Abstract
1. Intracellular pH (pHi) was recorded in isolated guinea-pig ventricular myocytes using the pH-sensitive fluoroprobe, carboxy-SNARF-1 (carboxy-seminaphthorhodafluor). 2. Addition and removal of 10 mM NH4Cl was used to induce an intracellular acid load in a myocyte perfused with HCO3(-)-buffered solution containing amiloride. Under these conditions, subsequent pHi recovery is known to rely upon Na(+)-HCO3- co-transport into the cell. The application of 0.5-5 microM adrenaline resulted in an inhibition of this pHi recovery. 3. In HEPES-buffered solution, where acid extrusion is mediated primarily by Na(+)-H+ antiport, pHi recovery from an acid load was stimulated by the application of adrenaline. 4. In HCO3-/CO2-buffered solution (no amiloride), when both acid-aquivalent extruders are activated by an intracellular acidification, adrenaline was found to slow pHi recovery. 5. When both carriers were inhibited in Na(+)-free, HCO3(-)-buffered medium, adrenaline had no effect on pHi, ruling out any effect of the catecholamine on background acid loading. 6. The voltage clamp technique was used to test if the inhibitory effect of adrenaline on amiloride-resistant, HCO3(-)-dependent pHi recovery was due to an efflux of HCO3- ions through catecholamine-activated anion channels. During pHi recovery, membrane depolarization, sufficient to reverse the electrochemical driving force acting on HCO3-, had no effect upon pHi recovery rate. 7. The above results show that adrenaline has direct but opposite effects on Na(+)-HCO3- co-transport and Na(+)-H+ antiport. In the presence of this agonist, the pHi dependence of Na(+)-HCO3- symport was shifted to the left along the pHi axis by 0.13 +/- 0.03 units (n = 4) whereas that for Na(+)-H+ antiport was shifted in the opposite direction by only 0.07 +/- 0.01 units (n = 3). Following an acid load, the net effect of adrenaline under physiological conditions was, therefore, a slowing of pHi recovery. 8. The application of extracellular ATP (ATPo, 10-50 microM) mimicked the effects of adrenaline on both Na(+)-H+ exchange and Na(+)-HCO3- symport. 