Abstract
Development of sequence-reading polyamides or “lexitropsins” with comparable DNA-binding affinities to cellular proteins raises the possibility of artificially regulated gene expression. Covalent linkage of polyamide ligands, with either a hairpin motif or crosslinking methylene bridge, has greatly improved binding affinity by ensuring their side-by-side register. Whereas hairpin polyamides have been investigated extensively, the optimized structure of crosslinked polyamides remains to be determined. This study examines a series of thiazole-imidazole-pyrrole (TIP) monomers and crosslinked dimers to evaluate the effects on selectivity and binding affinity of different N-terminal head groups attached to the leading thiazole ring and differing methylene linker lengths. Quantitative footprinting of a DNA sequence, containing potential match and mismatch sites for both maximum overlap and one-residue stagger binding modes, allowed measurement of binding constants at each putative site. Within an N-terminal amino TIP series, C7 and C8-linked compounds bound most strongly to these sites, whereas maximum binding affinity was observed for a C6 linker with a formyl head group. A C5 linker gave weak binding with either head group. A hydrogen or acetyl head group abrogated binding. Binding was confirmed by gel shift analyses. The highest specificity for the maximum overlap site over the one-residue stagger was observed with TIP-C7-amino. Selectivity of the leading thiazole was modulated by the head group, with N-terminal formyl TIP exhibiting up to 3-fold specificity for AGT over TGT, suggesting that N-formyl-thiazole may provide sequence discrimination of adenine over thymine. Moreover, the leading head group and methylene linker length significantly influences the binding characteristics of crosslinked polyamides.
Modification of the sequence specificity of DNA minor groove binders netropsin and distamycin from AT to GC (1, 2) has led to the development of sequence-reading polyamides or lexitropsins. Strict AT sequence recognition inherent in the naturally occurring tripyrrole carboxamides has been expanded by replacement of the guanine-excluding pyrrole ring with a number of structurally related heterocycles including imidazole (3). Following the demonstration that distamycin may bind in a 2:1 binding mode (4), oligopeptides containing imidazole were found also to bind cooperatively in this motif to GC tracts (5, 6).
The current generation of polyamides binds as antiparallel dimers to opposite strands of the minor groove with each constituent peptide ring contacting an individual nucleotide base. Covalent linkage of the two component oligopeptides has produced sequence-reading ligands of improved binding affinity (7–13). As a consequence, synthesis of small molecules capable of DNA sequence discrimination with equivalent binding affinities to those of DNA-binding proteins raises the possibility of artificial regulation of gene expression (14). Indeed, recent work has demonstrated that linked polyamides can modulate transcription of specific genes in vitro and in some cell culture situations (15–20).
Detailed studies of polyamide binding led to the development of pairing rules defining optimal combinations of imidazoles and pyrroles for binding at predetermined DNA sites (21–23). These rules were extended to address the problem of AT/TA discrimination through use of a 3-hydroxypyrrole unit for preferential thymine recognition, providing the first success in complete sequence recognition in the minor groove of DNA (24–26). Nevertheless, alternative solutions continue to be pursued in an effort to improve AT/TA discrimination, which include the potential use of thiazole as an adenine discriminator (27–29).
Accurate alignment of the peptide rings alongside each base pair is crucial to providing both sequence specificity and binding affinity for linked polyamides (6, 30, 31). Optimal length and geometry of the linkage is required to position both component ligands in close proximity to the base edges within the minor groove. To achieve this, two types of structures have been proposed: a hairpin, whereby the oligopeptides are linked head to tail by using a suitable “turn peptide” (8), and a crosslinked or stapled structure, where the central rings of two polyamide ligands are linked via a methylene bridge (7, 12, 32, 33). Whereas the binding affinity and selectivity of hairpin polyamides are well documented (34), the optimum conditions for methylene chain crosslinked polyamides remain to be defined.
For linked polyamides, two potential DNA-binding modes, an overlap and a staggered orientation, are possible (Fig. 1) (29). In the maximum overlap mode, favored by the hairpin linkage (9, 35), the absence of an amide on the leading ring holds the parallel peptide chains in specific register, whereby each ring stacks on an amide of its neighbor. In the one-residue stagger motif, polyamides with a leading amide head group can slip along the peptide chain by one amide yielding an extended reading frame. In this binding mode, polyamides also stack ring on amide; however, the first amide on each ligand overhangs. This sliding of the ligands in relation to each other allows further separation of the charged tails, reducing electrostatic repulsion by maximizing the distance between the charges.
Figure 1.
