Abstract
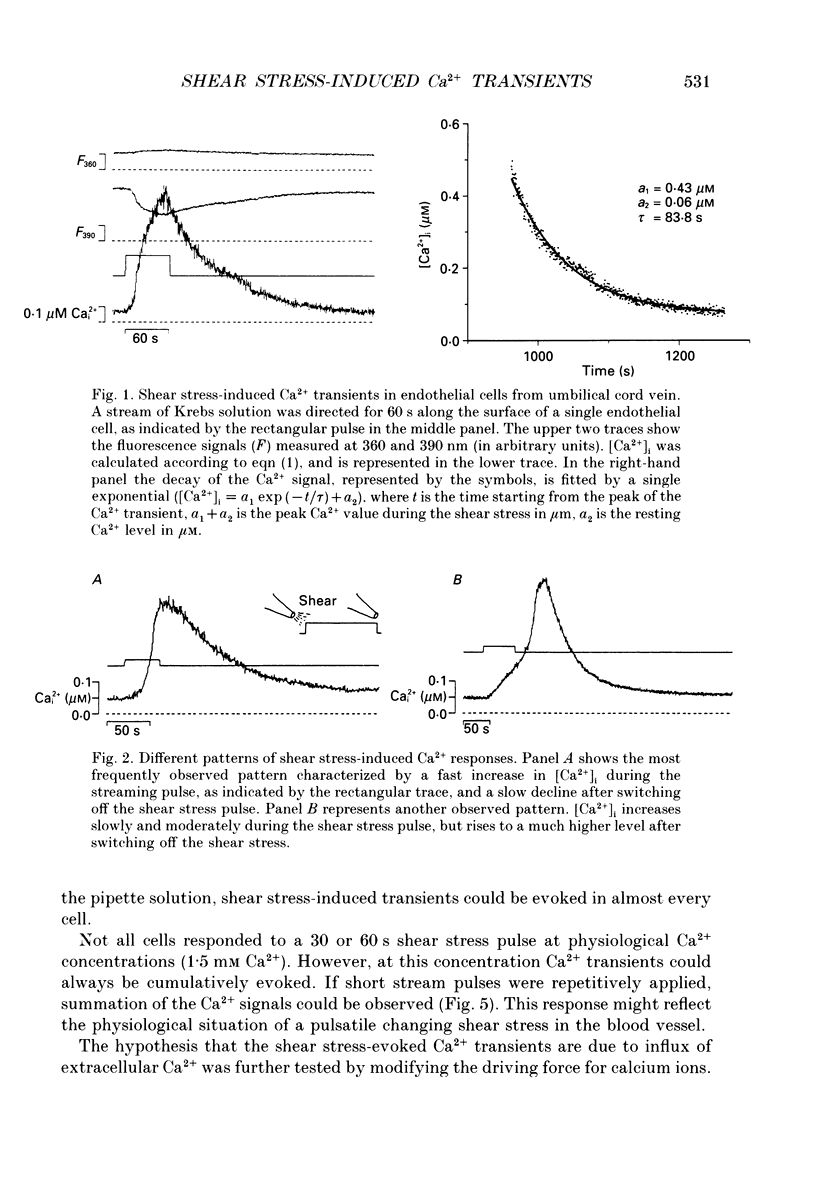
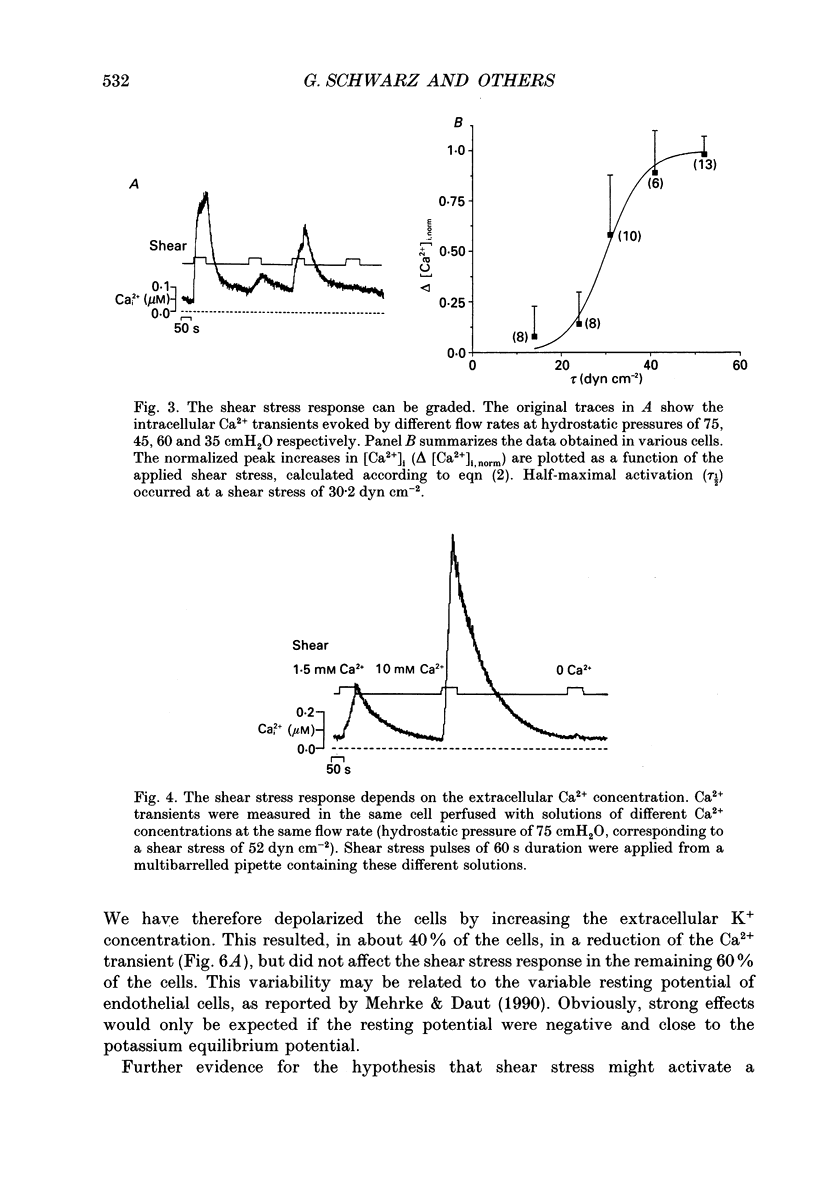
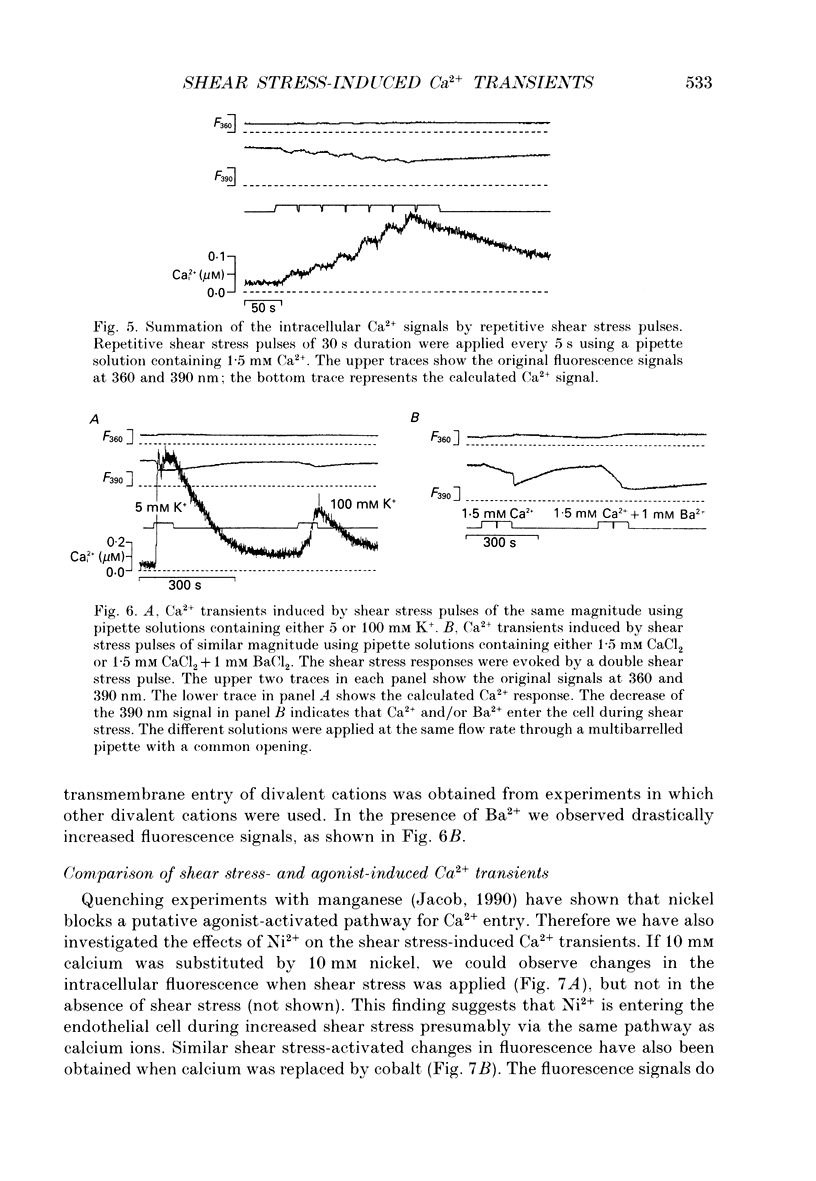
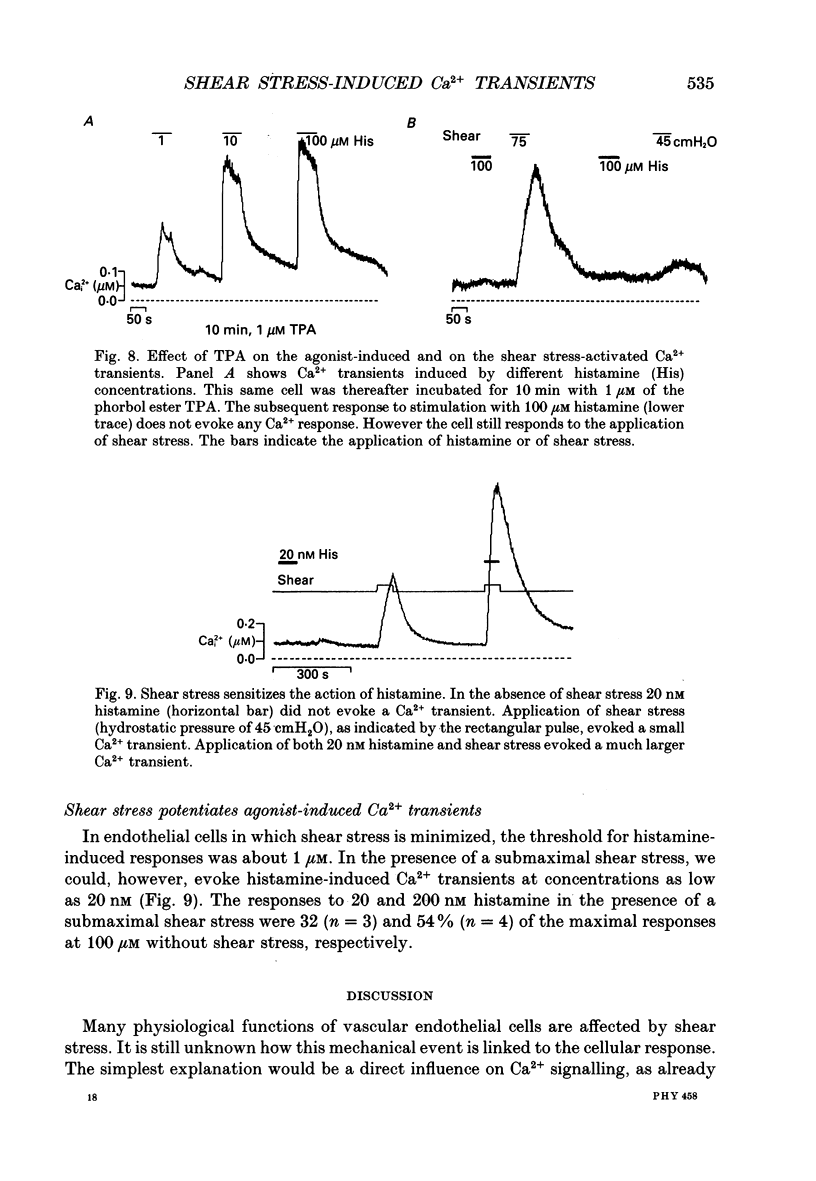
1. Changes of the free cytosolic Ca2+ concentration induced by shear stress were measured in Fura-2 acetoxymethyl ester-loaded endothelial cells from human umbilical cord veins. 2. We were able to induce Ca2+ transients in almost every cell by blowing a stream of physiological solution onto a single endothelial cell thereby inducing shear stress between 0 