Abstract
1. Identified neurones of the suboesophageal ganglia of Helix aspersa were loaded with tetramethylammonium (TMA+). Experimentally induced changes in cell water volume and membrane potential were measured continuously by monitoring changes in intracellular [TMA+] using ion-sensitive double-barrelled microelectrodes. The technique allowed measurements of cell water volume changes of less than 5%. 2. Exposure to hyperosmotic (up to +24%) or hyposmotic (up to about -10%) solutions caused reversible decreases and increases in cell water volume respectively, which agreed with near-ideal osmometric behaviour. Upon exposure to hyposmotic solutions whose osmolality was decreased by 30-40%, the cell water volume increased to maximum values below those expected for ideal osmometric behaviour and exhibited partial regulatory volume decrease. 3. The sodium pump was inhibited in twenty identified neurones by sustained exposure to 1 mM ouabain. In every case ouabain caused cell membrane depolarization, as expected for inhibition of an electrogenic sodium pump. 4. Upon pump inhibition most cells (n = 14) shrank by up to 13% of their initial water volume. In five of these cells, shrinkage was preceded by one or more short-lived swelling phases. In two other neurones short-lived swelling was followed by cell volume recovery without appreciable shrinkage. In four out of the twenty cells, there were no measurable volume changes. 5. The lack of an initial swelling phase in the cells that shrank, as well as the absence of detectable volume changes in some of the neurones, was not due to loss of ion-selective electrode sensitivity since predictable changes in cell volume elicited by osmotic challenges were monitored in the same cells. 6. It is concluded that neurones can be endowed with ouabain-insensitive mechanisms of volume control, whose activation following Na+ pump inhibition prevents them from short-term swelling and lysis.