Binding motifs of TIP. The box indicates which rings are side by side in the minor groove. (a) Maximum overlap motif. Th, thiazole; Im, imidazole; Py, pyrrole. The equal sign signifies an amide, and the plus sign signifies the charged tail. In the maximum overlap motif, the rings stack ring-on-amide. The DNA target sequence is indicated in capital letters, and the charged tails prefer adenine or thymine, a/t. (b) One residue stagger motif. This motif requires a leading amide shown in parentheses. Here, the polyamides slip relative to the maximum overlap, still stacking ring on amide, but moving the charged tails further apart. The one residue stagger motif has a longer reading frame but still preserves the same number of ring-on-amide interactions.
In this report, we present a detailed study on the effect of the N-terminal head group and methylene linker length on the binding affinity and specificity of a series of thiazole-imidazole-pyrrole (TIP) crosslinked polyamides (Fig. 2) by using quantitative DNase I footprinting and gel shift analyses.
Figure 2.
Molecular structures of distamycin A, the unlinked thiazole-imidazole-pyrrole polyamides, and the crosslinked thiazole-imidazole-pyrrole polyamides used in this study.
Materials and Methods
Quantitative DNase I Footprinting.
A 355-bp 5′ 32P-labeled PCR fragment was generated from the long terminal repeat region of plasmid pBSFIV34TF10, kindly provided by Tom Phillips (Vaccine Research Institute, San Diego, CA), using standard protocols. The labeled fragment was purified by agarose gel electrophoresis and isolated by using a Bio101 kit according to the manufacturer's instruction. Polyamides (prepared as in ref. 33) were incubated with 1,000 cps of 5′ single end-labeled fragment in 10 mM Tris, pH 7.0/1 mM EDTA/50 mM KCl/1 mM MgCl2/0.5 mM DTT/20 mM Hepes at room temperature for 30 min in a total volume of 50 μl. Cleavage was initiated by the addition of the drug-treated sample to 2 μl (0.1 unit) DNase I diluted in ice-cold 10 mM Tris, pH 7.0, from a stock solution (1 unit/μl, Promega) and 1 μl of a solution of 250 mM MgCl2/250 mM CaCl2. The reactions were performed at room temperature and stopped after 3 min by the addition of 100 μl of a stop mix containing 200 mM NaCl/30 mM EDTA, pH 8/1% SDS. The cleavage products were phenol/chloroform extracted and ethanol precipitated in the presence of 1 μl of glycogen (20 mg/ml, Roche Diagnostics), washed once in 80% ethanol and lyophilized dry. The samples were resuspended in formamide loading dye, denatured for 5 min at 90°C, cooled on ice, and electrophoresed at 2,000 V for 2 h on a 6% acrylamide denaturing gel (Sequagel, National Diagnostics). The gels were dried under vacuum at 80°C and exposed to film for 24 h (X-Omat, Kodak). Densitometry was carried out on a Bio-Rad GS-670 imaging densitometer.
Equilibrium association constants were determined for target-binding sites for each polyamide (36–39). Volume integrations of five target sites and a reference site allowed the apparent DNA target site saturation, θapp, to be calculated for each concentration of ligand using the following equation:
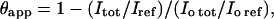 |
1 |
where Itot and Iref correspond to the integrated volumes of the target and reference sites, respectively. I0tot and I0ref are the integrated volumes for those sites in the absence of polyamide. The data were fitted to a Langmuir binding model (Eq. 2), where [L]tot is the total polyamide concentration, Ka is the equilibrium association constant, and θmin and θmax are the site-saturation values when the site is unoccupied or saturated, respectively.
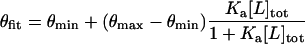 |
2 |
Values for θmin, θmax, and Ka were obtained by minimizing the difference between θapp and θfit, using a nonlinear optimization procedure with the constraint θmax > θmin. Goodness of fit was assessed by calculating a Pearson correlation coefficient between the actual and the model values. The confidence level was obtained by using a test statistic for a t distribution, and any fit with P > 0.05 was rejected. At least three sets of data were used in determining each association constant for five target match and mismatch sites.
Gel Shift Assays.
A DNA decamer and dodecamer oligomer were prepared by solid phase synthesis at a 5 μM scale on an Eppendorf ECOSYN D300 synthesizer. They were purified on a DE-52 ion exchange column using a KCl gradient, lyophilized, and resuspended in water to achieve the proper concentration. Polyamides were suspended in distilled water at the appropriate concentration.
Native gels (20%) using a 29:1 acrylamide:bis ratio were run at 4°C. The polyamides and the various DNA oligomers were incubated overnight at room temperature while shaking with 66 mM Mg(OAc)2 and 3.33 mM sodium cacodylate, pH 7.0. Dye and Ficoll were then added so that the final concentration/lane was 250 ng DNA/50 mM Mg(OAc)2/2.5 mM sodium cacodylate, pH 7.0/3.375% Ficoll/0.0125% xylene cyanol/0.0125% bromphenol blue in a volume of 20 μl. Gels were silver stained (40) by incubating the gel for 15 min with 1% HNO3 followed by 15 min with 0.2% AgNO3. The gels were then rinsed quickly with water followed by developer for 5–10 min. The gels were fixed with 10% acetic acid. After fixing, the acetic acid was removed by rinsing with distilled water before drying.