and 50 dyn cm-2. The Ca2+ response could be graded by varying the shear stress, and reached a half-maximal value at a shear stress of 30 dyn cm-2. 3. The shear stress responses critically depended on the extracellular Ca2+ concentration and were absent in a Ca(2+)-free solution. Repetitive application of short pulses of shear stress induced cumulative effects because of the slow decay of the shear stress Ca2+ responses (time constants 82.3 +/- 17.8 s from twenty-five cells). Application of a depolarizing high potassium solution to reduce the driving force for Ca2+ entry decreased the Ca2+ transients in some of the cells. 4. Application of shear stress in the presence of other divalent cations, such as nickel, cobalt or barium, always produced substantial changes in the ratio of the 390/360 nm fluorescence signal, indicating influx of these cations and subsequent quenching of the Fura-2 fluorescence. 5. Shear stress responses in the presence of 10 mM Ca2+ were completely blocked by application of 1 mM La3+. 6. Incubation of the cells with the phorbol ester 12-O-tetradecanoyl phorbol-13-acetate (TPA) did not alter the shear stress response, but completely blocked histamine-induced Ca2+ transients. 7. Small submaximal shear stress potentiated the Ca2+ transients induced by histamine. 8. We conclude that shear stress-dependent Ca2+ signals are induced by an influx of calcium that is not modulated via protein kinase C and not activated by membrane depolarization. The influx pathway is also permeable to divalent cations such as Ni2+, Co2+ and Ba2+, but is blocked by La3+.
