Abstract
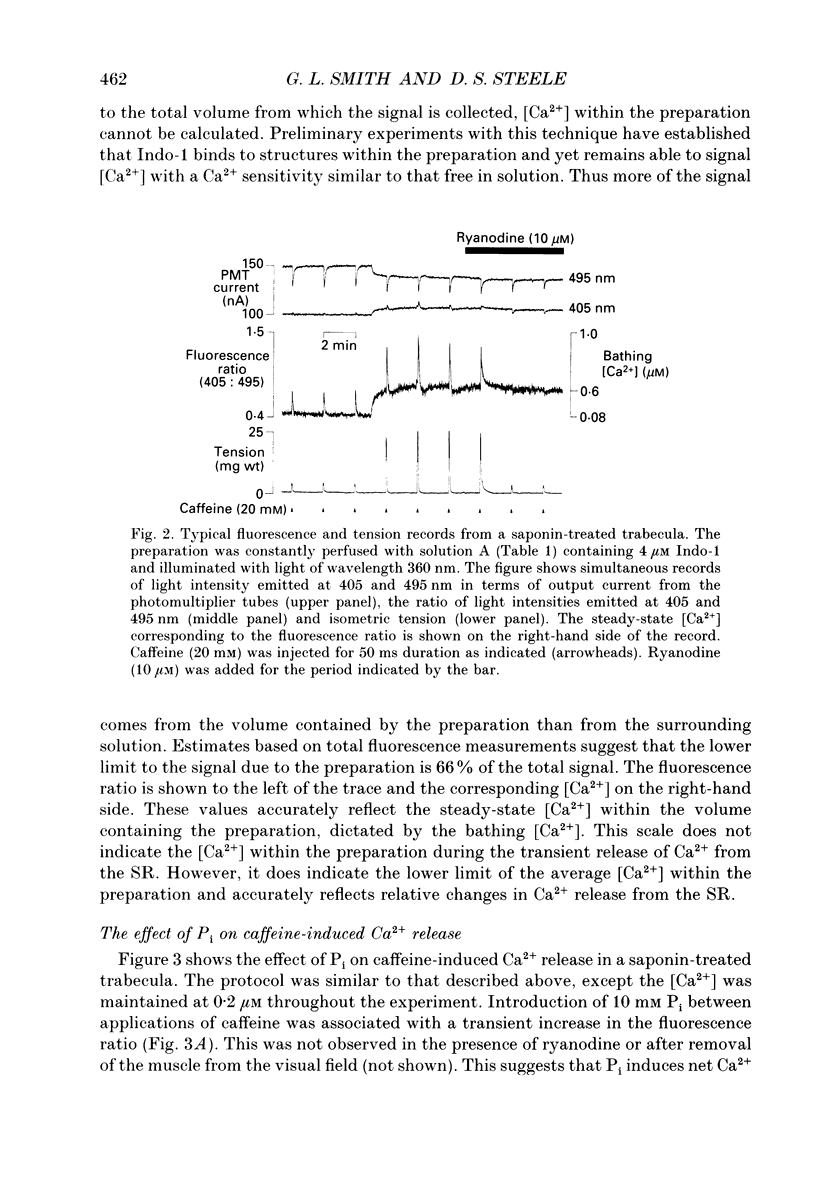
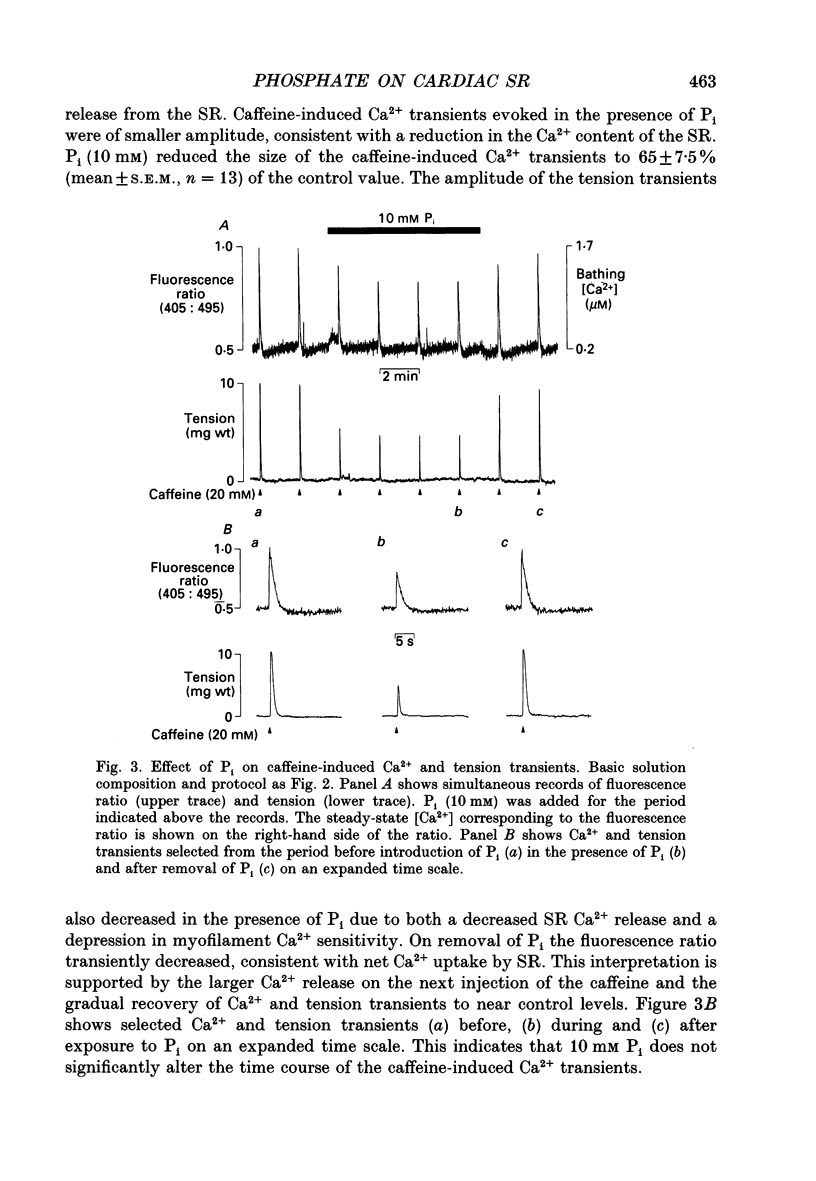
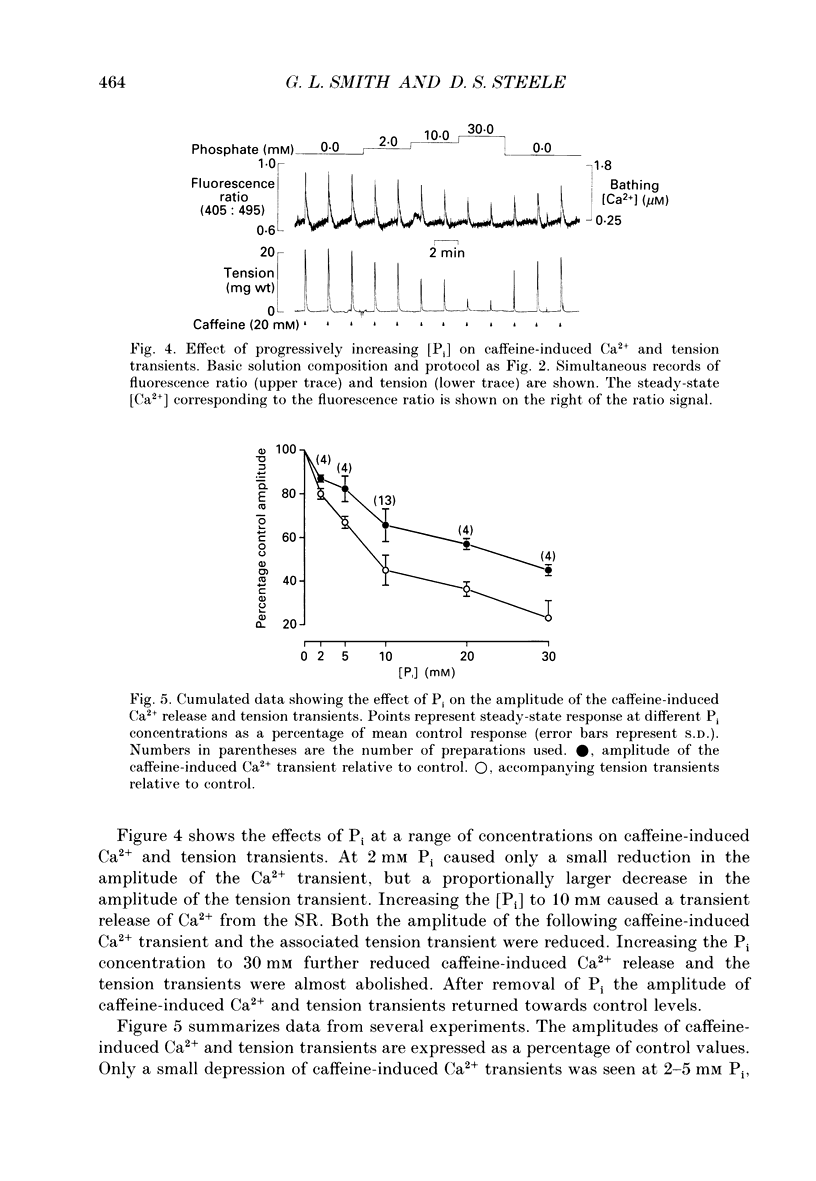
1. Measurements of [Ca2+] were made in saponin-permeabilized rat ventricular trabeculae using the fluorescent indicator Indo-1. Application of caffeine (20 mM) caused a transient rise in [Ca2+] within the preparation as a result of Ca2+ release from the sarcoplasmic reticulum (SR). The size of the caffeine-induced Ca2+ transient was related to the amount of Ca2+ accumulated by the SR prior to addition of caffeine. Caffeine-induced Ca2+ release was abolished by ryanodine (10 microM), an inhibitor of SR Ca2+ release. 2. At a bathing [Ca2+] of 0.2 microM, the amount of Ca2+ released from the SR on addition of caffeine was sufficient to generate a tension transient. Ca2+ and tension responses were stabilized by application of