Abstract
Background
Chronic obstructive pulmonary disease (COPD) is characterised by partially reversible airflow limitation. Many patients have little reversibility to short acting bronchodilators, but long acting bronchodilators are frequently advocated.
Objectives
To determine the effectiveness of long acting beta‐2 adrenoceptor agonists (LABAs) in COPD patients demonstrating poor reversibility to short‐acting bronchodilators.
Search methods
The Cochrane Airways Group Specialised Register was searched ('all years' to 2005) along with the reference lists from identified randomised controlled trials (RCTs).
Selection criteria
All RCTs comparing inhaled LABAs (salmeterol or formoterol) with placebo in the treatment of patients with stable, poorly reversible COPD. Studies were a minimum of four weeks in duration.
Data collection and analysis
Two authors independently performed data extraction and study quality assessment. If we required additional data, we contacted authors and pharmaceutical companies sponsoring the identified RCTs.
Main results
Twenty‐three published and unpublished studies (6061 participants) were included in the review. There was a significant change in forced expiratory volume in 1 second (FEV1) in favour of salmeterol 50 mcg twice daily (BID) of 51 mls (95% confidence intervals (CI) 32 to 70), end of study morning peak expiratory flow (PEF) 14.89 L/min (95% CI 10.86 to 18.91). Supplemental short‐acting bronchodilator usage was reduced by just under one puff per day. There were significant differences in the total, activity and impact domain scores of the St George's respiratory questionnaire in favour of salmeterol 50 mcg BID. Findings from other health status measurements and symptom scores were conflicting. There was no significant difference in exercise tolerance. The number of participants experiencing exacerbations was significantly reduced with salmeterol 50 mcg treatment compared with placebo (numbers needed to treat to benefit 24).
Authors' conclusions
This review shows that the treatment of patients with COPD with salmeterol 50 mcg produces modest increases in lung function. There were varying effects for other important outcomes such as health related quality of life or reduction in symptoms. However, there was a consistent reduction in exacerbations which may help people with COPD who suffer frequent deterioration of symptoms prompting healthcare utilisation. The strength of evidence for the use of salmeterol 100 mcg, formoterol 12 mcg, 18 mcg, 24 mcg was insufficient to provide clear indications for practice.
Keywords: Humans; Adrenergic beta‐Agonists; Adrenergic beta‐Agonists/therapeutic use; Albuterol; Albuterol/analogs & derivatives; Albuterol/therapeutic use; Bronchodilator Agents; Bronchodilator Agents/therapeutic use; Ethanolamines; Ethanolamines/therapeutic use; Formoterol Fumarate; Lung Diseases, Obstructive; Lung Diseases, Obstructive/drug therapy; Randomized Controlled Trials as Topic; Salmeterol Xinafoate
Plain language summary
Long‐acting beta2‐agonists for poorly reversible chronic obstructive pulmonary disease
This review aims to determine the effectiveness of long‐acting beta‐agonists, salmeterol or formoterol, in the treatment of COPD (emphysema/chronic bronchitis). These drugs improve airflow in the lungs, and enable people with COPD to get on with their daily activities. Twenty‐four studies (6061 participants) reported the effects of LABAs in people with COPD. People taking salmeterol 50 mcg daily do have fewer exacerbations than those on placebo, and some improvement in lung function and certain quality of life scores. The findings were not consistent enough to support a general recommendation for the use of these drugs in the group of people with COPD with minimal variation in their lung function, although there is some evidence of improvement in important outcomes and these findings require further exploration in additional trials.
Background
COPD is characterised by airways obstruction that is not fully reversible. Even though many patients have a poor response to short acting bronchodilators, long‐acting beta‐2 adrenoceptor agonists are increasingly recommended for administration to all patients with chronic obstructive pulmonary disease (COPD) as regular long term therapy. These drugs are beneficial as additive maintenance therapy in the reversible airflow obstruction which occurs in asthma (Ni Chroinin 2005). It is important to establish whether this class of drugs has any benefit in COPD, particularly as they are relatively expensive to the health system. There are clear cost implications for healthcare budgets of prescribing long‐acting beta agonists and thus it is important to evaluate if there are any clear clinical benefits of use in terms of lung function, exercise tolerance, quality of life, exacerbations and rescue short acting beta‐agonist use. We therefore aim to review the literature to determine the evidence of the efficacy of this medication in patients with COPD who have a poor spirometric response to short acting bronchodilator.
Objectives
To evaluate the effects of regular LABAs, administered via inhalation over at least four weeks to adults with COPD on:
Lung function, as measured by forced expiratory volume in 1 second (FEV1), Peak expiratory flow (PEF);
Exercise tolerance;
Quality of life;
Dyspnoea and symptoms;
Incidence of exacerbations;
Need for rescue salbutamol.
Methods
Criteria for considering studies for this review
Types of studies
Published, unpublished and ongoing RCTs, including parallel group and crossover design trials.
Types of participants
Participants with stable COPD without asthma as characterised by:
no recent infections, exacerbations, hospitalisations in the past month; and
FEV1 75% or less than predicted, FEV1/FVC less than 70% predicted; and
-
poor reversibility after a short dose of short acting beta‐2 agonist, defined as:
less than 15 % reversibility of FEV1 from baseline; or
less than 15 % reversibility of FEV1 as a % of the predicted normal value;
generally moderate severity of airways disease.
Types of interventions
Use of regular long‐acting beta‐2 adrenoceptor agonists (including salmeterol and formoterol or other), delivered via inhalation (metered dose inhaler or dry powder) for at least four weeks compared with placebo.
Types of outcome measures
Lung function tests, including FEV1 and PEF
Exercise tolerance, including six‐minute walk test
Health related quality of life (HRQL), including the St George's respiratory questionnaire (SGRQ), chronic respiratory diseases questionnaire (CRDQ), medical short form 36 (SF‐36), European quality of life questionnaire (EQ‐5D).