Full text
PDF


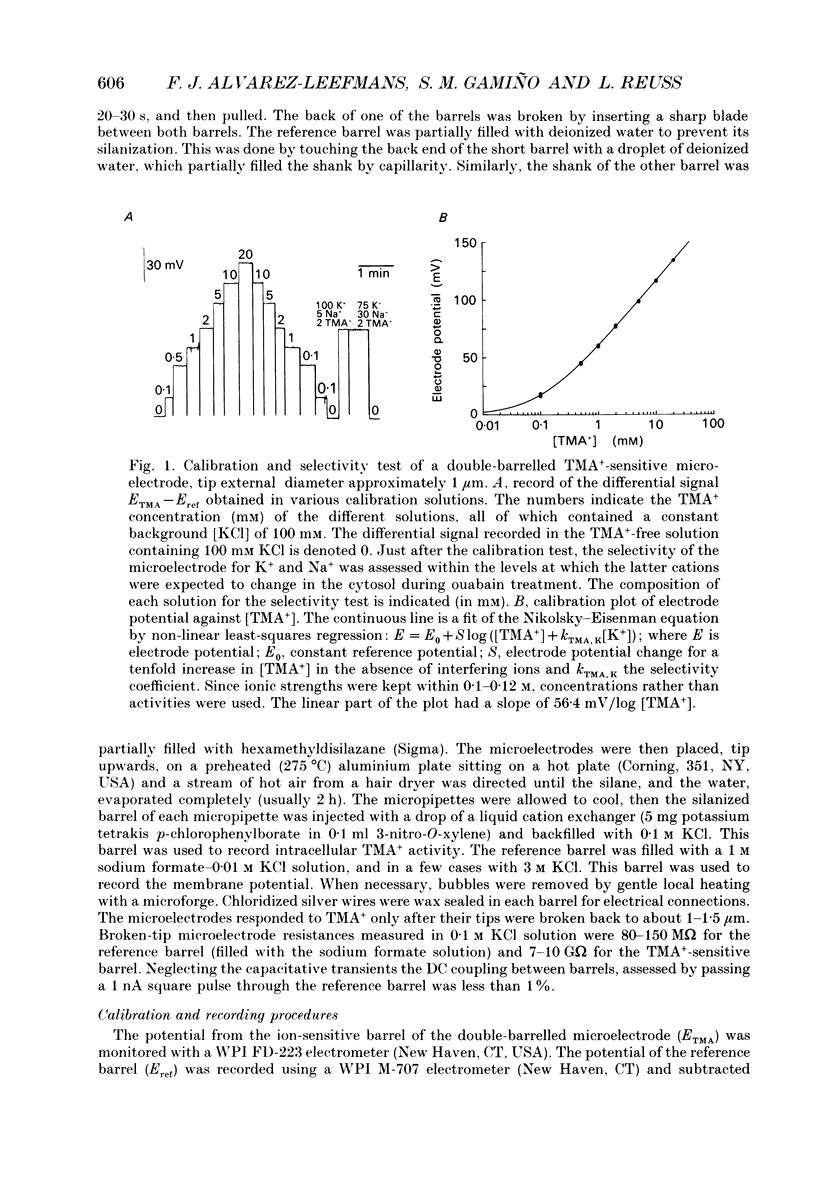

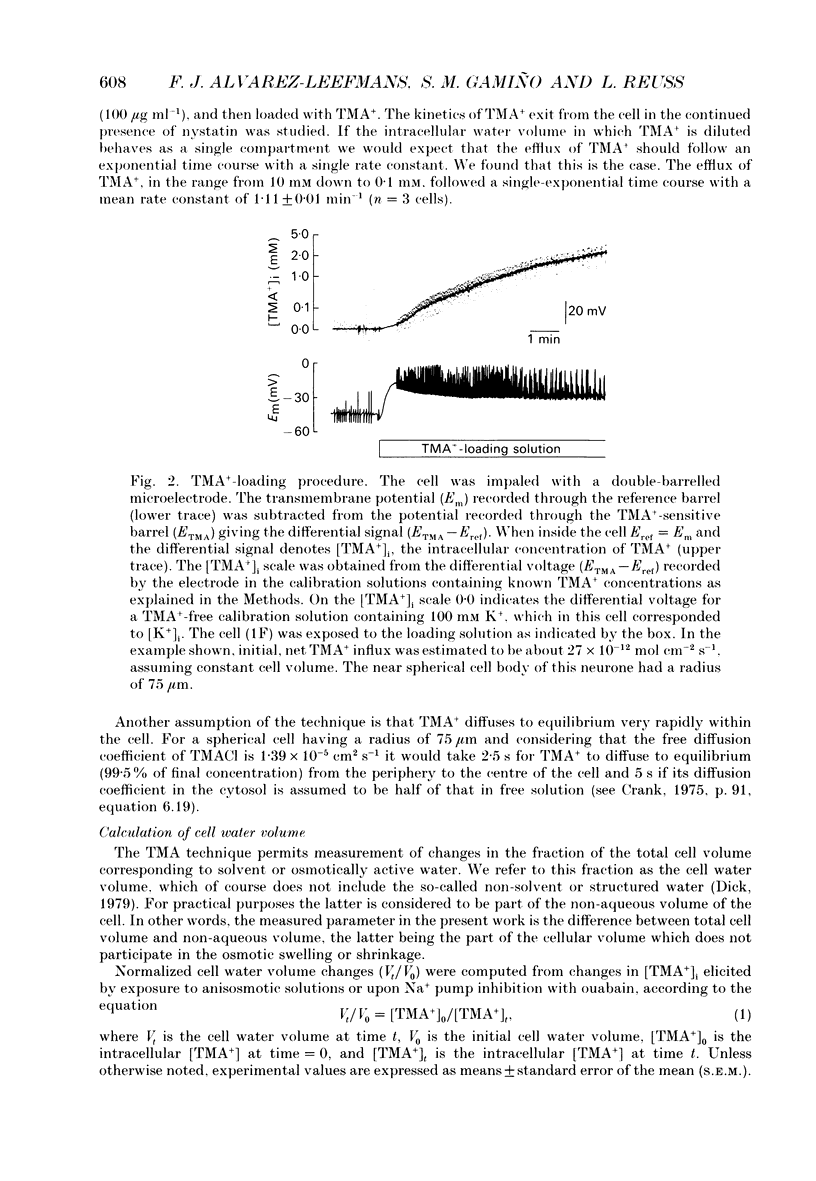

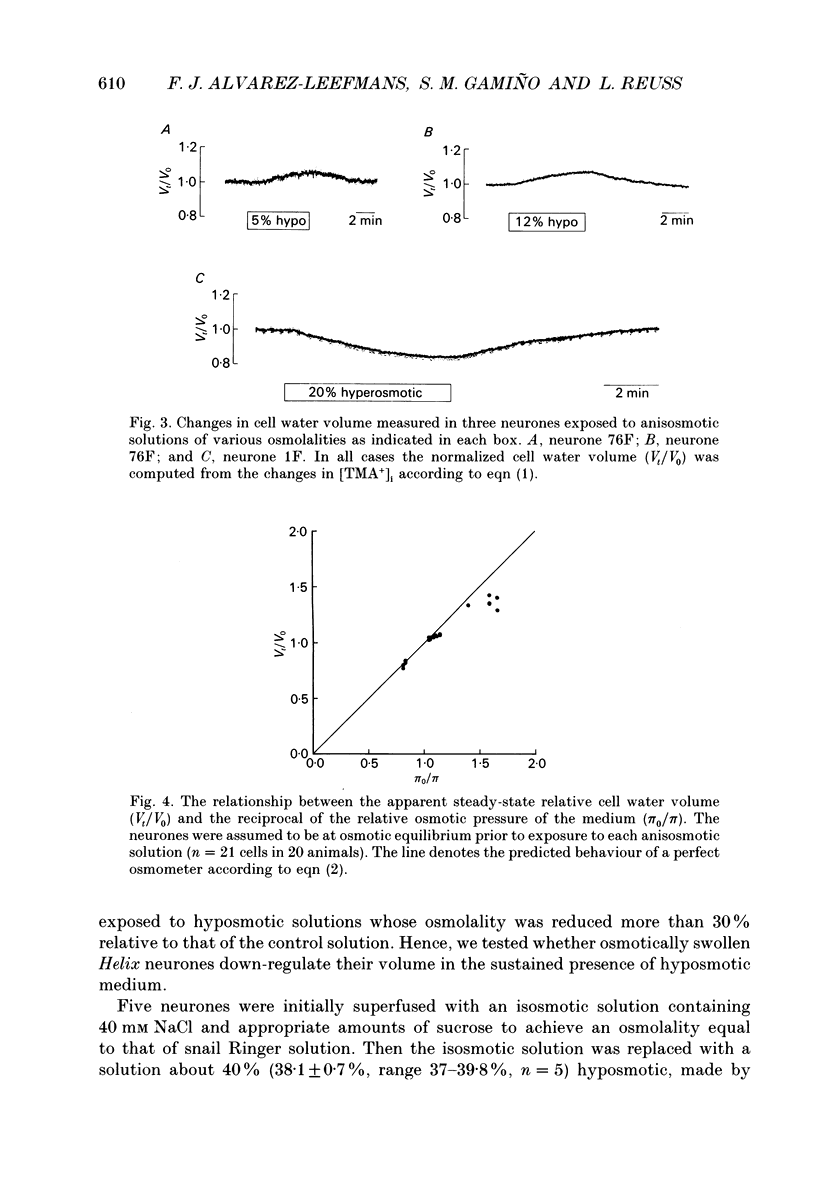

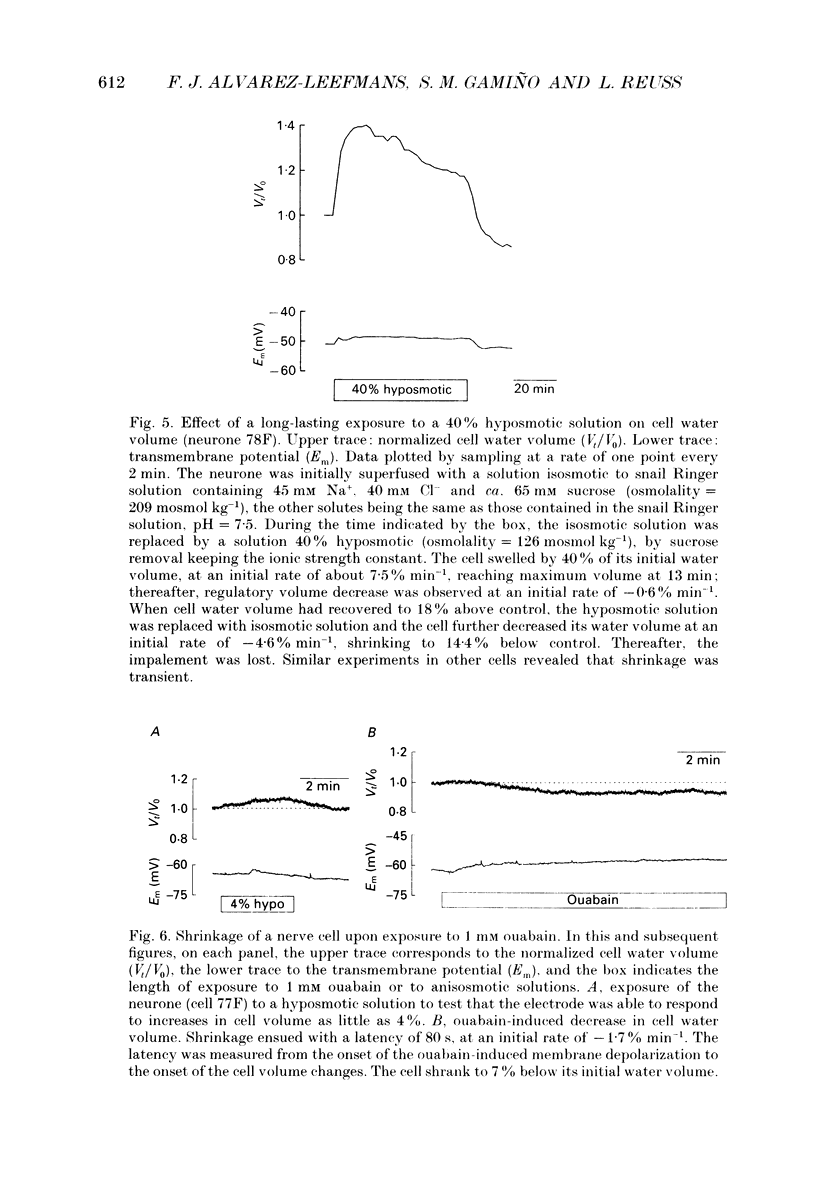
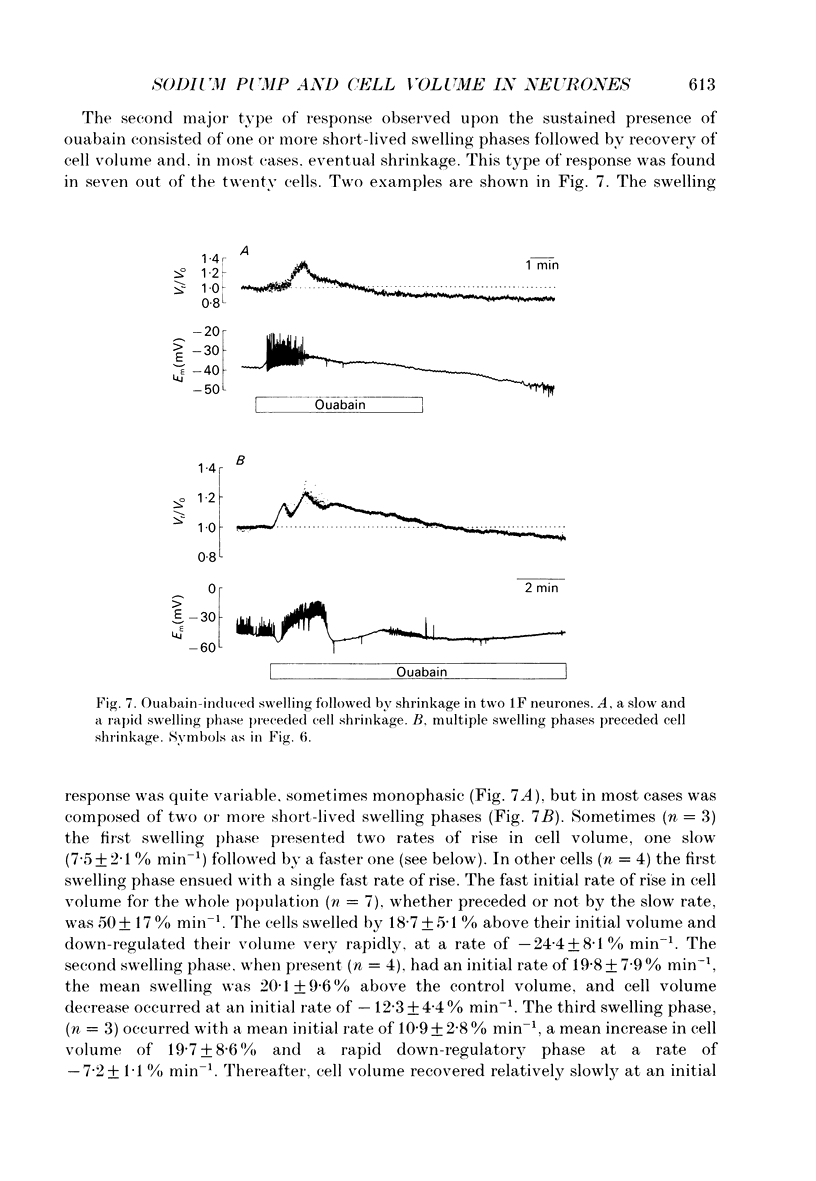
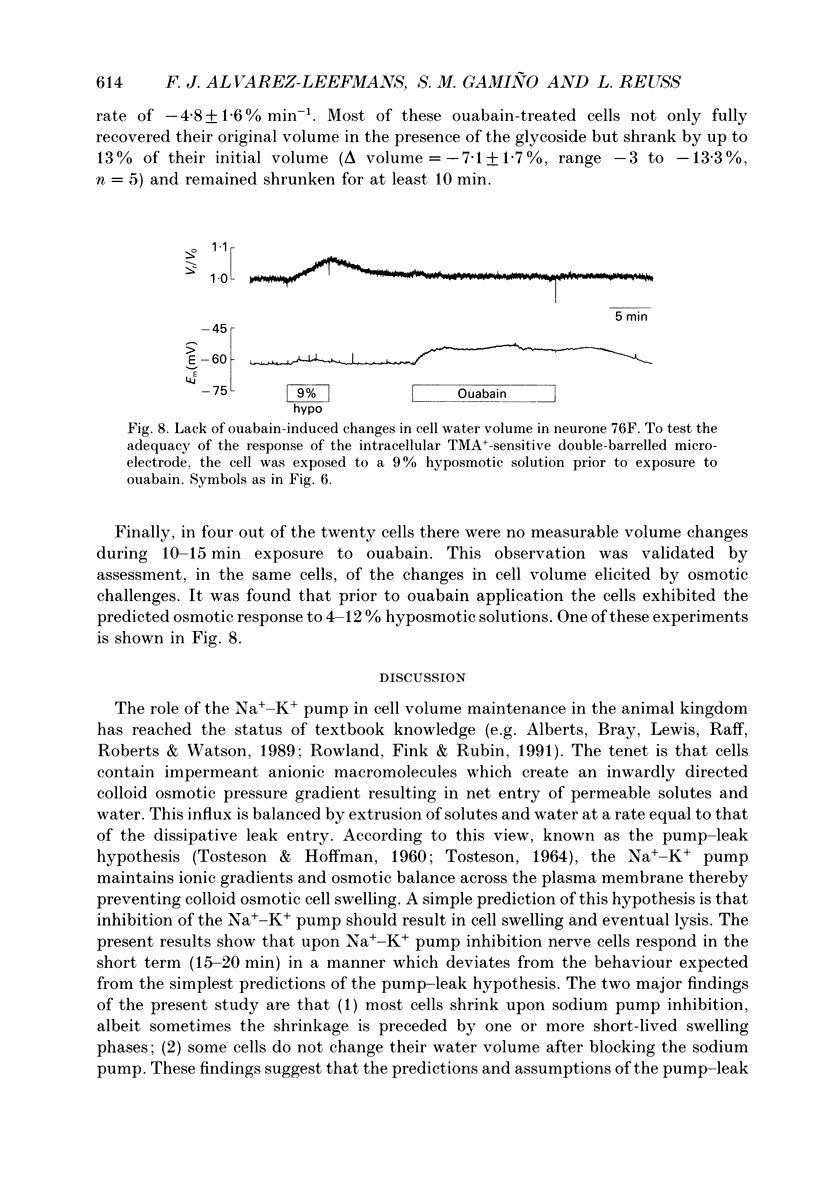





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Rink T. J. Intracellular free magnesium in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1984 Sep;354:303–317. doi: 10.1113/jphysiol.1984.sp015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P. Cell volume regulation in the nervous system. Ren Physiol Biochem. 1988 May-Oct;11(3-5):142–157. doi: 10.1159/000173159. [DOI] [PubMed] [Google Scholar]
- Barolet A. W., Andrews R., Morris M. E. Calibration of ion-selective microelectrodes: flow-system and analysis program for the IBM PC. J Neurosci Methods. 