caffeine at regular intervals (2 min). Addition of 10 mM inorganic phosphate (Pi) induced a transient increase in [Ca2+] within the preparation due to a net release of Ca2+ from the SR. The amplitude of subsequent caffeine-induced Ca2+ transients were reduced to 65 +/- 7.5% (mean +/- S.D., n = 13) of control. In addition, the accompanying tension transient fell to 45 +/- 6.9% of control. Removal of Pi caused a transient decrease in the [Ca2+] within the preparation consistent with a net increase in Ca2+ uptake by the SR. Subsequent caffeine-induced Ca2+ and tension transients returned to control levels. 3. Inclusion of Pi (2-30 mM) in the perfusing solution decreased the size of caffeine-induced Ca2+ and tension transients in a dose-dependent manner. 4. Addition of 10 mM ADP caused a transient increase in [Ca2+] and depressed subsequent caffeine-induced Ca2+ transients to a greater extent than 10 mM Pi. Despite the reduction in Ca2+ release from the SR, tension responses were larger in the presence of 10 mM ADP than under control conditions. This is a consequence of an increase in Ca(2+)-activated force by ADP. 5. A decrease in the amplitude of caffeine-induced Ca2+ transients also occurred on changing from a solution containing 1 mM ADP and 10 mM Pi to a solution with 10 mM ADP and 1 mM Pi. This confirms the previous observation that ADP is more effective than Pi at reducing caffeine-induced Ca2+ released from the SR. 6. Spontaneous oscillations of [Ca2+] and tension occurred in the presence of 0.5 microM Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



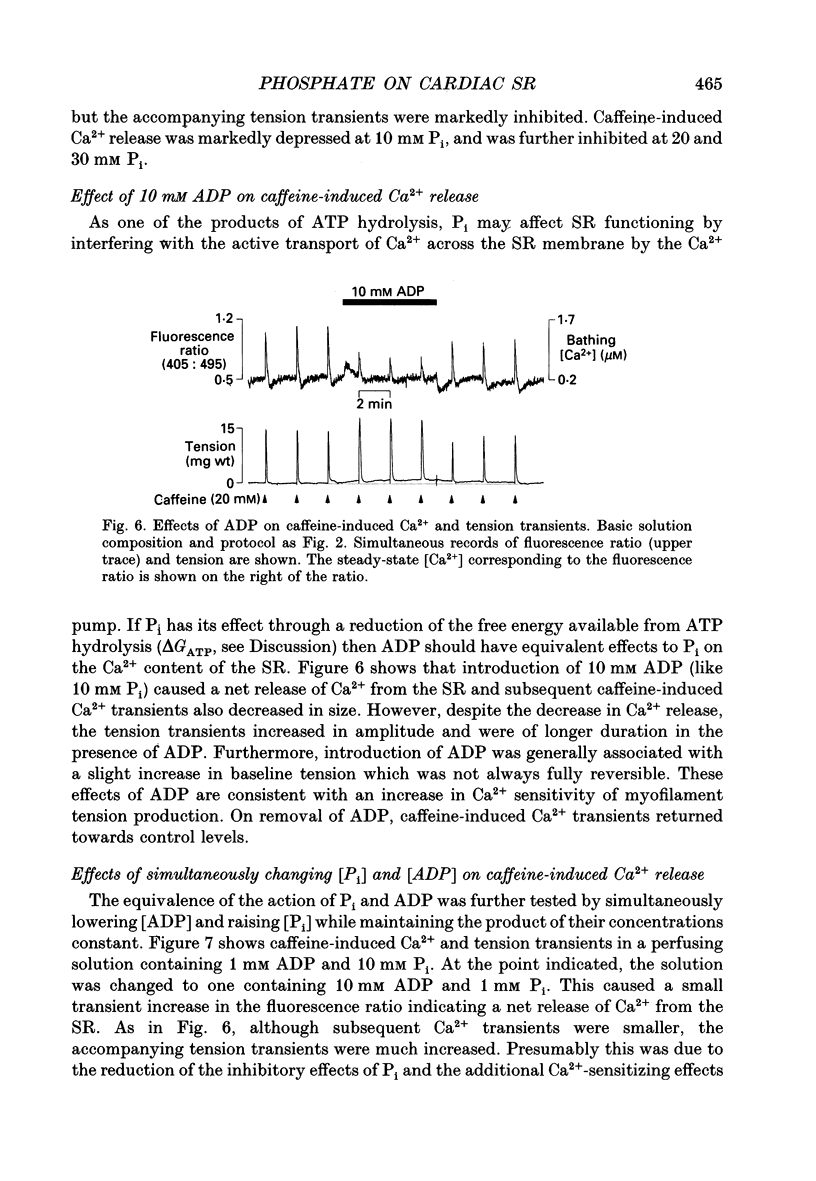
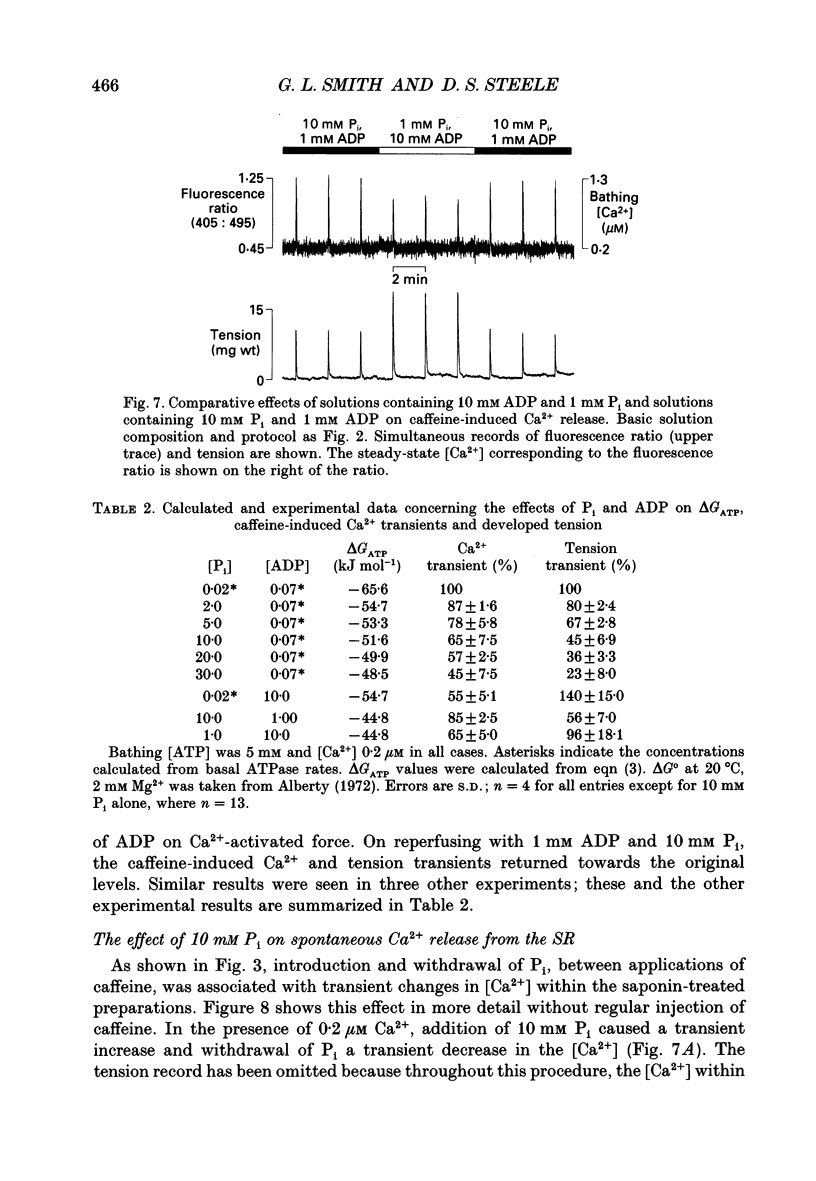
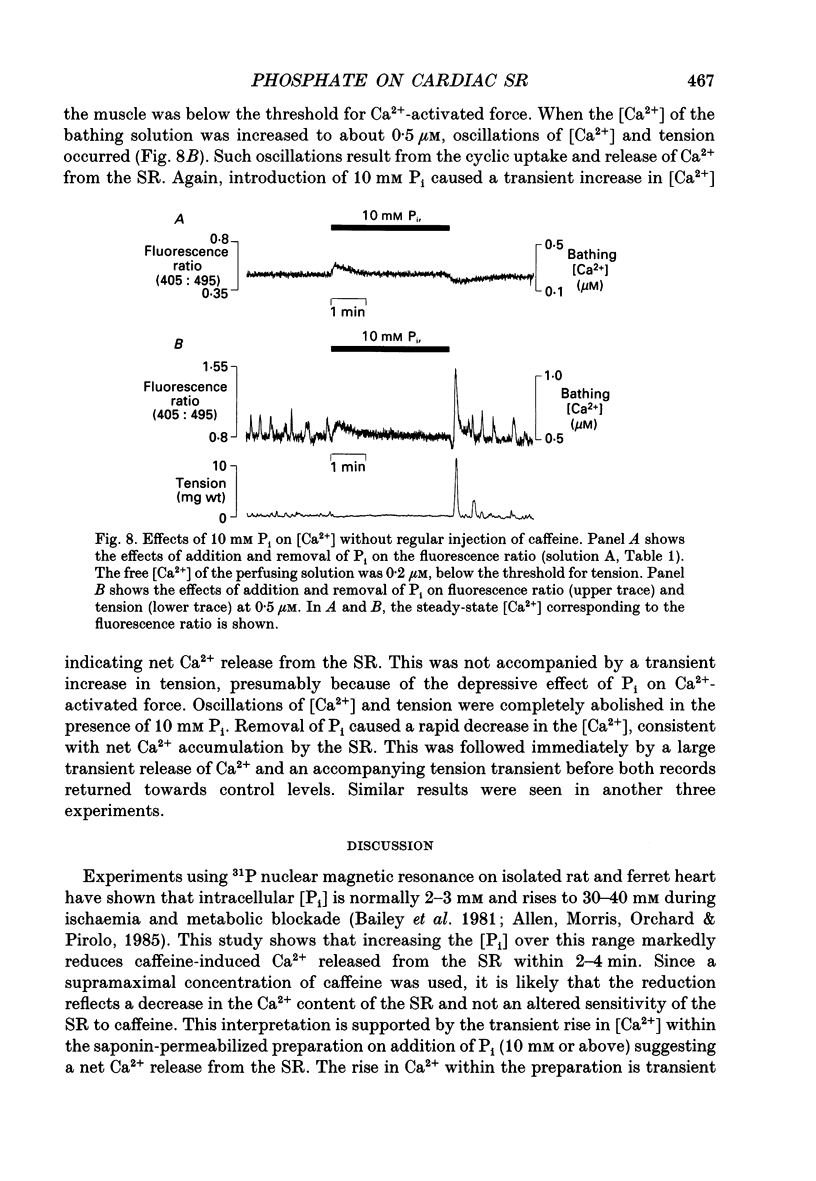