Dyspnoea and symptom measurements, including TDI, symptom scores
Exacerbations
Rescue medication use
Search methods for identification of studies
Electronic searches
The Trials Search Co‐coordinator searched the Cochrane Airways Group Specialised Register (July 2005). The Register is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and hand searching of respiratory journals and meeting abstracts. All records in the Register coded as 'COPD' were searched using the terms:
((beta* AND agonist*) AND long*) OR ((beta* AND adrenergic*) AND long*) OR (bronchodilator* AND long*) OR (salmeterol or formoterol or eformoterol or bambuterol or fenoterol)
Searching other resources
We checked the reference lists of identified RCTs to identify additional relevant references. Additional data were sought from online registers of published and unpublished clinical trial data (www.ctr.gsk.co.uk; www.astrazeneca.com; www.clinicalstudyresults.org).
Data collection and analysis
Selection of studies
Trials potentially suitable for inclusion in the review included all published and non‐published randomised controlled trials of parallel group or crossover design that were identified from titles, abstracts and keywords of references obtained in the literature search. We obtained full text versions of the studies wherever possible. Two authors assessed the suitability for inclusion, methodology and quality of the studies without consideration of the results. Differences were resolved by discussion.
Data extraction and management
Two authors extracted data from the trials independently, without blinding of authorship, onto standardised data extraction sheets. Qualitative data included the author, year of publication, setting, duration of study, baseline characteristics of participants and withdrawals. Quantitative data included dichotomous and continuous outcome measures. Standard errors were converted to standard deviations. We contacted authors and pharmaceutical companies sponsoring identified in an attempt to obtain missing and raw data. Data were estimated from graphs for some outcomes if values were not reported in the paper and we were unable to obtain the information from the authors.
Assessment of risk of bias in included studies
We assessed study quality according to whether studies met the following pre‐specified quality criteria (as yes, no or unclear, Handbook 2008):
Randomisation ‐ was sequence generation and allocation concealment adequate? Blinding ‐ were the treatments known to the patients, investigators and those assessing outcomes?
We also performed quality assessment of studies using the five point scale of Jadad 1996. Previous versions of this review applied both Jadad and Cochrane allocation concealment scores (Appendix 1).
Dealing with missing data
We have also attempted to minimise the impact of missing estimates of variance. If no standard deviations were reported in published or unpublished trial reports, and were not forthcoming following correspondence, we have imputed them based upon trials contributing to the same outcome. This has only been done if there are more than three studies with estimates of variance present in the analysis (see Table 1 for details of outcomes where this has been done). If only a P value has been reported and no standard deviations, we have estimated the standard error for the mean difference and entered the data as generic inverse variance (GIV).
1. Imputations.
| Comparison & Outcome | Study | Source of imputation |
| Change in FEV1(mls) from baseline to end of study (parallel study) | Gupta 2002 | SDs for Boyd 1997; Chapman 2002; Mahler 1999 and Hanania 2003 |
| Change in FEV1(mls) from baseline to end of study (parallel study) | SCO40030 | SDs for Boyd 1997; Chapman 2002; Mahler 1999 and Hanania 2003 |
Assessment of heterogeneity
Statistical heterogeneity was investigated using the I2 statistic (An I2 > 20% was considered statistically significant heterogeneity (Higgins 2003)). Pooled effect estimates were reported from fixed‐effect modelling, but if I2 > 20% we carried out random‐effects modelling, and we explored possible reasons for the variation between the studies.
Data synthesis
Data were entered into Review Manager software by one author and checked by a second author. For dichotomous outcomes, we expressed results of the analyses as an odds ratio (OR, 95% confidence intervals (CI)). For continuous outcomes, results of the analyses were expressed as a mean difference (MD, 95% CI) or a standardised mean difference (SMD, 95% CI). SMDs were utilised to conduct pooled analysis of outcomes if there was variation in the method of reporting of those outcomes. If a more positive outcome is favourable (e.g. FEV1, CRDQ) data were entered as positive values to generate positive MDs. In this case, the titles of the horizontal axes have been reversed so that effects that favour the treatment under review move to the right.
We have calculated a number needed to treat to benefit (NNTB) for efficacy outcomes and a number needed to treat to harm (NNTH) for safety variables. We have taken the combined control group event rate, and used this as an estimate of the baseline risk. We have used the pooled OR and 95% confidence intervals to estimate NNT from online visual Rx (www.nntonline.net).
The planned comparisons for meta‐analysis were:
Salmeterol versus placebo;
Formoterol versus placebo;
Other beta‐2 adrenoceptor agonist versus placebo
Subgroup analysis and investigation of heterogeneity
Subgroups identified for analysis a priori included:
Use of different long acting beta‐2 adrenoceptor agonists (salmeterol and formoterol, or other);
Different dosages;
Different study duration;
-
Reversibility as defined as:
less than 15 % reversibility of FEV1 from baseline;
less than 15 % reversibility of FEV1 as a % of the predicted normal value;
Different severity of airways disease.
A post‐hoc subgroup analysis related to (4) was also used to explore the consistency of effect between studies if the baseline reversibility met the inclusion criteria of the review, and in studies where this was not easy to ascertain (mainly unpublished trial reports, which did not provide extensive details of methodological design and entry criteria).
Results
Description of studies
Twenty‐three studies met the eligibility criteria of the review. These studies recruited 6061 participants.
There were 15 published RCTs identified describing the effects of LABAs, administered via inhalation over at least four weeks to adults with poorly reversible COPD. Two definitions for reversibility to a short‐acting beta agonist were considered in this review. Reversibility to a short acting beta agonist was defined as a less than 15% reversibility of FEV1 from baseline in eleven studies including the Ulrik 1995; Grove 1996; Boyd 1997; Mahler 1999; van Noord 2000; Rennard 2001; Rossi 2002; Gupta 2002; Mahler 2002; Celli 2003 and Hanania 2003 studies. Reversibility to a short acting beta agonist was defined as a less than 15% reversibility of predicted normal FEV1 in four studies including the Chapman 2002; Wadbo 2002; Calverley 2003 and Dal Negro 2003 studies. Four studies included participants with both reversible and poorly‐reversible COPD including the Rennard 2001; Mahler 2002; Rossi 2002 and Hanania 2003 studies and presented stratified results. From these studies, only the patient subgroups with poorly‐reversible COPD were included in this review.