Full text
PDF



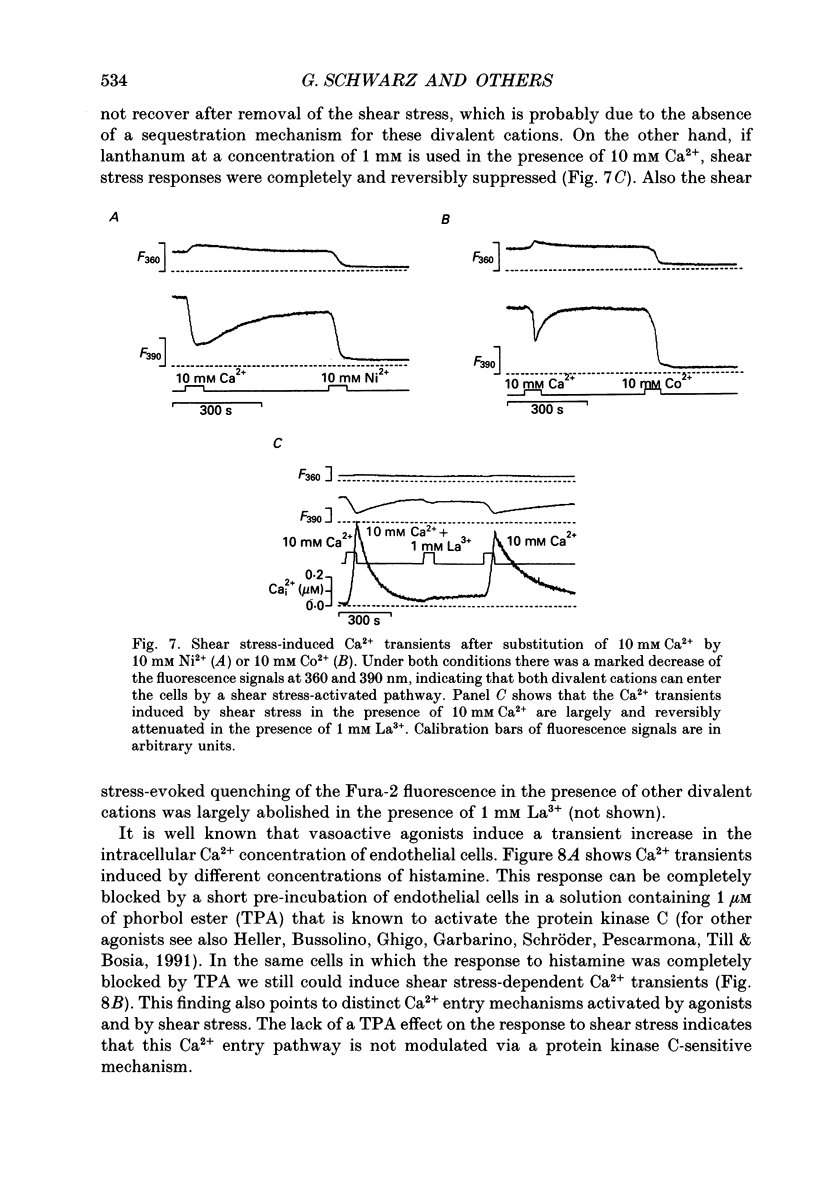



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando J., Komatsuda T., Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol. 1988 Sep;24(9):871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Coade S. B., Pearson J. D. Metabolism of adenine nucleotides in human blood. Circ Res. 1989 Sep;65(3):531–537. doi: 10.1161/01.res.65.3.531. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Dewey C. F., Jr, Bussolari S. R., Gordon E. J., Gimbrone M. A., Jr Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984 Apr;73(4):1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey C. F., Jr, Bussolari S. R., Gimbrone M. A., Jr, Davies P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981 Aug;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Diamond S. L., Eskin S. G., McIntire L. V. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989 Mar 17;243(4897):1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Goligorsky M. S. Mechanical stimulation induces Ca2+i transients and membrane depolarization in cultured endothelial cells. Effects on Ca2+i in co-perfused smooth muscle cells. FEBS Lett. 1988 Nov 21;240(1-2):59–64. doi: 10.1016/0014-5793(88)80340-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heller R., Bussolino F., Ghigo D., Garbarino G., Schröder H., Pescarmona G., Till U., Bosia A. Protein kinase C and cyclic AMP modulate thrombin-induced platelet-activating factor synthesis in human endothelial cells. Biochim Biophys Acta. 1991 Jun 7;1093(1):55–64. doi: 10.1016/0167-4889(91)90138-n. [DOI] [PubMed] [Google Scholar]
- Ives C. L., Eskin S. G., McIntire L. V. Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cell Dev Biol. 1986 Sep;22(9):500–507. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 1990 Jul;416(5):609–611. doi: 10.1007/BF00382697. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]


