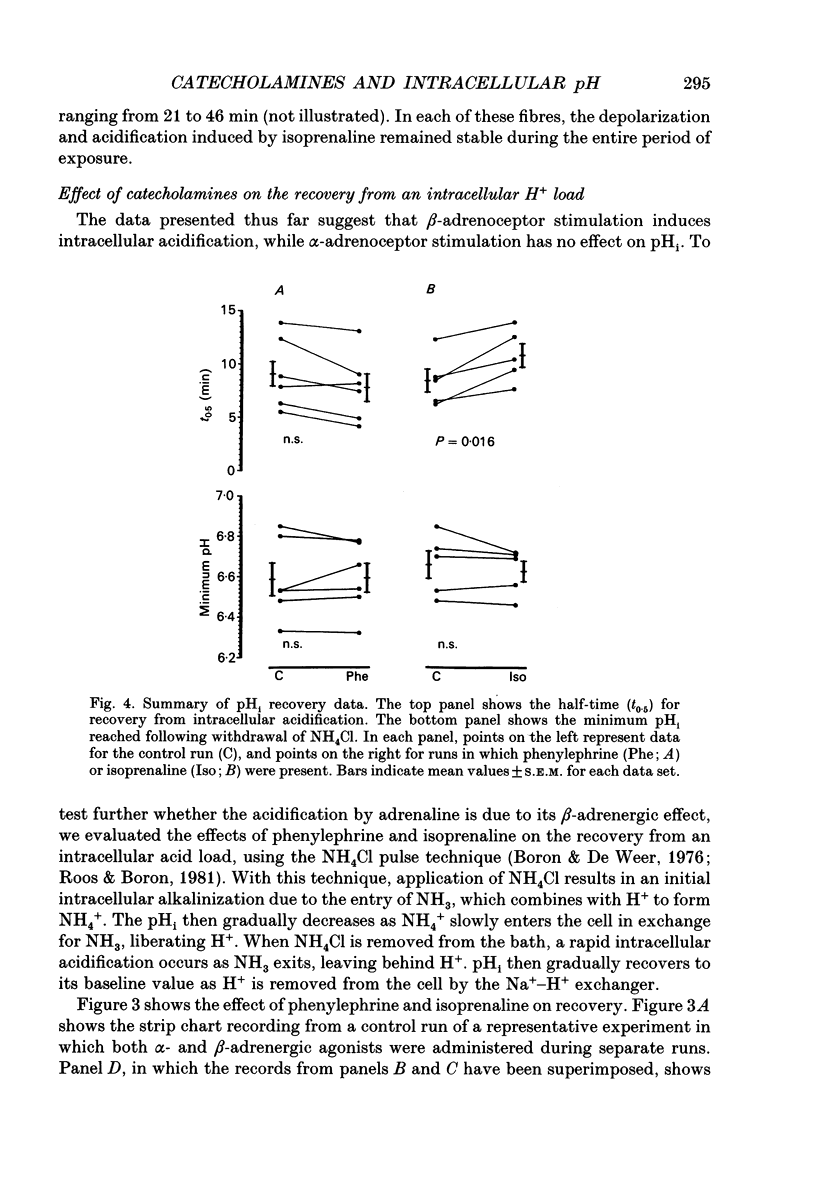
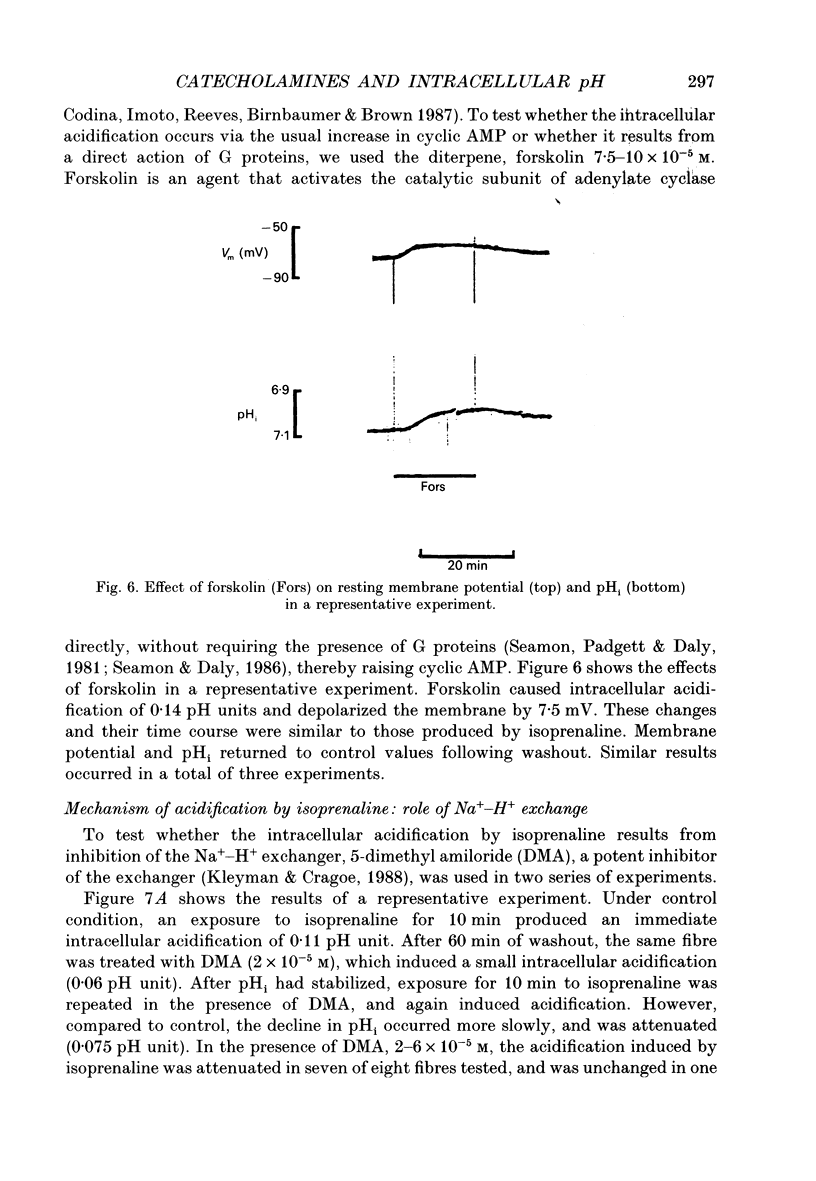
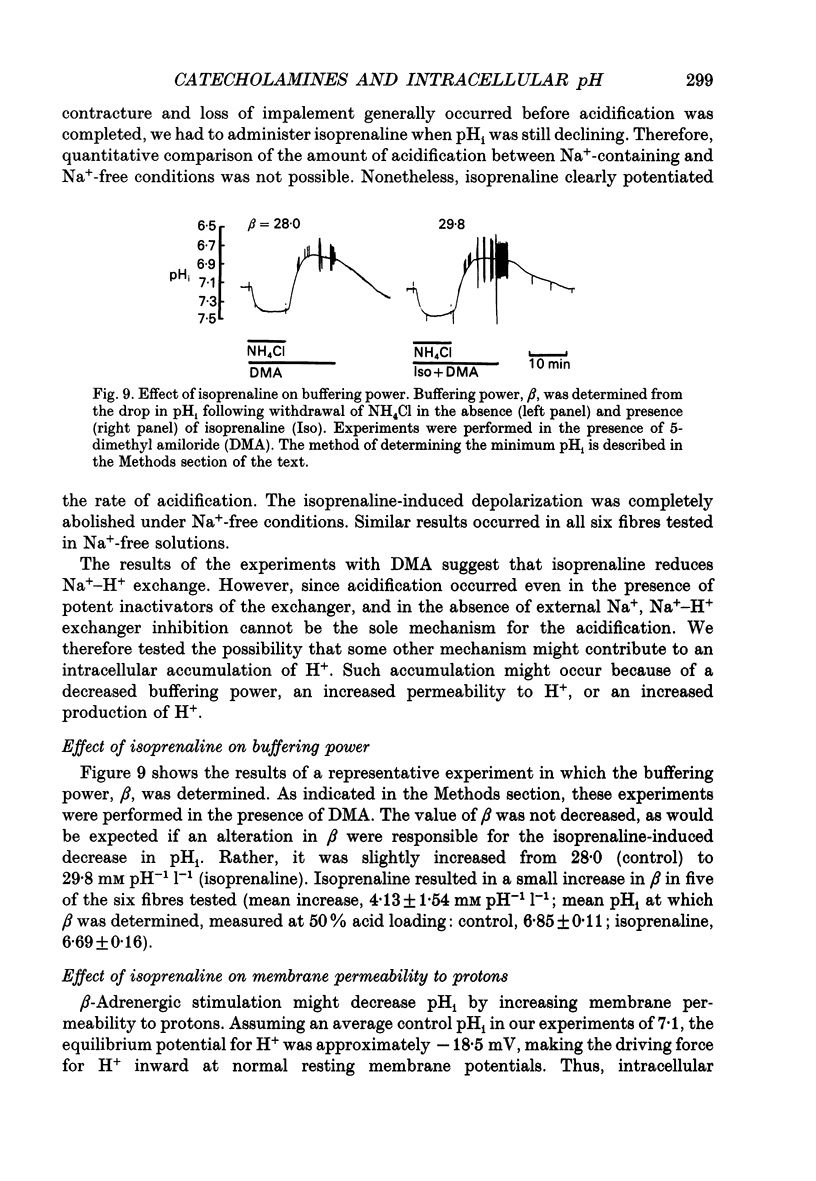
1. It has been reported that catecholamines affect intracellular pH (pHi) in a number of tissues, generally by altering the kinetics of the Na(+)-H+ exchanger. We postulated that catecholamines might affect pHi in cardiac tissue. We tested this in resting sheep cardiac Purkinje fibres by measuring transmembrane potential and pHi with standard and H(+)-sensitive microelectrodes. 2. Adrenaline and the beta-adrenergic agonist isoprenaline, both 5.0 x 10(-6) M, resulted in depolarization and intracellular acidification (adrenaline, 0.03 +/- 0.01 pH units, n = 8, P = 0.005; isoprenaline, 0.08 +/- 0.01 pH units, n = 17, P = 0.0001). The alpha-adrenergic agonist phenylephrine, at concentrations up to 200 microM, had no significant effect on membrane potential or pHi. 3. Isoprenaline significantly attenuated the half-time (t0.5) for pHi recovery from intracellular acidification induced via the NH4Cl pulse technique. Isoprenaline also attenuated the hyperpolarization that is normally seen at the onset of pHi recovery. Phenylephrine slightly reduced the t0.5 for recovery, although the reduction did not reach statistical significance. 4. Forskolin, 7.5-10 x 10(-5) M, an agent that raises intracellular cyclic adenosine 3',5'-monophosphate (cyclic AMP), also induced depolarization and acidification, similar to that induced by adrenaline and isoprenaline. 5. In the presence of the Na(+)-H+ exchange blocker 5-dimethyl amiloride, 2-6 x 10(-5) M, isoprenaline-induced acidification was blunted but not abolished. When administered in Na(+)-free Tyrode solution, isoprenaline-induced acidification was also not abolished. Buffering