Abstract
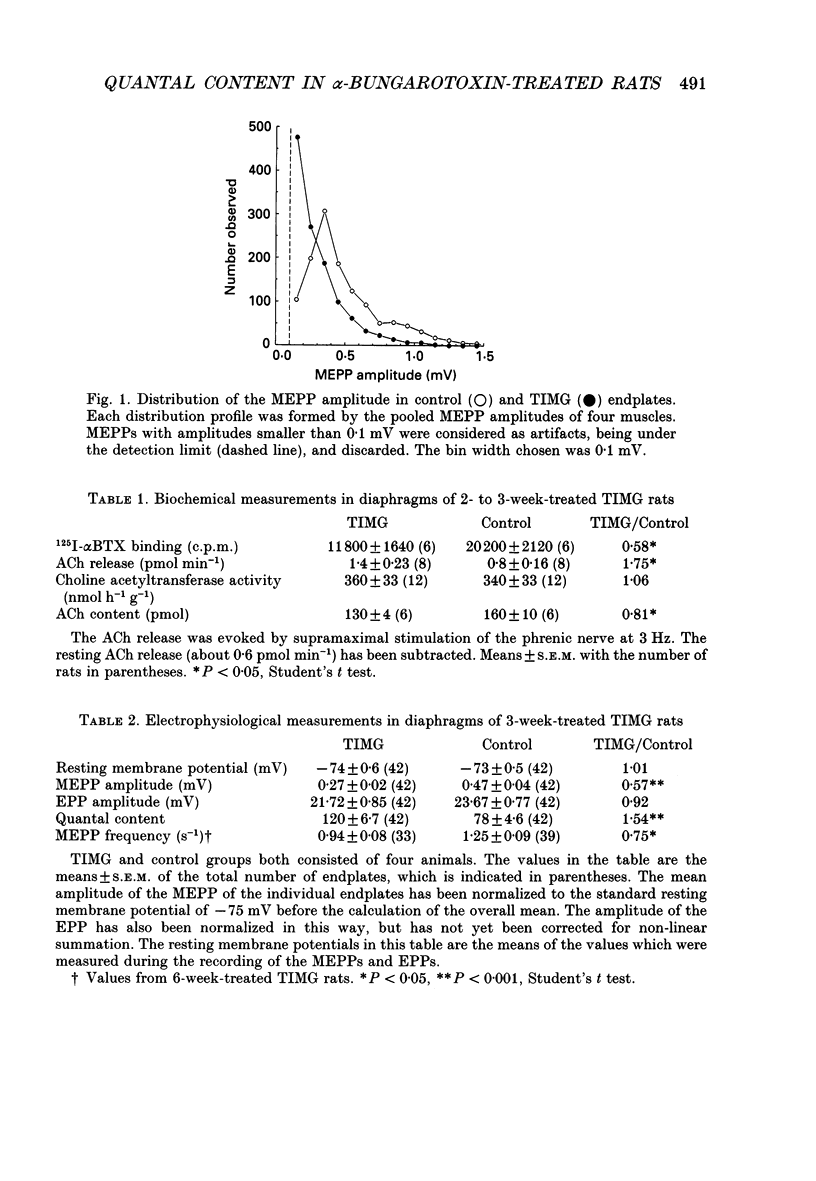
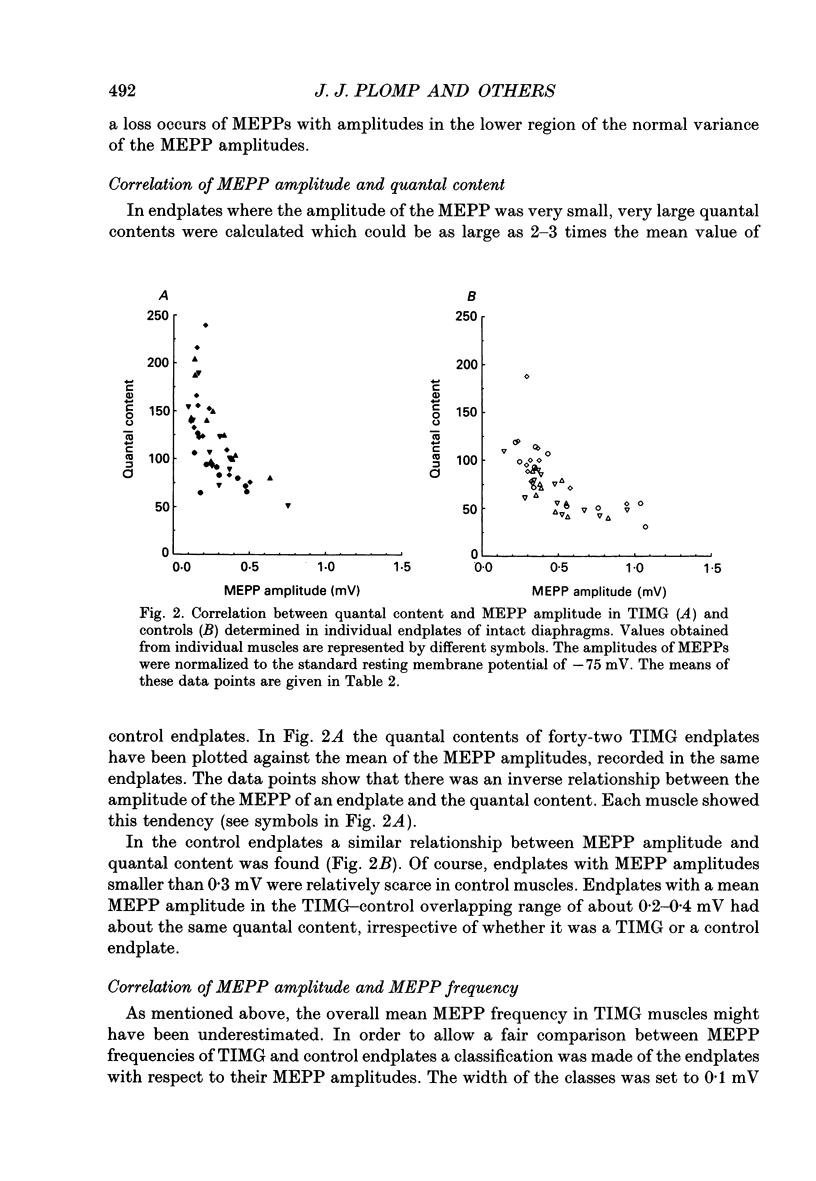
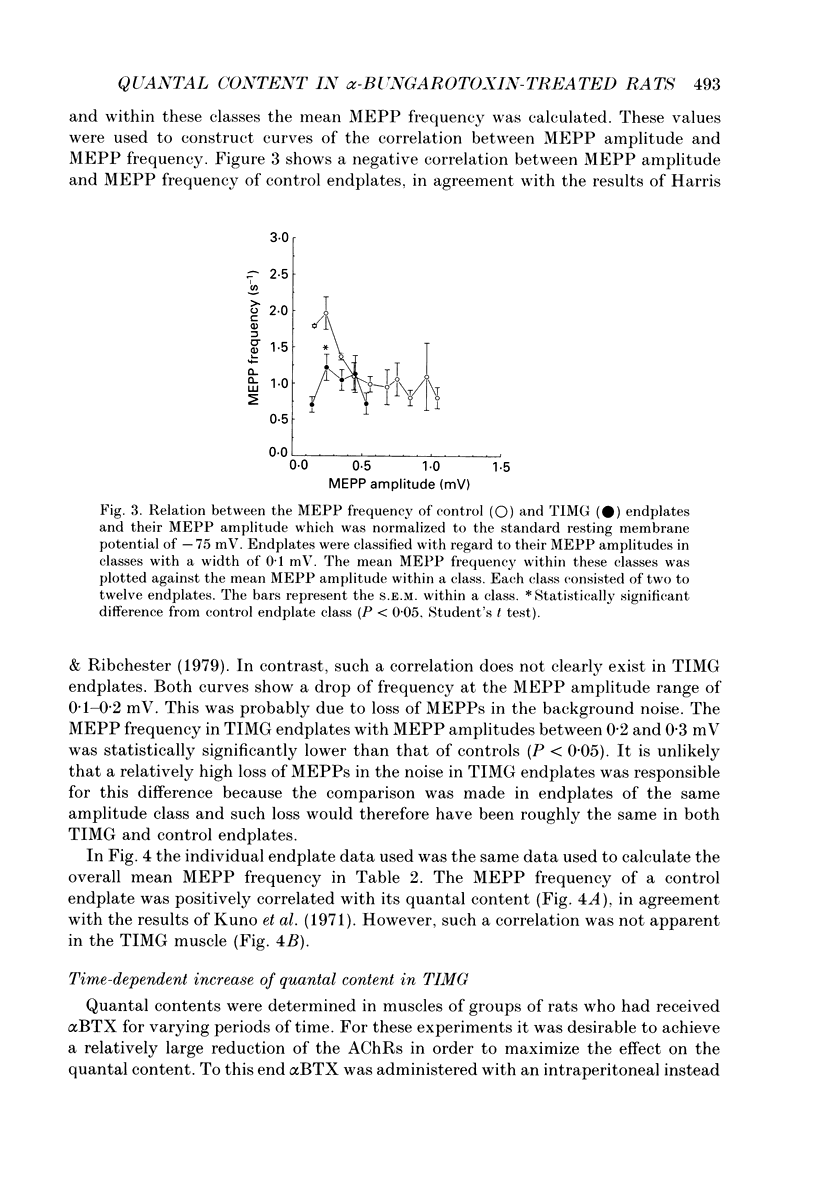
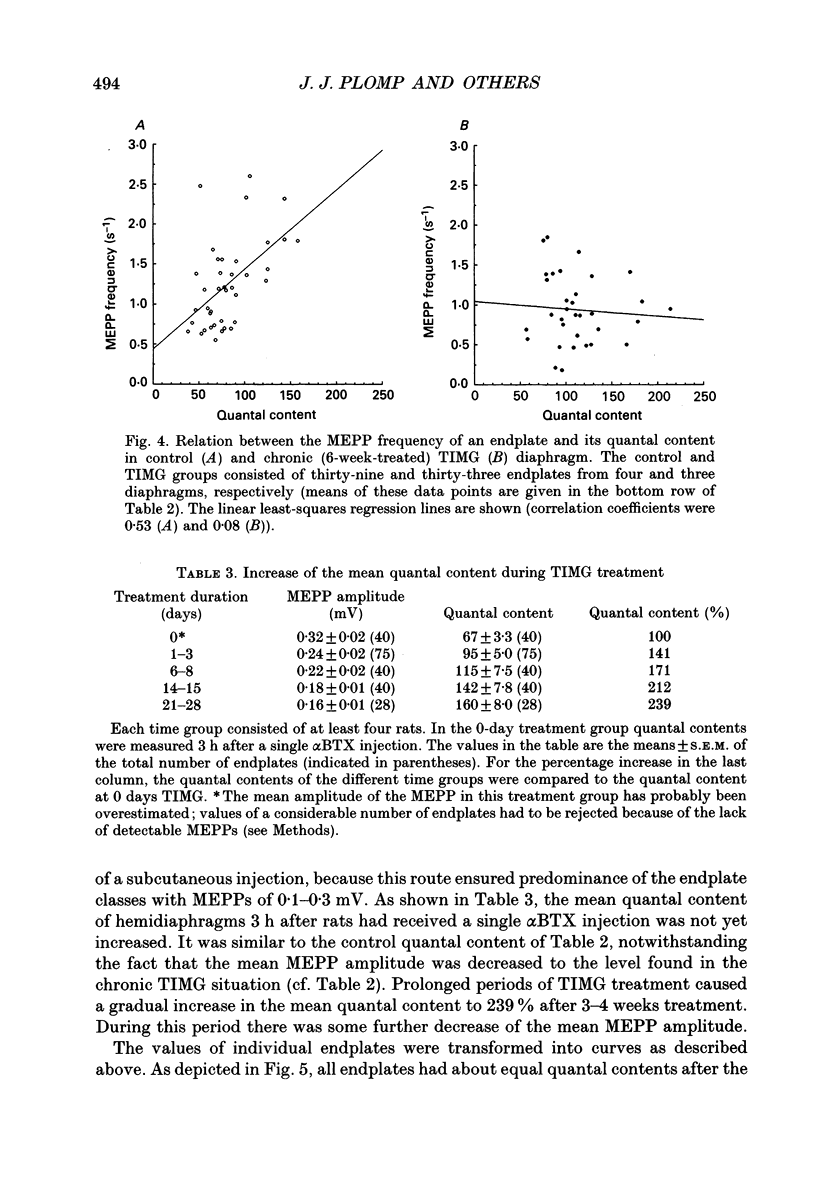
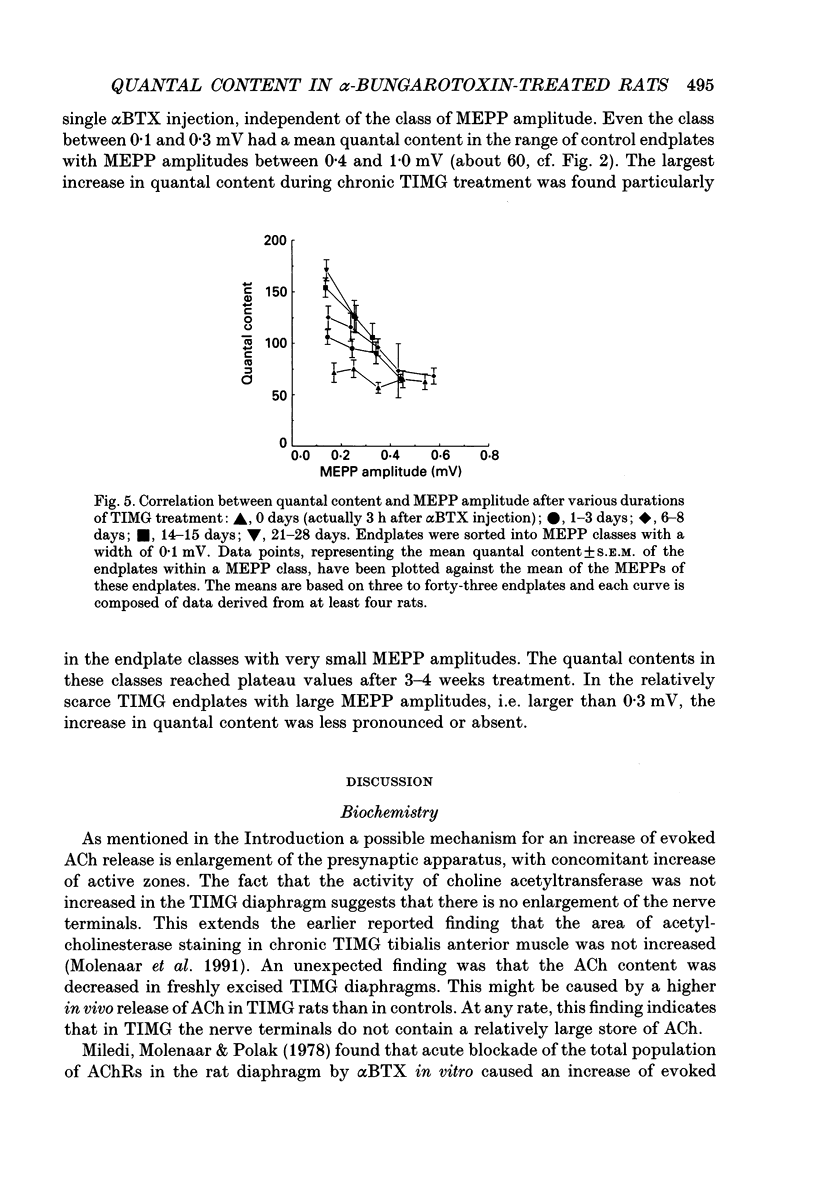
1. Rats were injected once every 48 h with alpha-bungarotoxin (alpha BTX) for periods up to 6 weeks. Injections caused weakness of facial muscles which lasted about 8 h. Hemidiaphragms were dissected for biochemical and electrophysiological measurements. 2. In muscles from animals treated for 2-3 weeks with toxin, the binding of 125I-alpha BTX was reduced to 58%, and the ACh content to 81% of control values. Choline acetyltransferase activity was unchanged. ACh release evoked by 3 Hz nerve stimulation was increased to 175% of control values. 3. The use of mu-conotoxin, which specifically blocks muscle action potentials, enabled the recording of full-sized endplate potentials (EPPs) and miniature endplate potentials (MEPPs) at normal muscle membrane potentials (-70 to -80 mV). The amplitude of MEPPs was decreased to 57% in muscles from