Abstract
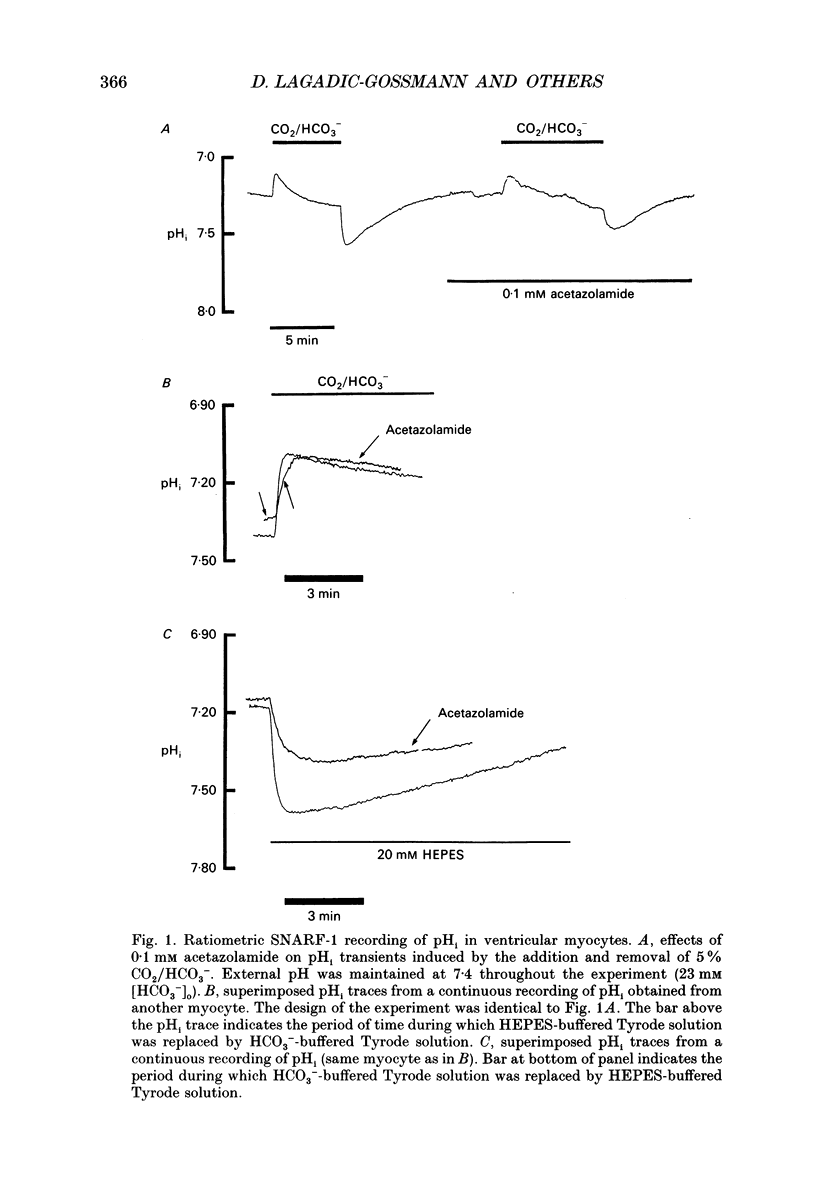
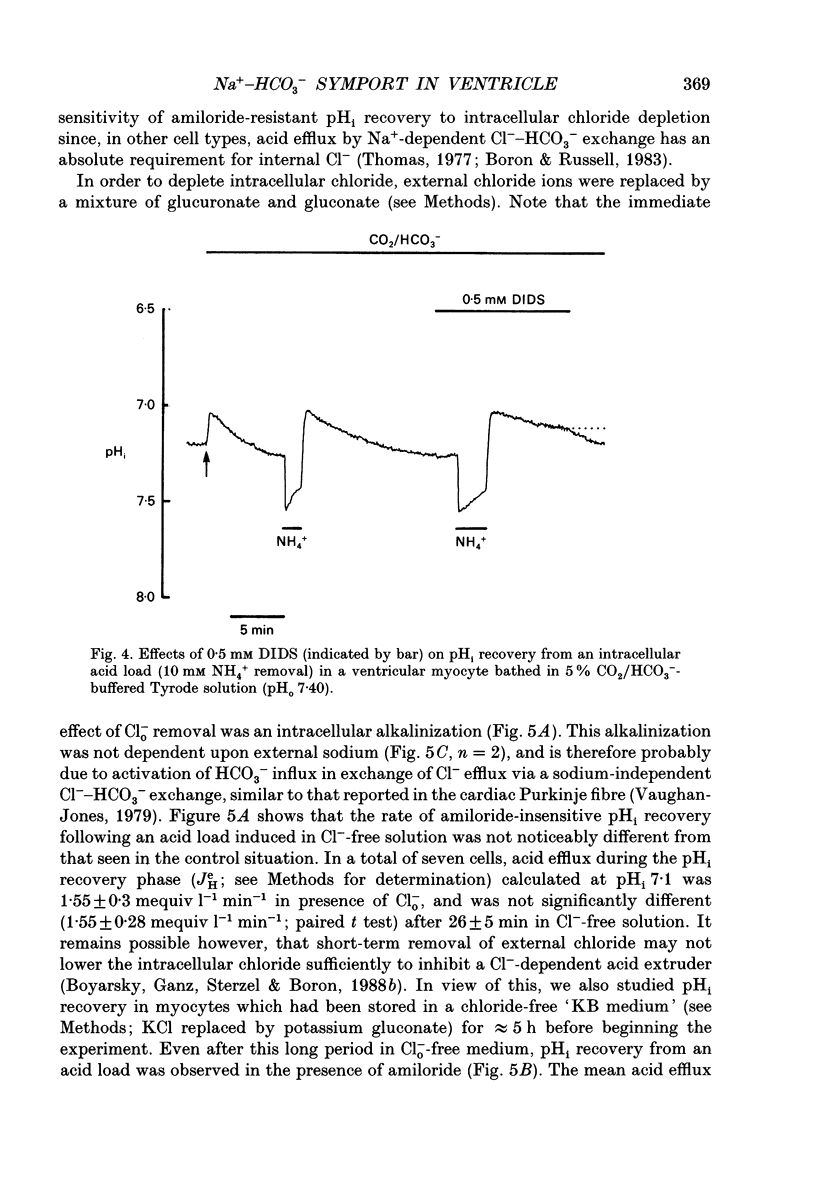
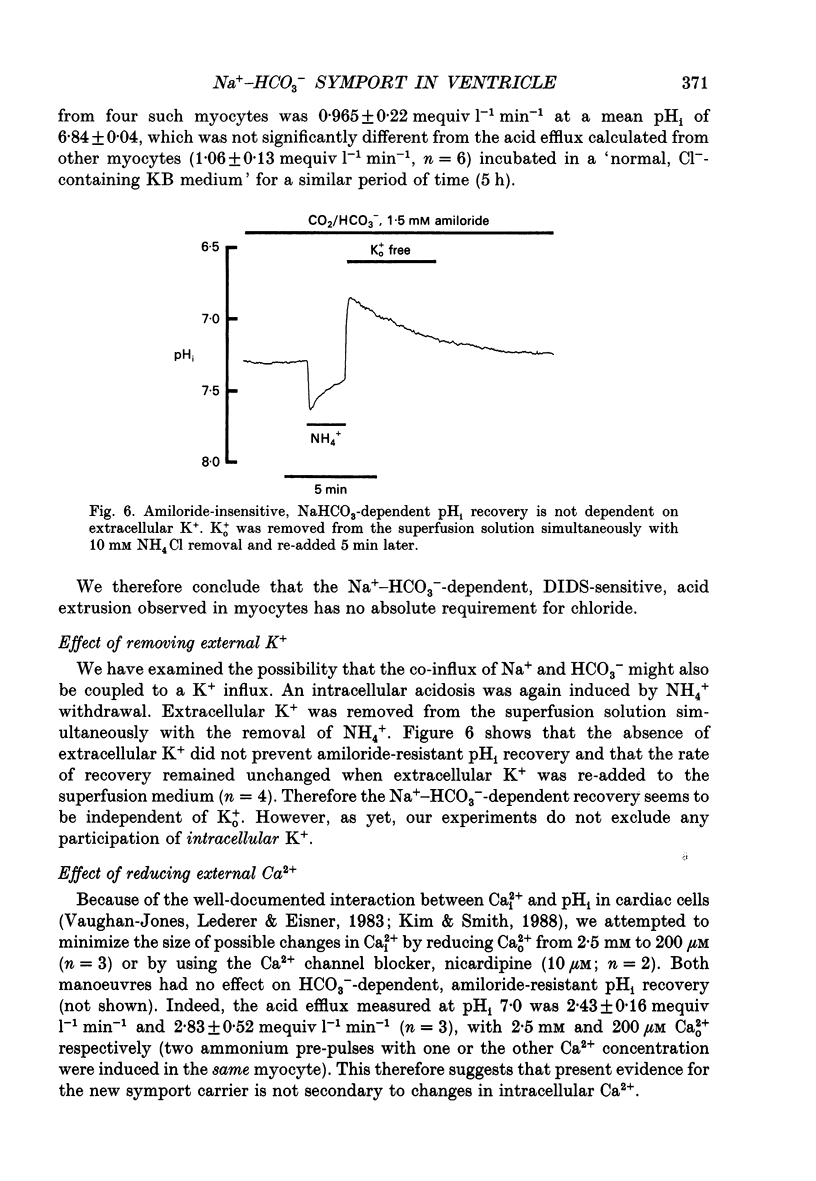
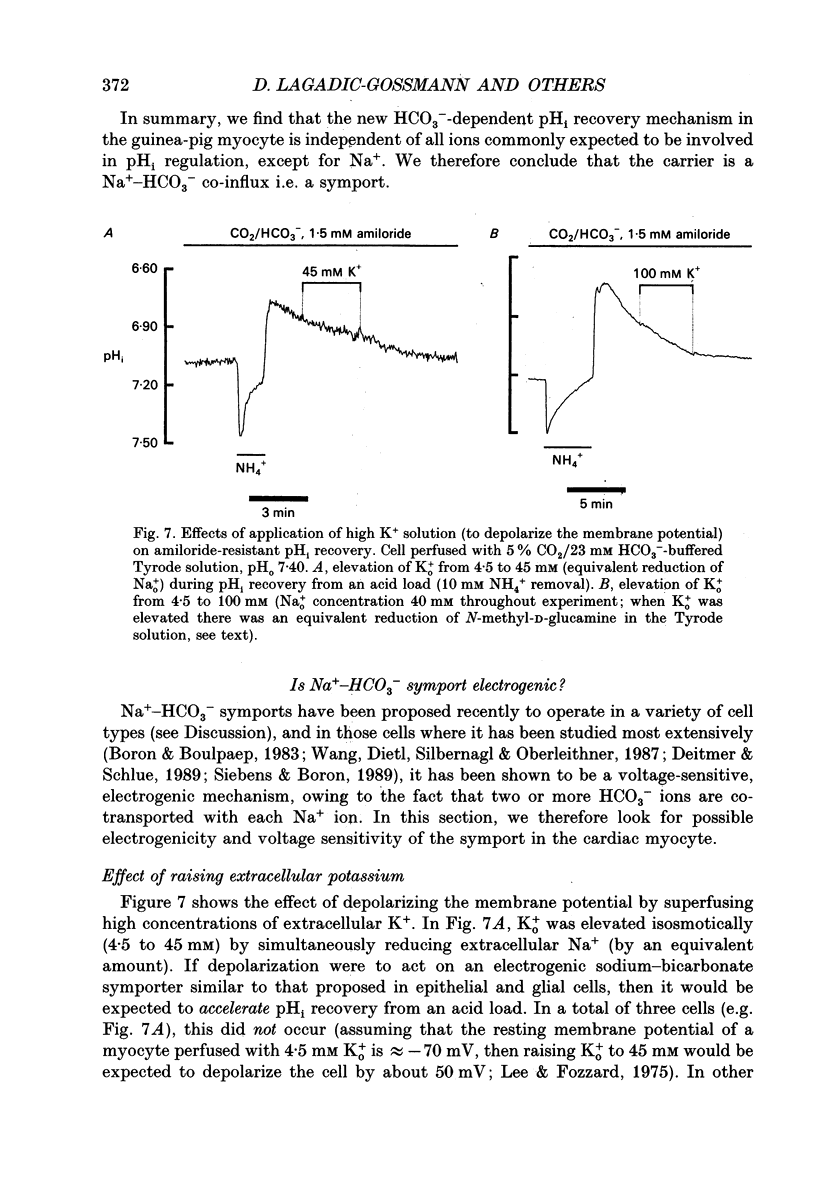
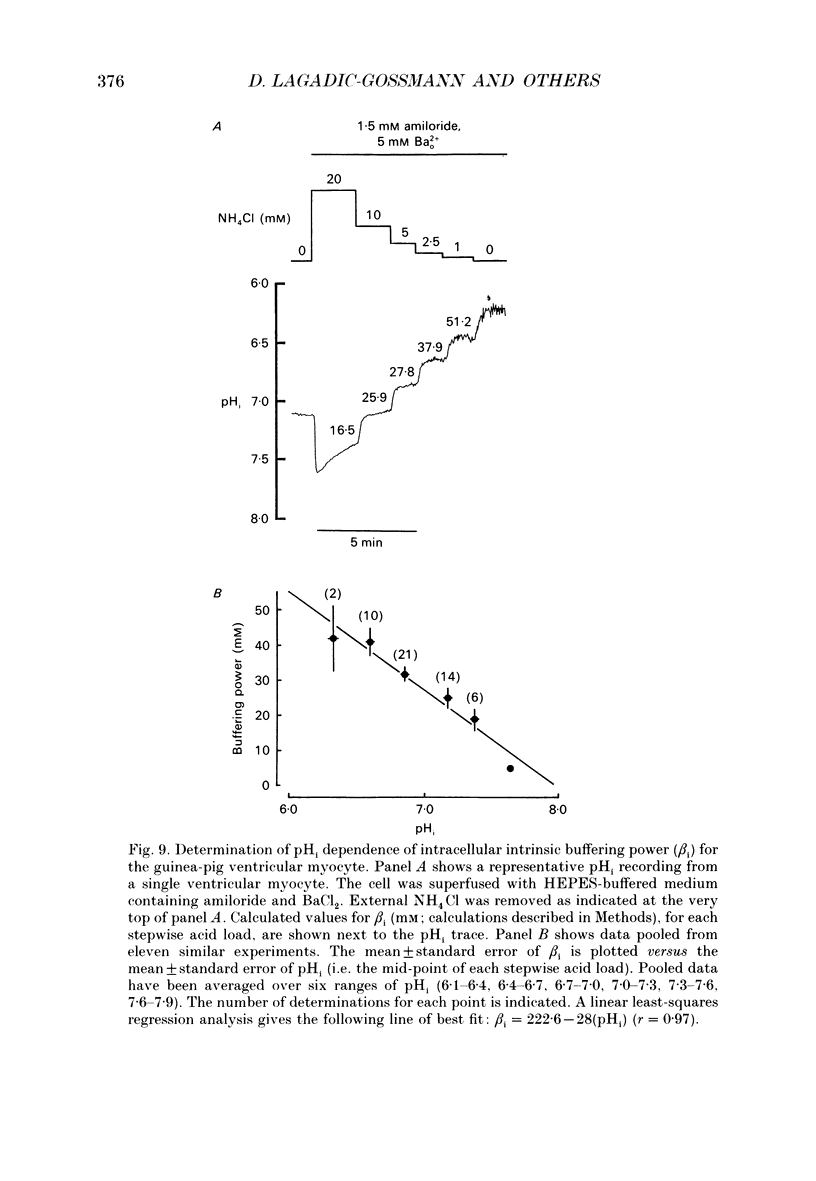
1. Intracellular pH (pHi) was recorded ratiometrically in isolated guinea-pig ventricular myocytes using the pH-sensitive fluoroprobe, carboxy-SNARF-1 (carboxy-seminaphthorhodafluor). 2. Following an intracellular acid load (10 mM NH4 Cl removal), pHi recovery in HEPES-buffered Tyrode solution was inhibited by 1.5 mM amiloride (Na(+)-H+ antiport blocker). In the presence of amiloride, switching from HEPES buffer to HCO3-/CO2 (pHo of both solutions = 7.4) stimulated a pHi recovery towards more alkaline levels. 3. Amiloride-resistant, HCO(3-)-dependent pHi recovery was inhibited by removal of external Na+ (replaced by N-methyl-D-glucamine), whereas removal of external Cl- (replaced by glucuronate, leading to depletion of internal Cl-), removal of external K+, or decreasing external Ca2+ by approximately tenfold