power, tested using the NH4Cl method, was not decreased by isoprenaline, but rather, was slightly increased. Reversal of H+ driving force across the cell membrane from the normally inward direction to outward (achieved by increasing pHo to 8.3-8.5 and depolarizing the membrane with 10 mM K+ solutions) did not prevent intracellular acidification from occurring in the presence of isoprenaline. When glycolysis was inhibited by a 60 min exposure to glucose-free solution containing 5.5 mM 2-deoxyglucose, acidification by isoprenaline was nearly abolished. 6. We conclude that, in resting sheep Purkinje fibres, beta- but not alpha-adrenergic stimulation results in intracellular acidification and depolarization, probably mediated via an increase in cyclic AMP. beta- but not alpha-adrenergic stimulation slows the rate of recovery from intracellular acidification and blunts the hyperpolarization associated with this recovery. 7. The intracellular acidification appears to be due both to partial inhibition of Na(+)-H+ exchange and to stimulation of glycolysis by beta-adrenergic agents.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S. W., Wang D. Y., Gong Q. Y., Lee C. O. Effect of norepinephrine on Na(+)-K+ pump and Na+ influx in sheep cardiac Purkinje fibers. Am J Physiol. 1990 Apr;258(4 Pt 1):C713–C722. doi: 10.1152/ajpcell.1990.258.4.C713. [DOI] [PubMed] [Google Scholar]
- Chao P., Ammann D., Oesch U., Simon W., Lang F. Extra- and intracellular hydrogen ion-selective microelectrode based on neutral carriers with extended pH response range in acid media. Pflugers Arch. 1988 Feb;411(2):216–219. doi: 10.1007/BF00582318. [DOI] [PubMed] [Google Scholar]