Results
Quantitative DNase I Footprinting.
DNase I footprinting allowed the determination of binding affinity and binding specificity for a series of monomer and crosslinked dimer polyamides (Fig. 2). The compounds contained one of four different N-terminal head groups, and the crosslinked compounds contained methylene linkers ranging from 5 to 8. A typical footprint titration for compound TIP-C7-amino is shown in Fig. 3A. Putative match and mismatch sites were present in the DNA sequence studied for both the maximum overlap and one-residue stagger binding modes (Fig. 1). Four of these sites are indicated in Fig. 3A. Association constants for these sites, and a further site at sequence position 971, were determined for all compounds and are given in Table 1. No significant binding was observed at any other DNA sequences within the DNA fragment tested, including at a stagger mode binding site with a single base mismatch.
Figure 3.
(A) Quantitative DNase I footprint titration experiment with TIP-C7-amino on the 5′ 32P-labeled 355-bp FIV34TF10 long terminal repeat fragment from plasmid pBSFIV34TF10, showing four binding sites. All reactions contain 1,000 cps of restriction fragment, 10 mM Tris, pH 7.0/1 mM EDTA/50 mM KCl/1 mM MgCl2/0.5 mM DTT/20 mM Hepes. Lane 1, untreated control; lane 2, DNase I-treated control; lanes 3–11, 10 nM, 25 nM, 50 nM, 75 nM, 100 nM, 250 nM, 500 nM, 1 μM TIP-C7-amino; lane 12, GA reaction; lane 13, T reaction. (B) DNase I footprint experiment with TIP-C6-formyl, TIP-C7-formyl, and TIP-C8-formyl on the 5′ 32P-labeled 355-bp FIV34TF10 long terminal repeat fragment from plasmid pBSFIV34TF10, showing four binding sites. All reactions contain 1,000 cps of restriction fragment, 10 mM Tris, pH 7.0/1 mM EDTA/50 mM KCl/1 mM MgCl2/0.5 mM DTT/20 mM Hepes. Lane 1, untreated control; lane 2, DNase I-treated control; lanes 3–6, 50 nM, 75 nM, 100 nM, 250 nM TIP-C6-formyl; lanes 7–10, 50 nM, 75 nM, 100 nM, 250 nM TIP-C7-formyl; lanes 11–14, 50 nM, 75 nM, 100 nM, 250 nM TIP-C8-formyl; lane 15, GA reaction; lane 16, T reaction.
Table 1.
Equilibrium association constants (Ka × 107 M−1) for the TIP polyamides
| Polyamide |
Ka ×
107 (M−1)
|
||||
|---|---|---|---|---|---|
| TAGTT (812) | TTGTA (890) | ATGTA (851) | CAGAT (971) | CACGTA (871) | |
| TIP-amino | 0.2 (± 0.04) | 0.1 (± 0.1) | 0.07 (± 0.07) | 0.06 (± 0.04) | 0.2 (± 0.2) |
| TIP-C5-amino | <0.1 | <0.1 | <0.1 | <0.01 | <0.01 |
| TIP-C6-amino | 0.2 (± 0.2) | 0.5 (± 0.4) | 0.3 (± 0.2) | 0.7 (± 0.5) | 0.3 (± 0.2) |
| TIP-C7-amino | 2.7 (± 1.6) | 2.3 (± 1.4) | 1.0 (± 0.4) | 2.8 (± 1.6) | 0.4 (± 0.1) |
| TIP-C8-amino | 3.1 (± 1.8) | 3.3 (± 2.1) | 1.6 (± 0.7) | 2.4 (± 0.7) | 1.1 (± 0.7) |
| TIP-C5-formyl | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| TIP-C6-formyl | 4.0 (± 1.8) | 2.4 (± 1.5) | 1.2 (± 0.85) | 3.4 (± 1.8) | 1.2 (± 0.8) |
| TIP-C7-formyl | 2.4 (± 1.0) | 1.2 (± 0.6) | 0.7 (± 0.02) | 1.2 (± 0.6) | 0.4 (± 0.1) |
| TIP-C8-formyl | 1.3 (± 0.4) | 1.0 (± 0.4) | 0.5 (± 0.2) | 1.7 (± 0.6) | 0.4 (± 0.1) |
| TIP-C5-H | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| TIP-C7-H | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| TIP-acetyl | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| TIP-C7-acetyl | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Values reported are the mean from at least three independent DNase I footprinting experiments, with the standard deviation indicated in parentheses.