animals treated for 3 weeks with alpha BTX. The mean of the quantal contents, calculated from the ratio of the corrected EPPs and the MEPPs, was increased to 154%. 4. Within individual muscles of both alpha BTX-treated and control rats, there was an inverse relationship between the quantal content of an endplate and its MEPP amplitude. 5. The MEPP frequency of endplates from control muscles was positively correlated with the quantal content. However, this correlation was not found in alpha BTX-affected muscles. 6. Three hours after a single injection of alpha BTX the amplitude of the MEPPs was reduced to about 60% of control values but no increase of the quantal content was found. During the first few days of alpha BTX treatment the quantal content gradually increased; it reached a plateau between 20 and 30 days. 7. The results suggest the existence of an adaptive mechanism, operating at individual endplates, in which retrograde signals at the motor nerve terminals modulate ACh release when neuromuscular transmission is endangered by block of acetylcholine receptors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstad J. A., Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pbarmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):373–390. [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Cruz L. J., Gray W. R., Olivera B. M., Zeikus R. D., Kerr L., Yoshikami D., Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985 Aug 5;260(16):9280–9288. [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio F., Fesce R., Cereser S., Favaro G., Fiori M. G. Spontaneous and nerve-evoked quantal transmission in regenerated motor terminals. Cell Biol Int Rep. 1989 Dec;13(12):1119–1126. doi: 10.1016/0309-1651(89)90025-8. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Miledi R., Molenaar P. C., Vincent A., Polak R. L., van Gelder M., Davis J. N. Acetylcholine in human muscle. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):475–480. doi: 10.1098/rspb.1976.0025. [DOI] [PubMed] [Google Scholar]