An additional subset of eight unpublished RCTs have also been identified following hand searching of the GSK online trials register (408DP‐03; Dauletbaev 2001; SCO40030; SMS40298; SMS40314; SMS40315; SMS40318; Stockley 2002). These studies are presented as short documents. In only Dauletbaev 2001 and SMS40318 were baseline entry criteria sufficiently described to permit the findings from these studies to contribute to the primary set of analyses. The remainder did not provide sufficiently detailed entry criteria in order to determine whether the studies recruited reversible, non‐reversible or mixed populations. For this reason they contribute data as part of a series of sensitivity analyses.
Trials that included participants with both reversible and poorly reversible COPD, and met all other inclusion criteria, but did not report outcomes in the poorly reversible COPD groups, were excluded from this review if we were unable to obtain data from authors or drug companies. These trials included Anderson 1997; Mahler 1997; Dahl 2001; Donohue 2002; Aalbers 2002; Brusasco 2003; Calverley 2003a; and Szafranski 2003. Six abstracts were identified from recent American Thoracic Society meetings and European Respiratory Society Annual Congresses as requiring further information to assess the suitability for inclusion in this review. One ongoing study protocol (TORCH) was identified as meeting the requirements for inclusion in the review but the results of the study are not yet available. This will be the largest (approximately 6,200 participants) randomised, double blind, placebo controlled parallel group study for salmeterol 50 mcg when the results are expected to become available in 2006.
Participants
The sample sizes of the parallel studies were variable, in the range of 144 to 674 participants. Total number of participants contributing data from these studies was 5979. The crossover studies had small sample sizes of 63 and 29 participants.
Interventions
Eleven parallel group studies investigated the effects of salmeterol 50 mcg (Boyd 1997; Mahler 1999; van Noord 2000; Rennard 2001; Chapman 2002; Gupta 2002; Mahler 2002; Calverley 2003; Celli 2003; Dal Negro 2003; Hanania 2003). One parallel group study investigated the effect of salmeterol 100 mcg (Boyd 1997). There were two crossover studies which investigated the effects of salmeterol 50 mcg (Ulrik 1995; Grove 1996)
There was one parallel group study that investigated the effect of formoterol 12 mcg (Rossi 2002), one parallel group study that investigated the effect of formoterol 18 mcg (Wadbo 2002) and one parallel group study that investigated the effect of formoterol 24 mcg (Rossi 2002).
Risk of bias in included studies
Methods of randomisation were described adequately or obtained from authors or study sponsors for all of the studies (Figure 1). All studies were described as double blind, and appropriate means of masking treatment were confirmed for all the studies. Based on correspondence with GSK, we were able to ascertain that usual processes for generating randomisation schedules and concealing allocation were at a low risk of bias (Appendix 2). On the basis of these judgements, the studies were well‐designed and at a low risk of bias in terms of allocation and blinding.
1.
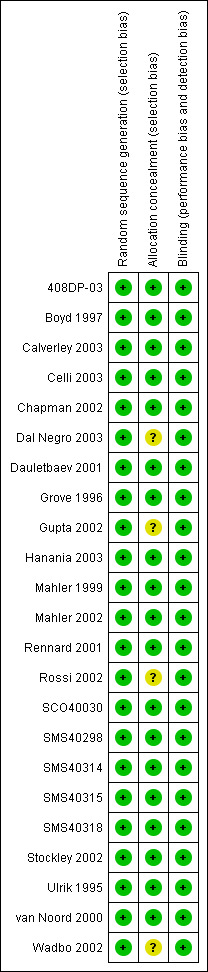
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Eight studies commented on the number of participants excluded from the trials (Calverley 2003; Celli 2003; Chapman 2002; Dal Negro 2003; Grove 1996; Hanania 2003; Mahler 2002; Ulrik 1995). Thirteen studies commented on withdrawals and dropouts (Ulrik 1995; Boyd 1997; Mahler 1999; van Noord 2000; Rennard 2001; Chapman 2002; Mahler 2002; Rossi 2002; Stockley 2002; Wadbo 2002; Calverley 2003; Celli 2003; Dal Negro 2003; Hanania 2003; 408DP‐03; Dauletbaev 2001; SCO40030; SMS40298; SMS40314; SMS40315; SMS40318).
Effects of interventions
Meta‐analysis was only possible for some outcomes because of variation in the reporting of study outcomes.
(1) Lung function
Meta‐analyses were performed for mean FEV1 (mls) change from baseline, end of study pre‐bronchodilator FEV1, and mean FEV1 change from baseline (% predicted) due to variation between the studies in the reporting of data.
(a) Salmeterol 50 mcg twice daily versus placebo
Change in FEV1 (mls)
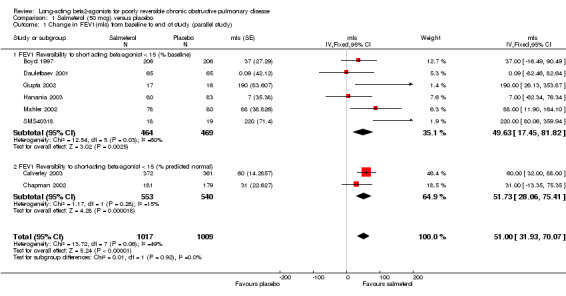
Boyd 1997; Calverley 2003; Chapman 2002; Dauletbaev 2001; Gupta 2002; Hanania 2003; Mahler 2002; SMS40318. There was a significant change in FEV1 (mls) in favour of salmeterol (MD 51 mls (95% CI 31.93 to 70.07), eight studies, N = 2026, Figure 2). There was a high degree of statistical heterogeneity (I2 49%). Celli 2003 reported no significant changes in FEV1, but no numerical data were provided.
2.
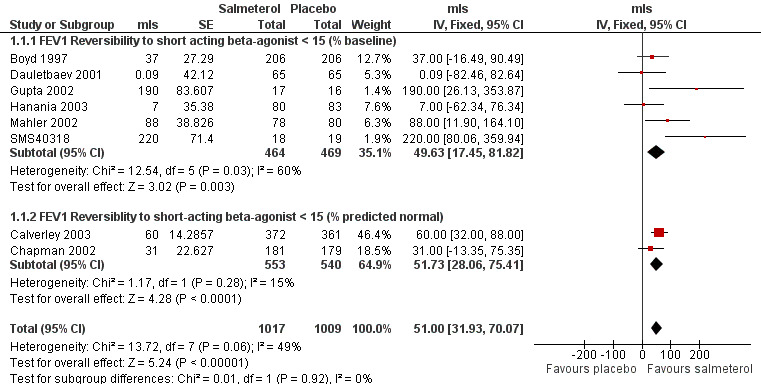
Forest plot of comparison: 1 Salmeterol (50 mcg) versus placebo, outcome: 1.1 Change in FEV1(mls) from baseline to end of study (parallel study).