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Bailey I. A., Williams S. R., Radda G. K., Gadian D. G. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem J. 1981 Apr 15;196(1):171–178. doi: 10.1042/bj1960171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrossi M. C., Houser S. R., Bahinski A., Lakatta E. G. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res. 1987 Oct;61(4):498–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Lipford G. B. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 10;818(3):373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Haynes D. H. Mechanism of Ca2+ transport by Ca2+-Mg2+-ATPase pump: analysis of major states and pathways. Am J Physiol. 1983 Jan;244(1):G3–12. doi: 10.1152/ajpgi.1983.244.1.G3. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y. Subcellular origin of the oxalate- or inorganic phosphate-stimulated Ca2+ transport by smooth muscle microsomes: revisitation of the old problem by a new approach using saponin. Biochim Biophys Acta. 1985 Sep 25;819(1):148–152. doi: 10.1016/0005-2736(85)90206-8. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. The effects of repeated exposure to anoxia on intracellular calcium, glycogen and lactate in isolated ferret heart muscle. Pflugers Arch. 1988 Nov;413(1):83–89. doi: 10.1007/BF00581232. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Morgan J. P. Differential effects of reoxygenation on intracellular calcium and isometric tension. Pflugers Arch. 1987 Aug;409(4-5):448–453. doi: 10.1007/BF00583800. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Mermier P., Hasselbach W. The effect of calcium and phosphate on the biphasic calcium uptake by the sarcoplasmic reticulum. Z Naturforsch C. 1975 Nov-Dec;30(6):777–780. [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H. Reperfusion injury and its pharmacologic modification. Circulation. 1989 Oct;80(4):1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V. Stimulation of Ca2+ efflux from sarcoplasmic reticulum by preincubation with ATP and inorganic phosphate. Biochem J. 1987 Nov 1;247(3):497–504. doi: 10.1042/bj2470497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Allen D. G. Effects of metabolic blockade on intracellular calcium concentration in isolated ferret ventricular muscle. Circ Res. 1988 Jun;62(6):1223–1236. doi: 10.1161/01.res.62.6.1223. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suko J., Hellmann G., Winkler F. The reversal of the calcium pump of cardiac sarcoplasmic reticulum. Basic Res Cardiol. 1977 Mar-Jun;72(2-3):147–152. doi: 10.1007/BF01906353. [DOI] [PubMed] [Google Scholar]