1989 Dec;30(3):263–266. doi: 10.1016/0165-0270(89)90137-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989 Aug;257(2 Pt 1):C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Cotton C. U., Weinstein A. M., Reuss L. Osmotic water permeability of Necturus gallbladder epithelium. J Gen Physiol. 1989 Apr;93(4):649–679. doi: 10.1085/jgp.93.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. Intracellular Na+ and Ca2+ in leech Retzius neurones during inhibition of the Na+-K+ pump. Pflugers Arch. 1983 May;397(3):195–201. doi: 10.1007/BF00584357. [DOI] [PubMed] [Google Scholar]
- Falke L. C., Misler S. Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3919–3923. doi: 10.1073/pnas.86.10.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Jensen P. K., Fisher R. S., Spring K. R. Feedback inhibition of NaCl entry in Necturus gallbladder epithelial cells. J Membr Biol. 1984;82(1):95–104. doi: 10.1007/BF01870736. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991 Jul;66(1):103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Kempski O., Staub F., von Rosen F., Zimmer M., Neu A., Baethmann A. Molecular mechanisms of glial swelling in vitro. Neurochem Pathol. 1988 Jul-Dec;9:109–125. doi: 10.1007/BF03160357. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Lambert J. D., Gayton R. J., Loker J. E., Walker R. J. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A Comp Physiol. 1975 Jan 1;50(1A):1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Leblond J., Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989 Jul;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- Lohr J. W., Grantham J. J. Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J Clin Invest. 1986 Nov;78(5):1165–1172. doi: 10.1172/JCI112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Schlue W. R. Effects of ouabain on intracellular ion activities of sensory neurons of the leech central nervous system. J Neurophysiol. 1991 Mar;65(3):736–746. doi: 10.1152/jn.1991.65.3.736. [DOI] [PubMed] [Google Scholar]
- Serve G., Endres W., Grafe P. Continuous electrophysiological measurements of changes in cell volume of motoneurons in the isolated frog spinal cord. Pflugers Arch. 1988 Apr;411(4):410–415. doi: 10.1007/BF00587720. [DOI] [PubMed] [Google Scholar]
- Strange K. Ouabain-induced cell swelling in rabbit cortical collecting tubule: NaCl transport by principal cells. J Membr Biol. 1989 Mar;107(3):249–261. doi: 10.1007/BF01871940. [DOI] [PubMed] [Google Scholar]
- Strange K. Volume regulation following Na+ pump inhibition in CCT principal cells: apical K+ loss. Am J Physiol. 1990 Mar;258(3 Pt 2):F732–F740. doi: 10.1152/ajprenal.1990.258.3.F732. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török T. L. Neurochemical transmission and the sodium-pump. Prog Neurobiol. 1989;32(1):11–76. doi: 10.1016/0301-0082(89)90027-0. [DOI] [PubMed] [Google Scholar]