The inclusion of several unpublished trials (including three where baseline reversibility criteria were not easily established: 408DP‐03; SCO40030; SMS40298) did not significantly alter the pooled effect estimate or increase the level of statistical heterogeneity (MD 55.13 mls (95% CI 38.16 to 72.09; 11 studies, N = 2644; I2 0). Visual inspection of the funnel plot with and without these studies suggested that publication bias only partially influences the distribution of studies around the fixed effect (Figure 3).
3.
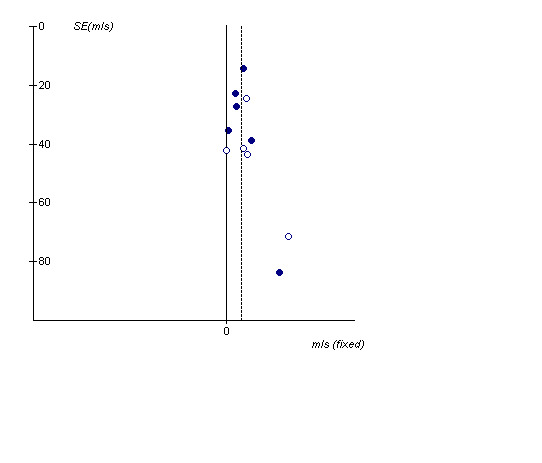
Funnel plot demonstrating the distribution of effect sizes around the 'fixed' effect (vertical dotted line). The 'hollow' dots represent studies which were unpublished, the 'complete' dots represent data from published clinical trials
Absolute FEV1 (litres)
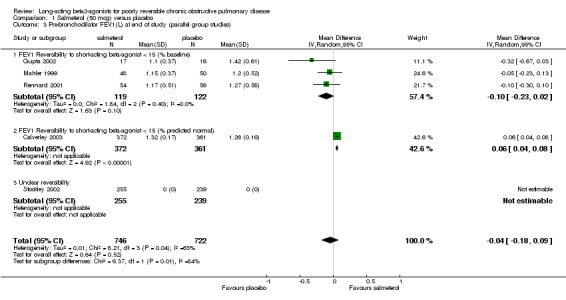
Mahler 1999; van Noord 2000; Rennard 2001; Gupta 2002; Calverley 2003 Pooled analysis showed no significant change in pre‐bronchodilator FEV1 with salmeterol (‐0.03 mls (95% CI ‐0.13 to 0.06), N = 1798. Statistical heterogeneity was high [I2 74.5%]. Additional studies contributing data for the subgroup of studies where baseline reversibility to short‐acting beta‐agonist was less than 15% predicted normal is required to see whether the observed difference between the subgroup estimates has validity.
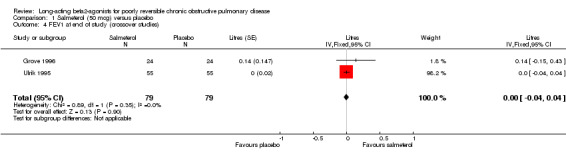
Two crossover studies involving 79 participants reported pre‐bronchodilator end of study FEV1 (Ulrik 1995; Grove 1996). Pooled analysis showed no significant difference between the two interventions (0 (95% CI ‐0.04 to 0.04)).
Change in FEV1 (% predicted)
One parallel group study involving 97 participants (van Noord 2000) reported a significant increase in mean FEV1 change from baseline (% predicted).
PEF (parallel group study)
Morning PEF (L/min) at end of study (van Noord 2000; Stockley 2002; Calverley 2003; Dal Negro 2003): There was a significant difference in favour of salmeterol (15.81 L/min (95% CI 11.96 to 19.67), N = 1467).
Night time PEF (L/min) at end of study: van Noord 2000 reported no significant difference in end of study night time PEF (L/min).
Change in PEF (L/min) morning (Chapman 2002; Dauletbaev 2001): There was a significant difference in favour of salmeterol (L/min 8.36 L/min (95% CI 2.88 to 13.85), N = 540).
PEF (crossover study)
Morning and night time (L/min) at end of study: Ulrik 1995 reported a significant difference in morning PEF (10 L/min (95% CI 3.34 to 16.66), N = 55). However, there was no significant difference in evening PEF (3 L/min (95% CI ‐3.08 to 9.08).
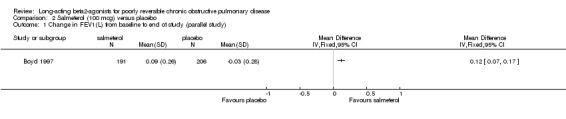
(b) Salmeterol 100 mcg versus placebo
Change in FEV1
Boyd 1997 reported a significant change in FEV1 in favour of salmeterol (MD 0.12 Litres (95% CI: 0.07 to 0.17), N = 412).
(c) Formoterol 12 mcg versus placebo
FEV1 (L)
Rossi 2002 reported no significant difference in pre‐bronchodilator FEV1.
PEF (parallel group study)
Rossi 2002 reported no significant difference in pre‐bronchodilator PEF.
(d) Formoterol 18 mcg versus placebo
Mean change (% predicted FEV1)
Wadbo 2002 reported a significant mean change from baseline in % predicted FEV1 in favour of formoterol (6.07% (95% CI 5.12, 6.92), N = 121).
Change in morning PEF (L/min)
Wadbo 2002 reported a significant change in morning PEF (l/min) favouring formoterol, although no estimates of variance were available.
(e) Formoterol 24 mcg versus placebo
FEV1 (L)
Rossi 2002 reported no significant difference in pre‐bronchodilator FEV1.
PEF (L/min), morning
Rossi 2002 reported no significant difference in morning PEF (L/min).