Binding Affinity.
Within the N-terminal amino series, the C7- and C8-linked compounds produced the strongest binding. Reduction in the methylene chain length to C6 produced a 15-fold decrease in binding affinity at the maximum overlap match site relative to the C7- and C8-linked compounds. Further reduction to a chain length of 5 methylenes produced weak overall binding. The unlinked TIP-amino bound with an affinity similar to that of the C6-linked compound.
In contrast, for the N-terminal formyl series, the C6 linker produced the strongest overall binding at its target site, having the greatest binding affinity (Ka = 4.0 × 107 M−1) for any compound in either series. The C7-linked formyl compound had an approximately 2-fold decreased binding affinity compared with the C6 compound at most binding sites. The C8-linked compound gave a further decrease in binding affinity. A comparison of the C6–C8 compounds is shown in Fig. 3B. Similarly to the amino series, the C5-linked formyl compound was a weak binder. No significant binding was observed when the TIP compounds contained an N-terminal hydrogen or acetyl head group in either the unlinked or crosslinked form.
Binding Specificity.
Comparison of the binding affinities at the individual binding sites allowed a determination of the relative binding of the TIP compounds between the maximum overlap (sites 812, 890, 851, and 971) and stagger (site 871) modes of binding. In addition, comparison of the four maximum overlap sites allowed an assessment of the discrimination of the compounds for A:T versus T:A sequences. Table 2 lists the ratios of binding at the stagger mode binding site (5′-ACGT-3′) and maximum overlap mismatch sites (5′-TGT-3′ and 5′-AGA-3′) compared with the potential maximum overlap match site (5′-AGT-3′).
Table 2.
Effects of linker length and head group on specificity of binding, where specificity is defined as Ka(AGT)/Ka(XGX)
| Polyamide | AGT/TGT (812/890) | AGT/TGT (812/851) | AGT/AGA (812/971) | AGT/ACGT (812/871) |
|---|---|---|---|---|
| TIP-amino | 2.0 | 2.9 | 3.3 | 1.0 |
| TIP-C6-amino | 0.4 | 0.7 | 0.3 | 0.7 |
| TIP-C7-amino | 1.2 | 2.7 | 1.0 | 6.8 |
| TIP-C8-amino | 0.9 | 1.9 | 1.3 | 2.8 |
| TIP-C6-formyl | 1.7 | 3.3 | 1.2 | 3.3 |
| TIP-C7-formyl | 2.0 | 3.4 | 2.0 | 6.0 |
| TIP-C8-formyl | 1.3 | 2.6 | 0.8 | 3.3 |
Within the amino series, only the C7 and C8-linked compounds showed specificity for the maximum overlap over the stagger mode of binding (Table 1). This was 7-fold in the case of TIP-C7-amino. The C6–C8 formyl compounds showed specificity for the maximum overlap mode, which was again maximal for the C7 compound (6-fold). The unlinked TIP-amino compound gave comparable binding at the maximum overlap match site and the one-residue stagger site. This compound, however, did show some discrimination between the maximum overlap match site and the three mismatch sites, indicating that the thiazole/pyrrole pair could discriminate between A:T and T:A. This specificity was also maintained with the formyl-linked compounds and was over 3-fold in some cases. The specificity for the match site was less with the linked amino compounds and absent in the case of the TIP-C6-amino.
Polyamide–DNA Gel Shift Assays.
Concurrent with the footprinting experiments, PAGE native gel shift experiments were conducted on two oligomers, which were eventually to be used in crystallization trials. The gel shift results gave an additional assessment of the binding ability of the various polyamides with different linkers and head group. A decamer, CCAACGTTGG, contained a single site 5′-ACGT-3′ for the one-residue stagger binding mode. A dodecamer, CGACTGCAGTCG, contained three binding sites: two maximum overlap sites, 5′-ACT-3′ and 5′-AGT-3′, and a further conceivable site, 3′-ACGT-5′, in the one residue stagger mode. But, reading in the reverse direction, 3′ to 5′ has been reported to occur only under special conditions, e.g., N-terminal acetylation or addition of a glycine into the tail region (41). Side-by-side polyamides tend to always read in a 5′ to 3′ direction.