had no inhibitory effect. These results suggest that the amiloride-resistant recovery is due to a Na(+)-HCO3- cotransport into the cell. 4. The stilbene derivative DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid, 500 microM) slowed Na(+)-HCO(3-)-dependent pHi recovery. 5. Intracellular pH increased in Cl(-)-free solution and this increase still occurred in Na(+)-free solution indicating that it is not caused via Na(+)-HCO3- symport and is more likely to be due to Cl- efflux in exchange for HCO3- influx on a sarcolemmal Cl(-)-HCO3- exchanger. The lack of any significant pHi recovery from intracellular acidosis in Na(+)-free solution suggests that this exchanger does not contribute to acid-equivalent extrusion. 6. Possible voltage sensitivity and electrogenicity of the co-transport were examined by using the whole-cell patch clamp technique in combination with SNARF-1 recordings of pHi. Stepping the holding potential from -110 to -40 mV did not affect amiloride-resistant pHi recovery from acidosis. Moreover, following an intracellular acid load, the activation of Na(+)-HCO3- co-influx (by switching from HEPES to HCO3-/CO2 buffer) produced no detectable outward current (outward current would be expected if the coupling of HCO3- with Na+ were > 1.0). 7. Intracellular intrinsic buffering power (beta i) was assessed as a function of pHi (beta i computed from the decrease of pHi following reduction of extracellular NH4 Cl in amiloride-containing solution). beta i in the ventricular myocyte increases roughly linearly with a decrease in pHi according the following equation: beta i = -28(pHi) +222.6. 8. Comparison of acid-equivalent efflux via Na(+)-HCO3- symport and Na(+)-H+ antiport showed that, following an intracellular acidosis, the symport accounts for about 40% of total acid efflux, the other 60% being carried by the antiport.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991 May;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D., Peers C., Nye P. C. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991 May;436:107–129. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Dart C., Vaughan-Jones R. D. Na(+)-HCO3- symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990 Feb;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Cellular mechanisms underlying calcium-proton interactions in cultured chick ventricular cells. J Physiol. 1988 Apr;398:391–410. doi: 10.1113/jphysiol.1988.sp017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Piwnica-Worms D., Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells. Na(+)-dependent Cl-/HCO3- exchange. J Gen Physiol. 1990 Dec;96(6):1247–1269. doi: 10.1085/jgp.96.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988 Feb 15;250(1):1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Loessberg P. A. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. II. Regulation of H+ and HCO3- transporters by Ca2(+)-mobilizing agonists. J Biol Chem. 1990 Aug 5;265(22):12813–12819. [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells. pHi regulation. J Gen Physiol. 1985 Jan;85(1):43–64. doi: 10.1085/jgp.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Siebens A. W., Boron W. F. Depolarization-induced alkalinization in proximal tubules. I. Characteristics and dependence on Na+. Am J Physiol. 1989 Feb;256(2 Pt 2):F342–F353. doi: 10.1152/ajprenal.1989.256.2.F342. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C748–C760. doi: 10.1152/ajpcell.1986.250.5.C748. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of intracellular pH in cardiac muscle. Ciba Found Symp. 1988;139:23–46. doi: 10.1002/9780470513699.ch3. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Na+-H+ exchange in isolated myocytes from adult rat heart. Am J Physiol. 1989 Aug;257(2 Pt 1):C207–C213. doi: 10.1152/ajpcell.1989.257.2.C207. [DOI] [PubMed] [Google Scholar]
- Wang W., Dietl P., Silbernagl S., Oberleithner H. Cell membrane potential: a signal to control intracellular pH and transepithelial hydrogen ion secretion in frog kidney. Pflugers Arch. 1987 Jul;409(3):289–295. doi: 10.1007/BF00583478. [DOI] [PubMed] [Google Scholar]