(2) Exercise tolerance
(a) Salmeterol 50 mcg versus placebo
Six minute walk test, distance (metres) at end of study (parallel group study)
Boyd 1997; Mahler 1999; Rennard 2001; Gupta 2002 There was no significant difference between salmeterol or placebo groups in distance walked (1.58 metres (95% CI ‐10.73 to 13.88), N = 659).
Six minute walk test, Borg score (< 3) for breathlessness at end of study
Boyd 1997 reported that significantly more patients in the salmeterol treated group had Borg scores less than three (three indicating moderate dyspnoea) compared with placebo (Peto OR 1.68 (95% CI 1.13 to 2.48), N = 412).
Six minute walk test, distance (m) at end of study (crossover study)
Grove 1996 reported no significant difference between salmeterol 50 mcg and placebo in the mean change in distance walked from baseline (1.93 (95% CI ‐15.40 to 19.26), N = 24).
Cycle ergometry (crossover study)
Grove 1996 reported no significant difference between salmeterol 50 mcg treatment and placebo for Borg scores and various physiological measurements including oxygen uptake, ventilation, heart rate and oxygen saturation (N = 24).
Six minute walk test, end of study post‐walk Borg dyspnoea scores
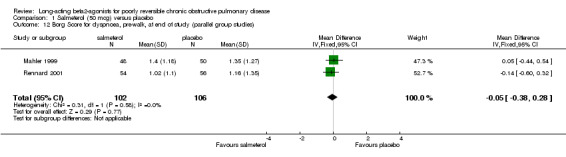
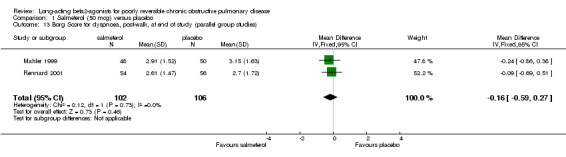
Borg dyspnoea scores were reported in two studies involving 208 participants (Mahler 1999; Rennard 2001). There was no significant difference between treatment groups (MD ‐0.16 (95% CI ‐0.59 to 0.27).
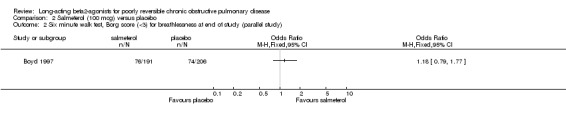
(b) Salmeterol 100 mcg versus placebo
Boyd 1997 reported no significant difference in proportions of patients with Borg scores less than three (Peto OR 1.18 (95% CI 0.79 to 1.77)). Boyd 1997 reported no significant difference between salmeterol 100 mcg and placebo in the distance walked at six minutes but did not provide data values.
(c) Formoterol 12 mcg versus placebo
No studies.
(d) Formoterol 18 mcg versus placebo
Wadbo 2002 reported change in shuttle walking distance from baseline which showed a significant increase in the formoterol group (14.13 (95% CI 11.28 to 16.98), N = 121).
Wadbo 2002 also reported post shuttle Borg scores which showed a significant improvement in favour of formoterol (‐0.47 (95% CI ‐0.54 to ‐0.40), N = 121).
(e) Formoterol 24 mcg versus placebo
No studies.
(3) Quality of life
(a) Salmeterol 50 mcg BID versus placebo
St Georges Respiratory Questionnaire (SGRQ)
Data from published and unpublished sources were available and contribute to the pooled analyses of the SGRQ domains. We report the pooled and subgroup findings of the studies where baseline reversibility criteria meet the inclusion criteria of the review (Boyd 1997; Calverley 2003; Celli 2003; Chapman 2002; Dauletbaev 2001; van Noord 2000), and studies where baseline reversibility of the study populations was unclear (SMS40298; SMS40314; SMS40315; Stockley 2002).
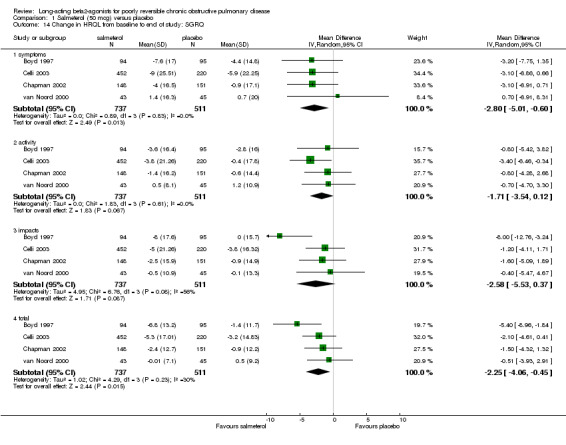
Change in total SGRQ score
Overall pooled estimate: ‐2.17 units (95% CI ‐2.88 to ‐1.46), 10 studies, N = 3607 Studies where baseline reversibility meets review inclusion criteria: ‐1.52 (95% CI ‐2.43 to ‐0.6), six studies, N = 2111. Unclear baseline reversibility: ‐3.19 units (95% CI ‐4.33 to ‐2.05), four studies, N = 1496. Difference between subgroup estimates: 1.67 units (95% CI 0.2 to 3.13), P = 0.025).
Change in symptoms domain
Overall pooled estimate: ‐2.17 (95% CI ‐3.31 to ‐1.02), seven studies, N = 2894 Subgroup of studies where baseline reversibility meets review criteria: ‐1.04 (95% CI ‐2.39 to 0.3), five studies, N = 1981. Unclear baseline reversibility: ‐5.13 (95% CI ‐7.32 to ‐2.94), two studies, N = 941. Difference between subgroup estimates: 4.09 (95% CI 1.52 to 6.66), P = 0.002
Change in activity domain
Overall pooled estimate: ‐2.13 (95% CI ‐3.16 to ‐1.10), eight studies, N = 3282 Subgroup of studies where baseline reversibility meets review criteria: ‐1.61 (95% CI ‐2.81 to ‐0.4), six studies, N = 2111 Unclear baseline reversibility: ‐3.56 (95% CI ‐5.55 to ‐1.56), two studies, N = 941 Difference between subgroup estimates: 1.95 (95% CI ‐0.38 to 4.28), P = 0.1
Change in impacts domain
Overall pooled estimate: ‐2.13 (95% CI ‐3.09 to ‐1.17), seven studies, N = 2922 Subgroup of studies where baseline reversibility meets review criteria: ‐2.03 (95% CI ‐3.15 to ‐0.91), five studies, N = 1981 Unclear baseline reversibility: ‐2.39 (95% CI ‐4.24 to ‐0.54), two studies, N = 941 Difference between subgroup estimates: 0.36 (95% CI ‐1.8 to 2.52), P = 0.74
The application of random‐effects modelling did not alter the significance of the pooled effect estimates irrespective of the presence of statistical heterogeneity.