Complete binding of the TIP-C7-amino at the one-residue stagger match site in the decamer, CCAACGTTGG, at a polyamide to DNA ratio of 2:1 and above was demonstrated (Fig. 4A). The unlinked TIP-amino also displayed complete binding at this site, but a higher polyamide to DNA ratio of 4:1 was necessary. In contrast, the TIP-C5-amino was unable to bind, even at an elevated 8:1 ratio of polyamide to DNA. Further gels using this oligomer were performed to establish the optimal length of methylene chain required for binding by the amino and formyl compound series. These indicated that within the N-terminal amino series, TIP-C7-amino and TIP-C8-amino produced the best binding for this site (Fig. 4B). The binding order was ascertained to be C7,C8 > unlinked > C6 > C5, respectively. In contrast, the N-terminal formyl polyamides showed optimal binding with a linker of six carbons (data not shown), and the binding order was C6 > C7 > C8 > C5.
Figure 4.
Gel shift where 0:1 indicates no polyamide, only DNA oligomer. (A) TIP with various head groups. Distamycin bound to AAAATCTCTA is a control, with the drug binding to AAAA. The remaining lanes show polyamide:DNA ratios for TIP:CCAACGTTGG, with a DNA concentration of 2 μM. The oligonucleotide contains a single ACGT one-residue stagger site. The head group and linker length is indicated. (B) TIP with amino head group. The two controls are unlinked TIP and 0:1, where 1 is the DNA sequence, CCAACGTTGG without polyamide. C5–C8 indicate the polyamide methylene linkers. (C) TIP with formyl head group. C5–C8 indicate the polyamide methylene linkers. The DNA sequence is CGACTGCAGTCG (1.7 μM), which contains three potential binding sites; a maximum overlap match site, AGT, a maximum overlap mismatch site, ACT, and a one-residue stagger site AGCT placed in the less favorable 3′ to 5′ direction. Note the diffuse bands in the shifted region, indicating multiple binding sites.
Gel shifts with the second oligomer, CGACTGCAGTCG, determined optimal binding in the maximum overlap mode. The preferred sequence for this dodecamer in the overlap motif is AGT (Fig. 1), as the alignment of drug rings places an Im/Im pair next to a GC base pair. The Im/Im pair is redundant and can read either CG or GC. Assays with the N-terminal amino compounds using this oligomer resulted in the same order of overall binding as seen with the decamer above. With the formyl head group (Fig. 4C), the C6 linker proved to be optimal, and once more the compounds followed the binding order observed with the one-residue stagger motif.
With this DNA sequence, which has two binding sites in the maximum overlap motif, the gel shifts showed a smear indicating a number of conformers. This smearing was present on gels with either head group, amino or formyl. As the Im/Im pair in the TIP dimer is redundant and may read either GC or CG, this may account for two of the bands, ACT and AGT, in the diffused shifted region. Some of the lanes indicate a third band (Fig. 4C), which could be explained by having two polyamides bind concurrently.
The formyl head group tested in the gel shifts had a lower affinity for the DNA in both binding motifs. This was indicated by an increase in the drug to DNA ratio needed for effective binding. Optimal binding for the amino head group occurred at a 2:1 drug:DNA ratio for the ACGT sequence, whereas the best binding with the formyl occurred at a 4:1 ratio. However, the C6-formyl had a high affinity for AGT in the footprint results.
Discussion
The binding affinities of a series of thiazole-imidazole-pyrrole polyamide monomers and crosslinked dimers were determined to evaluate the effects on selectivity and binding of the introduction of different N-terminal head groups attached to the leading thiazole ring, together with variation in the linking methylene chain length. Quantitative DNase I footprint titrations of a DNA sequence, containing putative match and mismatch sites for both the maximum overlap and one residue stagger binding modes, allowed the measurement of binding constants at each site. The presence of alternative mismatches for a given match site enabled specificity of binding to be determined and, consequently, the potential base recognition properties of the thiazole to be assessed. Concurrent gel shift experiments examined binding to short oligomers containing match sites for both modes of binding.
Quantitative DNase I footprinting demonstrated that within the N-terminal amino-TIP series, the C7 and C8-linked compounds bound most strongly to the putative binding sites. No significant binding was observed at sites other than these. The TIP-C7-amino proved the most selective, with up to a 7-fold selectivity for the maximum overlap match site, AGT. A significant decrease in both affinity and selectivity was seen in this series with reduced methylene chain lengths of five or six carbons. Alteration of the leading head group to a formyl resulted in an optimal methylene linker of six carbons. TIP-C6-formyl produced the strongest binding with a 3-fold specificity for the overlap match site over the one-residue stagger site. Reduced binding was seen with the TIP-C7-formyl, which nevertheless showed up to a 6-fold specificity for the overlap match site. The C8-linked formyl demonstrated a decrease in both binding affinity and specificity. Gel shift experiments strongly supported the footprinting data, giving the same order of binding affinity in both the amino and formyl series. Overall, however, the formyl series demonstrated a lower affinity for the DNA in both binding modes, requiring an elevated drug to DNA ratio. This is consistent with the CD experiments of Burckhardt and coworkers (42) comparing C7-linked compounds with an amino or formyl head group.