- Danziger R. S., Sakai M., Lakatta E. G., Hansford R. G. Interactive alpha- and beta-adrenergic actions of norepinephrine in rat cardiac myocytes. J Mol Cell Cardiol. 1990 Jan;22(1):111–123. doi: 10.1016/0022-2828(90)90976-9. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A. P., Milligan C. L. Myocardial intracellular pH in a perfused rainbow trout heart during extracellular acidosis in the presence and absence of adrenaline. J Exp Biol. 1986 Sep;125:347–359. doi: 10.1242/jeb.125.1.347. [DOI] [PubMed] [Google Scholar]
- Gesek F. A., Strandhoy J. W. Dual interactions between alpha 2-adrenoceptor agonists and the proximal Na(+)-H+ exchanger. Am J Physiol. 1990 Mar;258(3 Pt 2):F636–F642. doi: 10.1152/ajprenal.1990.258.3.F636. [DOI] [PubMed] [Google Scholar]
- Giovannini P., Seydoux J., Girardier L. Evidence for a modulating effect of Na+/H+ exchange on the metabolic response of rat brown adipose tissue. Pflugers Arch. 1988 Mar;411(3):273–277. doi: 10.1007/BF00585114. [DOI] [PubMed] [Google Scholar]
- Imoto Y., Yatani A., Reeves J. P., Codina J., Birnbaumer L., Brown A. M. Alpha-subunit of Gs directly activates cardiac calcium channels in lipid bilayers. Am J Physiol. 1988 Oct;255(4 Pt 2):H722–H728. doi: 10.1152/ajpheart.1988.255.4.H722. [DOI] [PubMed] [Google Scholar]
- Isom L. L., Cragoe E. J., Jr, Limbird L. E. Alpha 2-adrenergic receptors accelerate Na+/H+ exchange in neuroblastoma X glioma cells. J Biol Chem. 1987 May 15;262(14):6750–6757. [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Morimoto A., Sakata Y., Watanabe T., Murakami N. Leucocytosis induced in rabbits by intravenous or central injection of granulocyte colony stimulating factor. J Physiol. 1990 Jul;426:117–126. doi: 10.1113/jphysiol.1990.sp018129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E. Effect of catecholamines on Na/H exchange in vascular smooth muscle cells. J Cell Biol. 1986 Nov;103(5):2053–2060. doi: 10.1083/jcb.103.