Medical Short Form 36 (SF‐36)
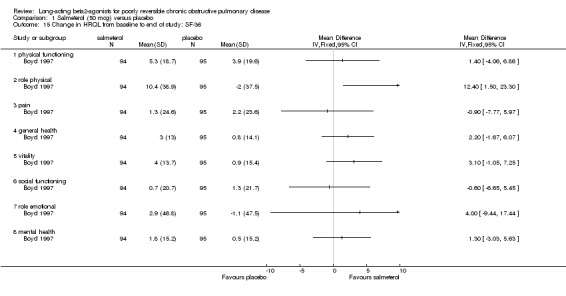
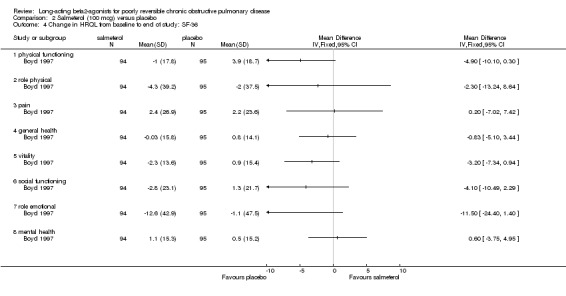
The SF‐36 was administered in two studies. Positive scores on the SF‐36 indicate an improvement in quality of life, and negative scores indicate deterioration. One study involving 189 participants (Boyd 1997) reported the change in SF‐36 domain scores. General health as measured by the SF‐36 showed significant improvement in only one of the eight components, Role Physical (MD 12.4 (95% CI: 1.5 to 23.3)).
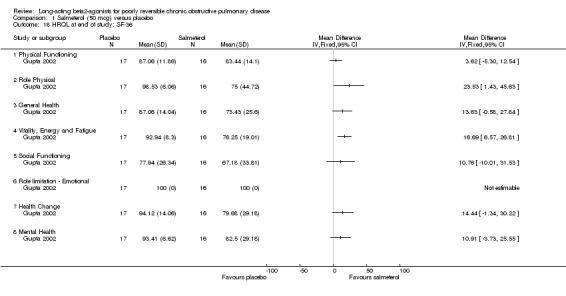
One parallel group study involving 33 participants (Gupta 2002) reported end of study SF‐36 domain scores. There were significant changes in favour of salmeterol in the role ‐ physical (MD 23.53, 95% CI 1.43 to 45.63), vitality energy and fatigue (MD 16.69, 95% CI 6.57 to 26.81) domains.
The Chronic Respiratory Diseases Questionnaire (CRDQ)
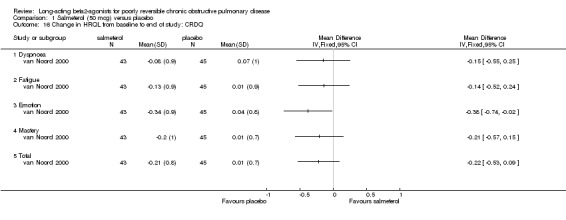
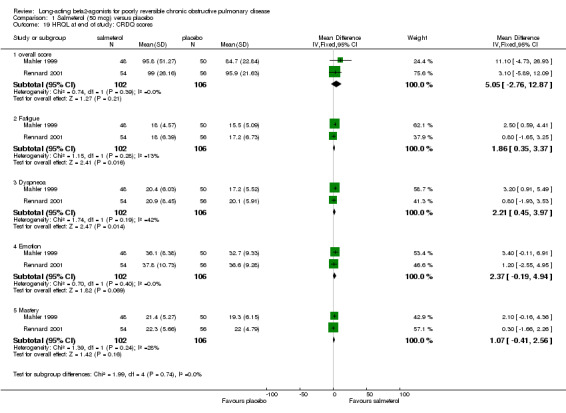
van Noord 2000; Mahler 1999; Rennard 2001
Two studies involving 208 participants reported end of study CRDQ domain scores (Mahler 1999; Rennard 2001). Significant treatment differences were demonstrated in the Fatigue (MD 1.86 (95% CI 0.35 to 3.37), I2 = 12.9%) and Dyspnoea (MD 2.21 (95% CI 0.45 to 3.97), I2 = 42.5%) domains which were not observed in the overall score (MD 5.05 (95% CI ‐2.76 to 12.87), Emotion (MD 2.37 (95% CI ‐0.19 to 4.94) and Mastery domains (MD 1.07 (95% CI ‐0.41 to 2.56), I2 = 27.8%) domains.
Mahler 1999 reported that a significantly greater proportion of participants in the salmeterol group demonstrated an increase in overall CRDQ score of 10 points, exceeding the MCD for Total score (Reidelmeier 1996) in the poorly reversible stratum of participants. This result was not confirmed by van Noord 2000.
European Quality of Life Questionnaire (EQ‐5D)
Celli 2003 reported the change in the weighted health index EQ‐5D did not reach statistical significance between salmeterol and placebo. Data were not presented in the publication, and have not been forthcoming from the study investigators.
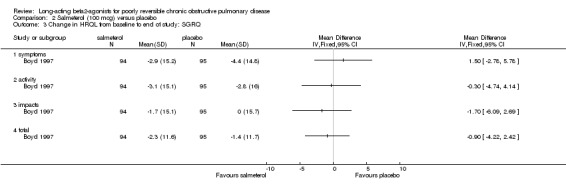
(b) Salmeterol 100 mcg versus placebo
None of the SGRQ or SF‐36 scores improved significantly on salmeterol 100 mcg (Boyd 1997).
(c) Formoterol 12 mcg versus placebo
St Georges Respiratory Questionnaire (SGRQ)
One parallel group study involving 173 participants reported mean change in SGRQ scores and reported no significant changes in any of the four domains (symptoms, activity, impacts, totals) (Rossi 2002).