Certain linkage and head group combinations proved particularly weak binders in both footprinting and gel shift experiments. For example, weak binding was observed with a five-carbon linker for either the N-terminal formyl or amino compound series. No binding was observed for C5 and C7-linked compounds lacking an N-terminal head group (hydrogen only) or with unlinked and C7-linked polyamides with an acetyl head group. These observations suggest that the presence of an N-terminal hydrogen or acetyl may abrogate binding in crosslinked polyamides, as was observed previously with C7-linked compounds by Burckhardt and coworkers (42). This is not the case, however, for hairpin polyamides, which bind efficiently with an N-terminal hydrogen (34).
Whereas polyamides linked using a hairpin motif have been extensively investigated (34), optimized binding conditions for crosslinked polyamides remain to be defined. Several studies of crosslinking in polyamides have demonstrated significant improvements in binding on linkage of the individual polypeptide ligands (7, 13, 42). The detailed assessment of linkage with a range of possible linkers presented here indicates a C6 linker with an N-terminal formyl, and a C7 linker with an N-terminal amino provides suitable alignment of the polyamide dimer within the minor groove. This is lost when the linker is reduced to five carbons, possibly because comprehensive contact of the dimeric ligand with both strands of the DNA is restricted. Furthermore, a C5 linker provides unfavorable binding compared with an unlinked monomer, suggesting that although suitable linkage enhances binding, suboptimal linkage may diminish effective binding.
This study reveals that the nature of the N-terminal moiety has a profound influence on the binding characteristics of crosslinked polyamides. Both the footprinting and gel shift data indicate that the use of a hydrogen or acetyl head group may inhibit binding. Furthermore, replacement of an amino head group with a formyl alters the optimal linker required for binding. Therefore, there may be a combined effect on polyamide binding by the linkage and head group. Polyamide specificity may also be modulated in this way, resulting in an overall improved specificity for match site binding with an N-terminal formyl.
It is significant that both of the successful head groups, amino and formyl, have an NH group capable of hydrogen bonding with DNA, whereas the unsuccessful head groups, –H and acetyl, do not. In a side-by-side unlinked di-imidazole complex with leading formyl groups (28), both of the formyl NH make hydrogen bonds with the floor of the minor groove: 2.94 Å from cytosine O2 or 2.80 Å from guanine N3 (see Table 1 of ref. 28). Hydrogen-bonded interactions of the head group with DNA seem to be required for crosslinked side-by-side binding, although such an interaction is not demanded for a hairpin complex (34).
The thiazole ring, tested here in the terminal position of the polyamide as a reading element for adenine, bound its AGT target in the maximum overlap motif with both the amino and formyl head groups. The overlap motif pairs the thiazole ring with pyrrole; thus, thiazole reads adenine and pyrrole thymine as expected. The footprinting results with TIP-C6-formyl showed a preference for AGT over TGT by up to 3-fold. Furthermore, the footprinting indicated that AGT is strongly favored over ACGT, the one-residue stagger motif, in which thiazole is next to cytosine and imidazole is next to guanine. Therefore, thiazole seems to favor adenine moderately in a Th/Py pair, with poor CG specificity when paired with imidazole.
Our results therefore indicate that the thiazole moiety provides moderate binding and discrimination of adenine when in the polyamide N-terminal position. A previous study concluded that a thiazole/pyrrole pair binds poorly to all four Watson–Crick base pairs (43). However, in that study the thiazole was placed internally in a hairpin. Positioning of the heterocyclic moiety within a polyamide may be crucial. Indeed, the successful T/A discrimination seen with hydroxypyrrole sited internally within the hairpin motif has not been found when hydroxypyrrole is placed at the terminal position (44). Binding affinities of hairpins containing hydroxypyrrole vary considerably based on the position of the hydroxypyrrole ring within the hairpin motif (24). Calculation of relative binding affinities of imidazole and hydroxypyrrole rings by Goodsell and coworkers (45) indicates that the hydroxypyrrole ring binds with a lower affinity for AT base pairs compared with the affinity of the imidazole ring for GC pairs. In the present study, the specificity for adenine observed with thiazole in the terminal position is comparable with the specificity for thymine provided by a hydroxybenzamide/pyrrole pair in eight ring hairpin polyamides (44). The specificity of thiazole placed internally with a crosslinked polyamide remains to be determined.