9. Adenosine (50 microM) and ADP (50 microM) did not induce any inhibition of Na(+)-HCO3- symport, suggesting that the inhibition induced by ATP was not mediated through P1 or P2-purinergic receptors. 10. We conclude that Na(+)-H+ antiport and Na(+)-HCO3- symport are both coupled to adrenaline and ATPo receptors. Activation of these receptors switches acid-equivalent extrusion from a situation dependent on both HCO3- and H+ ions to one nearly exclusively dependent upon H+.
Full text
PDF















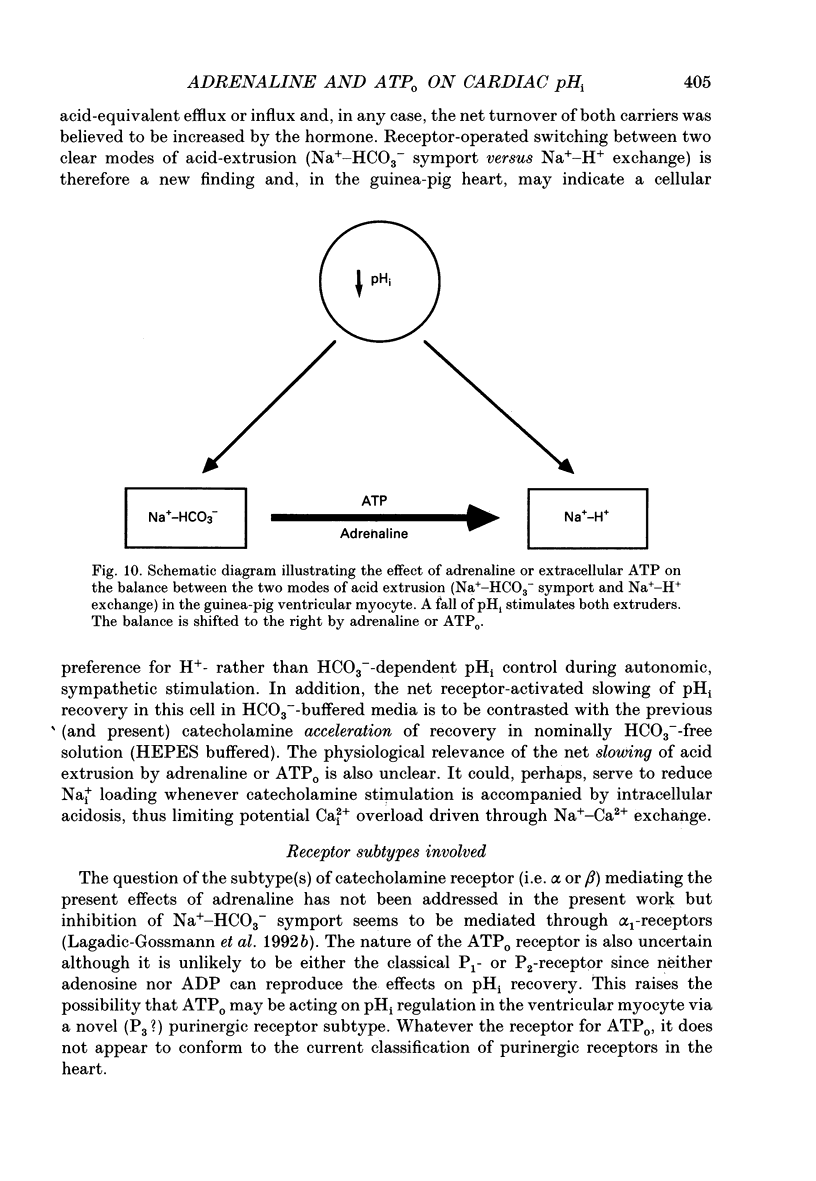


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
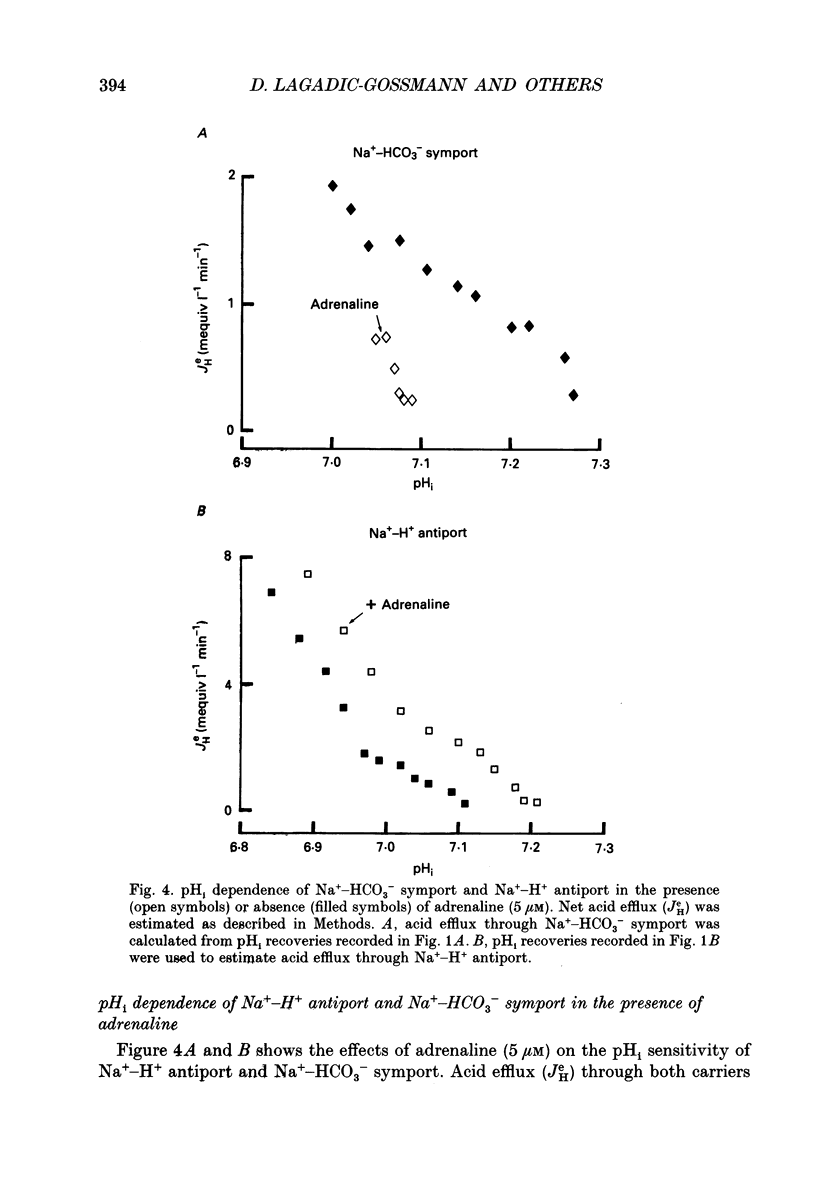
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
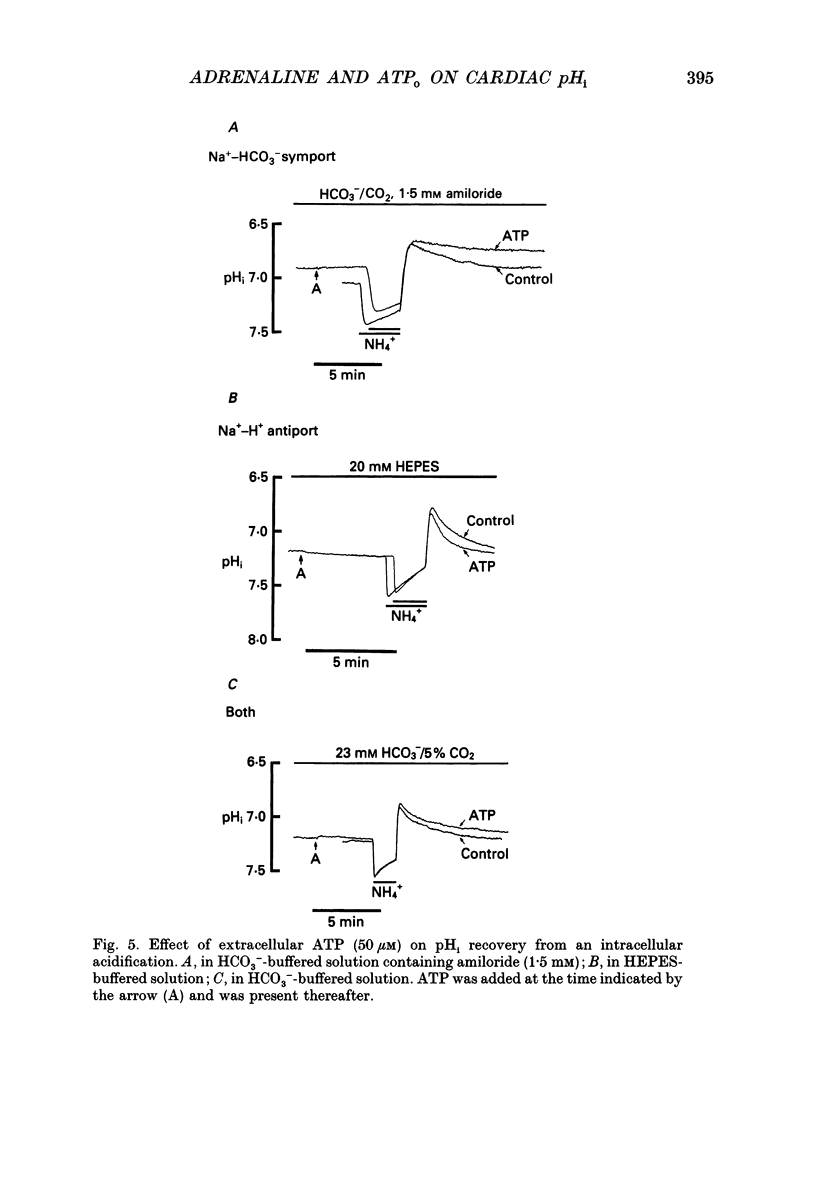
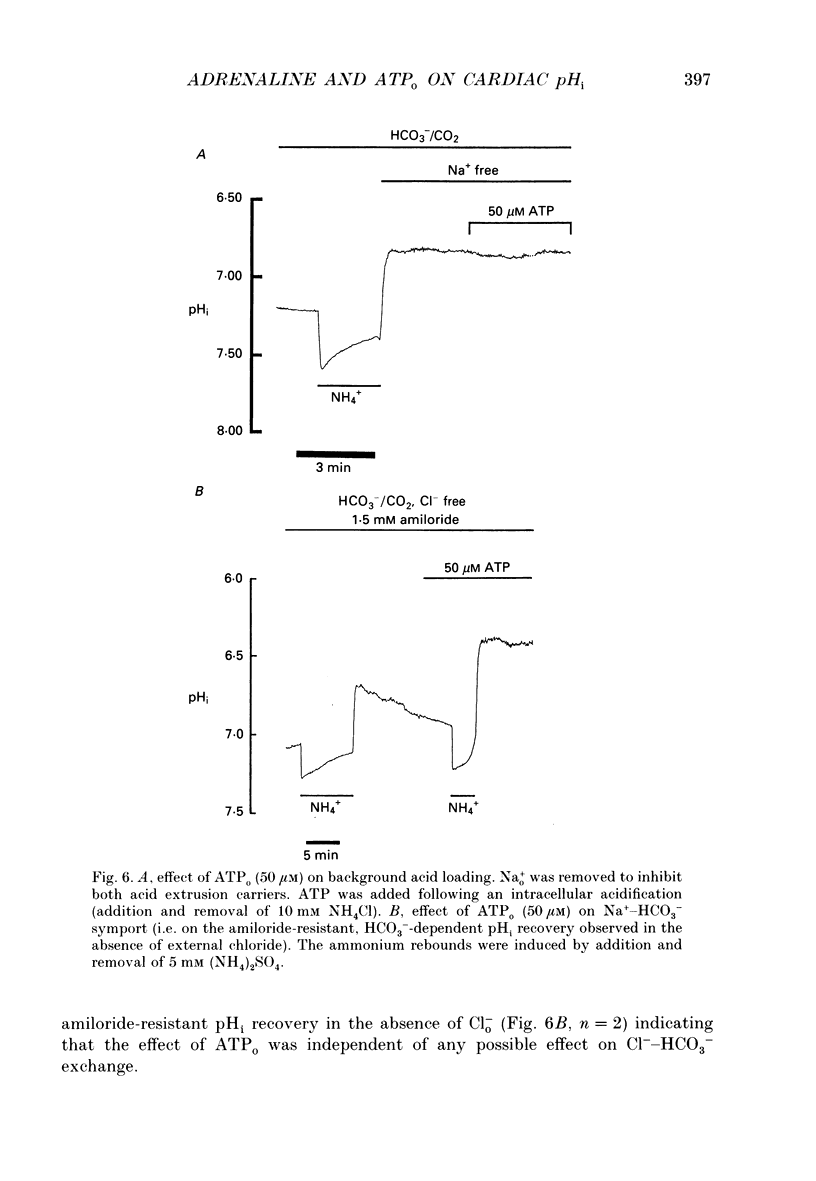
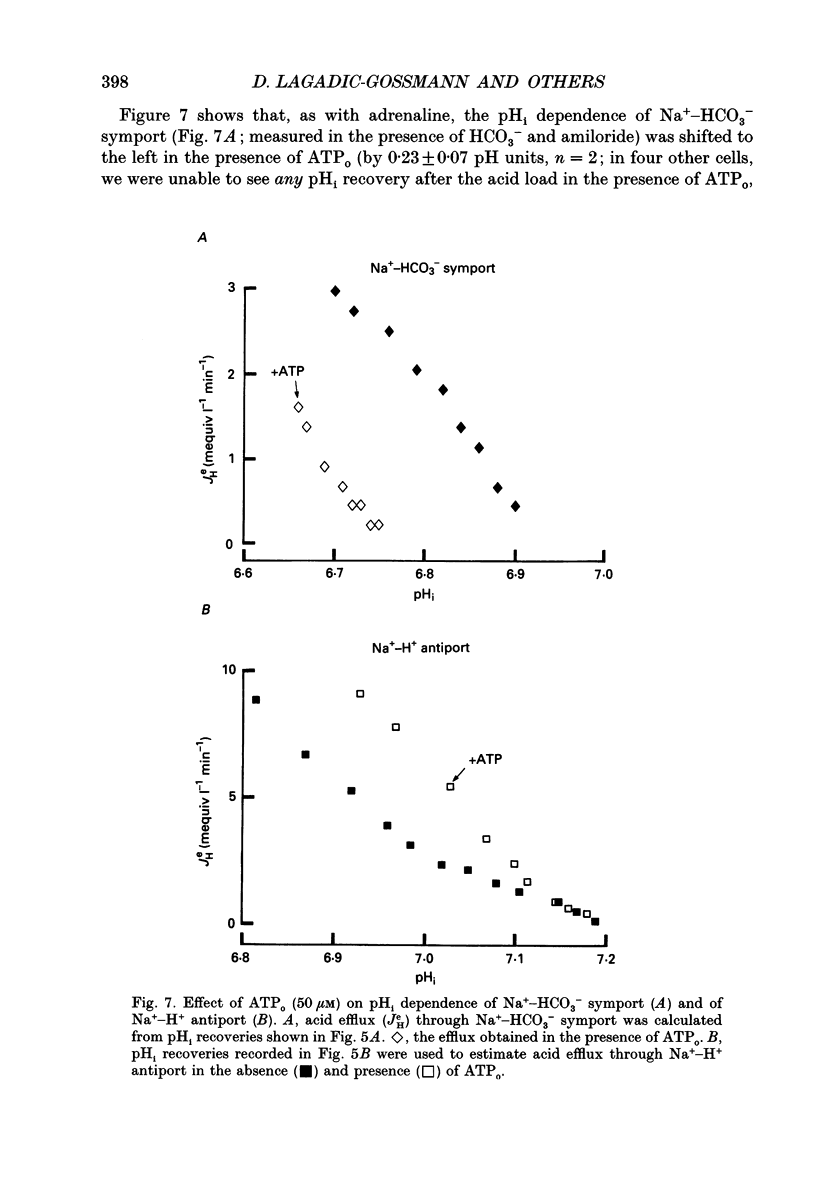
- Bonanno J. A. K(+)-H+ exchange, a fundamental cell acidifier in corneal epithelium. Am J Physiol. 1991 Mar;260(3 Pt 1):C618–C625. doi: 10.1152/ajpcell.1991.260.3.C618. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Dart C., Vaughan-Jones R. D. Na(+)-HCO3- symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Iwakura K., Hori M., Watanabe Y., Kitabatake A., Cragoe E. J., Jr, Yoshida H., Kamada T. Alpha 1-adrenoceptor stimulation increases intracellular pH and Ca2+ in cardiomyocytes through Na+/H+ and Na+/Ca2+ exchange. Eur J Pharmacol. 1990 Sep 4;186(1):29–40. doi: 10.1016/0014-2999(90)94057-5. [DOI] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984 May;16(5):389–394. doi: 10.1016/s0022-2828(84)80610-0. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Buckler K. J., Vaughan-Jones R. D. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A., Sakata Y., Watanabe T., Murakami N. Leucocytosis induced in rabbits by intravenous or central injection of granulocyte colony stimulating factor. J Physiol. 1990 Jul;426:117–126. doi: 10.1113/jphysiol.1990.sp018129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Loessberg P. A. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. II. Regulation of H+ and HCO3- transporters by Ca2(+)-mobilizing agonists. J Biol Chem. 1990 Aug 5;265(22):12813–12819. [PubMed] [Google Scholar]
- Otani H., Otani H., Uriu T., Hara M., Inoue M., Omori K., Cragoe E. J., Jr, Inagaki C. Effects of inhibitors of protein kinase C and Na+/H+ exchange on alpha 1-adrenoceptor-mediated inotropic responses in the rat left ventricular papillary muscle. Br J Pharmacol. 1990 Jun;100(2):207–210. doi: 10.1111/j.1476-5381.1990.tb15783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Scamps F., Vassort G. Extracellular ATP-induced acidification leads to cytosolic calcium transient rise in single rat cardiac myocytes. Biochem J. 1991 Feb 15;274(Pt 1):55–62. doi: 10.1042/bj2740055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Vassort G. Extracellular MgATP activates the Cl-/HCO3- exchanger in single rat cardiac cells. J Physiol. 1991 Dec;444:241–256. doi: 10.1113/jphysiol.1991.sp018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of intracellular pH in cardiac muscle. Ciba Found Symp. 1988;139:23–46. doi: 10.1002/9780470513699.ch3. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]