(d) Formoterol 18 mcg versus placebo
St Georges Respiratory Questionnaire (SGRQ)
One parallel group study involving 121 participants (Wadbo 2002) reported mean SGRQ scores change from baseline and found no significant changes between formoterol and placebo in symptoms, impacts, activity and total SGRQ scores.
(e) Formoterol 24 mcg versus placebo
St Georges Respiratory Questionnaire (SGRQ)
Rossi 2002 reported mean SGRQ scores change from baseline and found no significant changes in any of the four domains (symptoms, activity, impacts, totals).
(4) Dyspnoea and Symptom Scores
Several measures of dyspnoea were used.
(a) Salmeterol 50 mcg versus placebo
Baseline dyspnoea index (BDI) The baseline dyspnoea index was used by one parallel group study involving 33 participants which reported end of study scores (Gupta 2002). There was a significant difference in favour of salmeterol (MD 0.88 (95% CI 0.07 to 1.69).
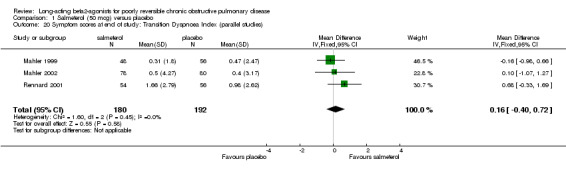
Transitional dyspnoea index (TDI)
Mahler 1999; Rennard 2001; Mahler 2002; SMS40314; SMS40315 There was no significant difference between salmeterol and placebo in the published studies where (MD 0.16 (95% CI ‐0.40 to 0.72), three studies, N = 372). Pooled analysis with two unpublished studies gave a significant difference in favour salmeterol (MD 0.43 (95% CI 0.06 to 0.81), N = 934). However, there was moderate statistical heterogeneity (I2 37.3%), and the pooled effect estimate was non‐significant with random‐effects modelling (0.41 (95% CI ‐0.07 to 0.9).
Symptom scores (pre‐walk Borg scores)
No significant difference (MD ‐0.05 (95% CI ‐0.38 to 0.28), N = 208.
van Noord 2000 reported that mean daytime symptom scores were significantly reduced by salmeterol treatment (MD ‐0.3 (95% CI ‐0.58 to ‐0.02) but not mean night time scores (MD 0.10 (95% CI ‐0.18 to 0.38).
Symptom scores (shortness of breath, chest tightness, cough and sputum production)
Three published studies (Mahler 1999; Rennard 2001; Calverley 2003) and one unpublished study (Stockley 2002) reported data for one or more of these four symptom domains. With data available from Stockley 2002 there were significant differences in shortness of breath (SMD ‐0.18 (95% CI ‐0.28 to ‐0.08), but no significant difference in cough (SMD ‐0.08 (95% CI ‐0.17 to 0.02). For the remaining outcomes where only data from published studies were available, there were no significant differences in either chest tightness (SMD ‐0.12 (95% CI ‐0.40 to 0.15) and sputum production (SMD (‐0.09, 95% CI:‐0.23, 0.06).
Night symptoms
Mahler 1999; Rennard 2001 No significant differences in dyspnoea (MD ‐0.03 (95% CI ‐0.28 to 0.23), cough (MD ‐0.02 (95% CI ‐0.28 to 0.24) and chest tightness (MD ‐0.03 (95% CI ‐0.17 to 0.11). Dal Negro 2003 reported overall symptoms scores which was not statistically significant [MD ‐0.70, 95% CI ‐1.49 to 0.09].
Boyd 1997 reported statistically significant differences in favour of salmeterol in median day and night time symptom scores. Chapman 2002 reported no significant difference between salmeterol and placebo for median daytime and night‐time symptom scores. Median day and night time symptom scores were reported to be significantly lower in the salmeterol treatment period compared to the placebo period in a crossover study (Ulrik 1995).
(b) Salmeterol 100 mcg versus placebo
Boyd 1997 reported no significant difference in the number of participants with lower Borg breathlessness scores between salmeterol and placebo (Peto OR 1.18 (95% CI 0.79 to 1.77)). This study reported statistically significant differences in favour of salmeterol in median day and night time symptom scores.
(c) Formoterol 12 mcg versus placebo
Rossi 2002 reported no significant difference in median symptom scores between treatment groups.
(d) Formoterol 18 mcg versus placebo
Wadbo 2002 reported a significant reduction in favour of formoterol in shortness of breath at night (MD ‐0.17 (95% CI ‐0.19 to ‐0.15), daytime shortness of breath (MD ‐0.21 (95% CI ‐0.24 to ‐0.18), night‐time cough (MD ‐0.12 (95% CI ‐0.14 to ‐0.10), daytime cough (MD ‐ 0.11 (95% CI ‐0.13 to ‐0.09) and sleep (MD ‐0.12 (‐0.14 to ‐0.10).
(e) Formoterol 24 mcg versus placebo
Rossi 2002 reported no significant difference in median symptom scores between treatment groups.
(5) Exacerbations and withdrawal due to lack of efficacy
(a) Salmeterol 50 mcg versus placebo
Number of participants experiencing exacerbations (parallel study)
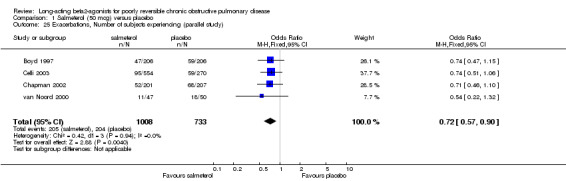
Boyd 1997; van Noord 2000; Chapman 2002; Celli 2003; Stockley 2002; SMS40298 The odds of experiencing an exacerbation were significantly reduced with salmeterol compared with placebo (OR 0.72 (95% CI 0.57 to 0.90, N = 1741). Including data from unpublished studies also gave a significant result (0.8 (95% CI 0.67 to 0.95). Assuming a baseline risk of around 28%, this gave a NNTB of 24 (95% CI 14 to 98, see Figure 4).
4.