Differences in the two types of polyamides, hairpin versus crosslinked, complicate direct comparison of the thiazole ring in the two linkage motifs. In the hairpin motif, there is effective binding in the absence of a head group. In contrast, in the crosslinked motif the head group attached to the leading ring may significantly modulate its binding characteristics, with formyl enhancing adenine selectivity and acetyl or hydrogen inhibiting binding for crosslinked polyamides with a leading thiazole ring. Additionally, the number of constituent rings in the dimer, six in this case and eight in the hairpin, may significantly affect the affinity of binding. We suggest that N-formyl-thiazole may provide sequence discrimination in crosslinked polyamides, with thiazole favoring adenine over thymine. Moreover, we have demonstrated the important influence of the leading head group in combination with the length of methylene linker on the binding characteristics of crosslinked polyamides.
Acknowledgments
We thank Dr. Aiden Flynn for assistance with the numerical analysis. This work was supported by National Institutes of Health Grant GM-31299 (to R.E.D.) and Cancer Research Campaign Program Grant SP2000/0402 (to J.A.H.).
Abbreviation
- TIP
thiazole-imidazole-pyrrole
References
- 1.Kopka M L, Yoon C, Goodsell D, Pjura P, Dickerson R E. Proc Natl Acad Sci USA. 1985;82:1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lown J W, Krowicki K, Bhat U G, Skorobogaty A, Ward B, Dabrowiak J C. Biochemistry. 1986;25:7408–7416. doi: 10.1021/bi00371a024. [DOI] [PubMed] [Google Scholar]
- 3.Krowicki K, Lown J W. J Org Chem. 1987;52:3493. [Google Scholar]
- 4.Pelton J G, Wemmer D E. Proc Natl Acad Sci USA. 1989;86:5723–5727. doi: 10.1073/pnas.86.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade W S, Mrksich M, Dervan P B. J Am Chem Soc. 1992;114:8783–8794. [Google Scholar]
- 6.Dwyer T J, Geierstanger B H, Bathini Y, Lown J W, Wemmer D E. J Am Chem Soc. 1992;114:5911–5919. [Google Scholar]
- 7.Chen Y H, Lown J W. J Am Chem Soc. 1994;116:6995–7005. [Google Scholar]
- 8.Mrksich M, Parks M E, Dervan P B. J Am Chem Soc. 1994;116:7983–7988. [Google Scholar]
- 9.Parks M E, Baird E E, Dervan P B. J Am Chem Soc. 1996;118:6147–6152. [Google Scholar]
- 10.Baliga R, Baird E E, Herman D M, Melander C, Dervan P B, Crothers D M. Biochemistry. 2001;40:3–8. doi: 10.1021/bi0022339. [DOI] [PubMed] [Google Scholar]
- 11.Mrksich M, Dervan P B. J Am Chem Soc. 1993;115:9892–9899. [Google Scholar]
- 12.Mrksich M, Dervan P B. J Am Chem Soc. 1994;116:3663–3664. [Google Scholar]
- 13.Chen Y H, Yang Y, Lown J W. J Biomol Struct Dyn. 1996;14:341–355. doi: 10.1080/07391102.1996.10508129. [DOI] [PubMed] [Google Scholar]
- 14.Trauger J W, Baird E E, Dervan P B. Nature (London) 1996;382:559–561. doi: 10.1038/382559a0. [DOI] [PubMed] [Google Scholar]
- 15.Gottesfeld J M, Neely L, Trauger J W, Baird E E, Dervan P B. Nature (London) 1997;387:202–205. doi: 10.1038/387202a0. [DOI] [PubMed] [Google Scholar]
- 16.Mapp A K, Ansari A Z, Ptashne M, Dervan P B. Proc Natl Acad Sci USA. 2000;97:3930–3935. doi: 10.1073/pnas.97.8.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson L A, Gulizia R J, Trauger J W, Baird E E, Mosier D E, Gottesfeld J M, Dervan P B. Proc Natl Acad Sci USA. 1998;95:12890–12895. doi: 10.1073/pnas.95.22.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson L A, Trauger J W, Baird E E, Ghazal P, Dervan P B, Gottesfeld J M. Biochemistry. 1999;38:10801–10807. doi: 10.1021/bi9912847. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson L A, Trauger J W, Baird E E, Dervan P B, Graves B J, Gottesfeld J M. J Biol Chem. 1999;274:12765–12773. doi: 10.1074/jbc.274.18.12765. [DOI] [PubMed] [Google Scholar]