- Ito Y., Miledi R., Vincent A., Newsom-Davis J. Acetylcholine receptors and end-plate electrophysiology in myasthenia gravis. Brain. 1978 Jun;101(2):345–368. doi: 10.1093/brain/101.2.345. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980 Dec;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Newsom-Davis J., Polak R. L., Vincent A. Choline acetyltransferase in skeletal muscle from patients with myasthenia gravis. J Neurochem. 1981 Nov;37(5):1081–1088. doi: 10.1111/j.1471-4159.1981.tb04657.x. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Oen B. S., Plomp J. J., Van Kempen G. T., Jennekens F. G., Hesselmans L. F. A non-immunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol. 1991 Apr 10;196(1):93–101. doi: 10.1016/0014-2999(91)90413-k. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L., Miledi R., Alema S., Vincent A., Newsom-Davis J. Acetylcholine in intercostal muscle from myasthenia gravis patients and in rat diaphragm after blockade of acetylcholine receptors. Prog Brain Res. 1979;49:449–458. doi: 10.1016/S0079-6123(08)64657-9. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., Cruz L. J. Diversity of Conus neuropeptides. Science. 1990 Jul 20;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Sun Y. A., Poo M. M. Evoked release of acetylcholine from the growing embryonic neuron. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2540–2544. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori M., Sakato S., Okumura S. Presynaptic function modified by acetylcholine-receptor interaction in experimental autoimmune myasthenia gravis. J Neurol Sci. 1984 Nov-Dec;66(2-3):245–253. doi: 10.1016/0022-510x(84)90013-3. [DOI] [PubMed] [Google Scholar]
- Vincent A. Immunology of acetylcholine receptors in relation to myasthenia gravis. Physiol Rev. 1980 Jul;60(3):756–824. doi: 10.1152/physrev.1980.60.3.756. [DOI] [PubMed] [Google Scholar]
- Xie Z. P., Poo M. M. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y., Abe T., Satake M. Mu-conotoxins share a common binding site with tetrodotoxin/saxitoxin on eel electroplax Na channels. J Neurosci. 1987 May;7(5):1498–1502. doi: 10.1523/JNEUROSCI.07-05-01498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