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Shigeto N., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells: secondary stimulation of electrogenic transport during recovery from intracellular acidosis. J Mol Cell Cardiol. 1986 Nov;18(11):1109–1116. doi: 10.1016/s0022-2828(86)80036-0. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Korth M. Voltage-dependent effects of isoproterenol on cytosolic Ca concentration in rat heart. Am J Physiol. 1987 Apr;252(4 Pt 2):H697–H703. doi: 10.1152/ajpheart.1987.252.4.H697. [DOI] [PubMed] [Google Scholar]
- Terris S., Wasserstrom J. A., Fozzard H. A. Depolarizing effects of catecholamines in quiescent sheep cardiac Purkinje fibers. Am J Physiol. 1986 Nov;251(5 Pt 2):H1056–H1061. doi: 10.1152/ajpheart.1986.251.5.H1056. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Barnabei O. Norepinephrine and potassium fluxes in cardiac Purkinje fibers. Pflugers Arch. 1971;322(4):287–303. doi: 10.1007/BF00587747. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Eisner D. A., Lederer W. J. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):1015–1032. doi: 10.1085/jgp.89.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Catecholamine effects on intracellular sodium activity and tension in dog heart. Am J Physiol. 1982 Nov;243(5):H670–H675. doi: 10.1152/ajpheart.1982.243.5.H670. [DOI] [PubMed] [Google Scholar]
- Weissberg P. L., Little P. J., Cragoe E. J., Jr, Bobik A. The pH of spontaneously beating cultured rat heart cells is regulated by an ATP-calmodulin-dependent Na+/H+ antiport. Circ Res. 1989 Apr;64(4):676–685. doi: 10.1161/01.res.64.4.676. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]