- 20.Chiang S Y, Burli R W, Benz C C, Gawron L, Scott G K, Dervan P B, Beerman T A. J Biol Chem. 2000;275:24246–24254. doi: 10.1074/jbc.M000820200. [DOI] [PubMed] [Google Scholar]
- 21.Mrksich M, Dervan P B. J Am Chem Soc. 1993;115:2572–2576. [Google Scholar]
- 22.Geierstanger B H, Mrksich M, Dervan P B, Wemmer D E. Science. 1994;266:646–650. doi: 10.1126/science.7939719. [DOI] [PubMed] [Google Scholar]
- 23.Mrksich M, Dervan P B. J Am Chem Soc. 1995;117:3325–3332. [Google Scholar]
- 24.White S, Szewczyk J M, Turner J M, Baird E E, Dervan P B. Nature (London) 1998;391,Suppl.:468–471. doi: 10.1038/35106. [DOI] [PubMed] [Google Scholar]
- 25.Kielkopf C L, White S, Szewczyk J M, Turner J M, Baird E E, Dervan P B, Rees D C. Science. 1998;282:111–115. doi: 10.1126/science.282.5386.111. [DOI] [PubMed] [Google Scholar]
- 26.Kielkopf C L, Bremer R E, White S, Szewczyk J M, Turner J M, Baird E E, Dervan P B, Rees D C. J Mol Biol. 2000;295:557–567. doi: 10.1006/jmbi.1999.3364. [DOI] [PubMed] [Google Scholar]
- 27.Rao K E, Shea R G, Yadagiri B, Lown J W. Anticancer Drug Design. 1990;5:3–20. [PubMed] [Google Scholar]
- 28.Kopka M L, Han G W, Goodsell D S, Chiu T K, Walker W L, Lown J W, Dickerson R E. Structure, Motion, Interaction and Expression of Biological Macromolecules, Proc. 10th Conversation. New York: Adenine Press; 1998. pp. 177–191. [Google Scholar]
- 29.Kopka M L, Goodsell D S, Han G W, Chiu T K, Lown J W, Dickerson R E. Structure (Cambridge, UK) 1997;5:1033–1046. doi: 10.1016/s0969-2126(97)00255-4. [DOI] [PubMed] [Google Scholar]
- 30.Mrksich M, Wade W S, Dwyer T W, Geierstanger B H, Wemmer D E, Dervan P B. Proc Natl Acad Sci USA. 1992;89:7586–7590. doi: 10.1073/pnas.89.16.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwyer T J, Geierstanger B H, Mrksich M, Dervan P B, Wemmer D E. J Am Chem Soc. 1993;115:9900–9906. [Google Scholar]
- 32.Chen Y H, Lown J W. Heterocycles. 1995;8:1691–1707. [Google Scholar]
- 33.Sharma S K, Tandon M, Lown J W. J Org Chem. 2000;65:1102–1107. doi: 10.1021/jo991571g. [DOI] [PubMed] [Google Scholar]
- 34.Dervan P B, Burli R W. Curr Opin Chem Biol. 1999;3:688–693. doi: 10.1016/s1367-5931(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 35.Parks M E, Baird E E, Dervan P B. J Am Chem Soc. 1996;118:6153–6159. [Google Scholar]
- 36.Brenowitz M, Senear D F, Shea M A, Ackers G K. Methods Enzymol. 1986;130:132–181. doi: 10.1016/0076-6879(86)30011-9. [DOI] [PubMed] [Google Scholar]
- 37.Brenowitz M, Senear D F, Shea M A, Ackers G K. Proc Natl Acad Sci USA. 1986;83:8462–8466. doi: 10.1073/pnas.83.22.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senear D F, Brenowitz M, Shea M A, Ackers G K. Biochemistry. 1986;25:7344–7354. doi: 10.1021/bi00371a016. [DOI] [PubMed] [Google Scholar]
- 39.Zhan Z-Y J, Dervan P B. Bioorg Med Chem. 2000;8:2467–2474. doi: 10.1016/s0968-0896(00)00182-6. [DOI] [PubMed] [Google Scholar]
- 40.Palfner K, Kneba M, Hiddemann W, Bertram J. BioTechniques. 1995;19:926–929. [PubMed] [Google Scholar]
- 41.Hawkins C A, Pelaez de Clairac R, Dominey R N, Baird E E, White S, Dervan P B, Wemmer D E. J Am Chem Soc. 2000;122:5235–5236. [Google Scholar]
- 42.Burckhardt G, Fortsch I, Simon H, Birch-Hirschfeld E, Kittle L, Schutz H, Sharma S K, Lown J W, Zimmer C. J Biomol Struct Dyn. 2000;11:355–363. doi: 10.1080/07391102.2000.10506641. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen D H, Szewczyk J W, Baird E E, Dervan P B. Bioorg Med Chem. 2001;9:7–17. doi: 10.1016/s0968-0896(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 44.Ellervik U, Wang C C C, Dervan P B. J Am Chem Soc. 2000;122:9354–9360. [Google Scholar]
- 45.Walker W L, Kopka M L, Goodsell D S. Biopolymers. 1997;44:323–334. doi: 10.1002/(SICI)1097-0282(1997)44:4<323::AID-BIP2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]