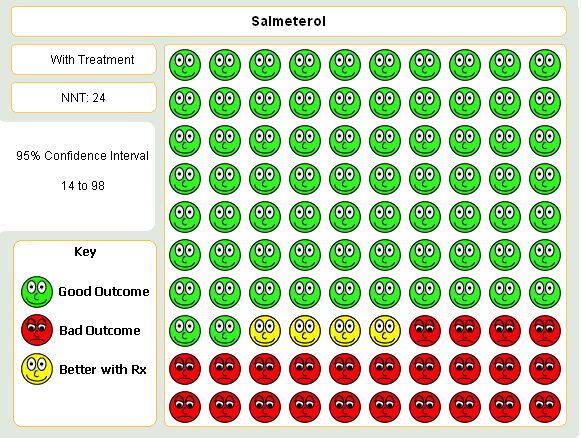
Graphic to demonstrate that 24 people with COPD would need to be treated with salmeterol 50mcg BID in order to prevent one exacerbation in the short term.
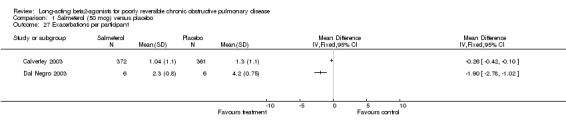
Two parallel group studies (Calverley 2003; Dal Negro 2003) reported the mean number of exacerbations per year. Whilst both studies reported significant differences in favour of salmeterol there was a high level of statistical heterogeneity (I square 92.3%). This may reflect a more homogenous, but selected group of patients in Dal Negro 2003.
Number of participants experiencing exacerbations (crossover study)
Ulrik 1995 demonstrated no significant differences between treatment groups in the number of participants experiencing exacerbations while on treatment (OR 1.0 (95% CI: 0.14 to 7.33).
Study withdrawal due to lack of efficacy
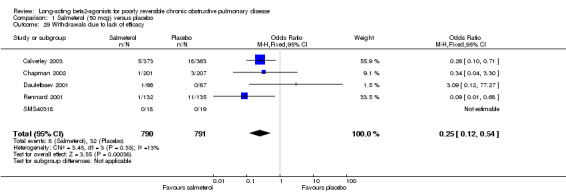
There was a significant reduction in withdrawal due to lack of efficacy in participants treated with salmeterol (Peto OR 0.29 (95% CI 0.15 to 0.54, five studies, N = 1581)). When data from studies with unclear reversibility criteria were included, the effect remained significant (OR 0.44 (95% CI 0.29 to 0.68), N = 3363). Assuming a baseline risk of around 4%, this gives a NNTB of 46 (95 % CI 36 to 81, see Figure 5).
5.
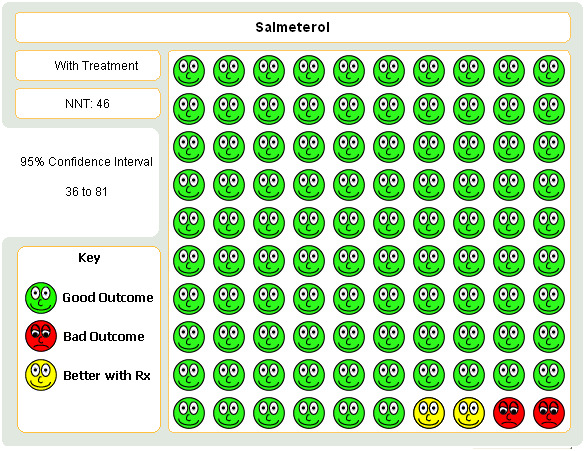
Graphic to demonstrate that 46 people with COPD would need to be treated with salmeterol 50mcg BID in order to prevent one withdrawal due to lack of efficacy.
(b) Salmeterol 100 mcg versus placebo
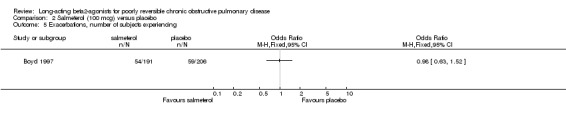
Boyd 1997 reported no significant difference in the odds of exacerbations between salmeterol and placebo (Peto OR 0.98 (95% CI 0.64 to 1.52).
(c) Formoterol 12 mcg versus placebo
Rossi 2002 reported no significant difference in the odds of exacerbations between formoterol placebo.
(d) Formoterol 18 mcg versus placebo
No studies
(e) Formoterol 24 mcg versus placebo
Rossi 2002 reported no significant difference in the odds of exacerbations between formoterol placebo.
(6) Rescue medication use
(a) Salmeterol 50 mcg versus Placebo
Percentage of days and nights with additional salbutamol use van Noord 2000 reported a significantly lower percentage of days and nights with additional salbutamol use in favour of salmeterol (MD ‐40.0% (95% CI ‐54.61 to ‐25.39) and (MD ‐16.0% (95% CI ‐29.61 to ‐2.39) respectively).
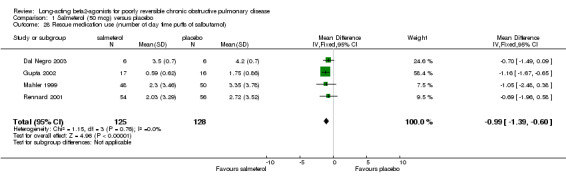
Number of daytime puffs of salbutamol (Mahler 1999; Rennard 2001, Gupta 2002, Dal Negro 2003; Stockley 2002) There was a significant difference in favour of salmeterol in the mean number of daytime puffs of short‐acting beta agonist (MD ‐0.78 (95% CI ‐1.03 to ‐0.53), N = 877). This finding was not affected by the inclusion of data from a study with unclear baseline reversibility (Stockley 2002).
Other One study reported a significant increase in the number of nights without short‐acting beta agonist use in favour of salmeterol (Ulrik 1995). Boyd 1997 reported that median daytime salbutamol use was significantly lower in salmeterol treated participants compared with placebo (P < 0.001) and that the median percentage reduction in rescue medication use favoured salmeterol (P = 0.014). Chapman 2002 did not find a significant difference between salmeterol and placebo in the use of rescue salbutamol.
(b) Salmeterol 100 mcg versus placebo
Boyd 1997 reported that median daytime salbutamol 100 mcg use was significantly lower in salmeterol treated participants compared with placebo (P < 0.001), and that the night‐time percentage reduction in salbutamol use in salmeterol treated participants was significantly lower than in placebo treated participants (P = 0.005).
(c) Formoterol 12 mcg & 24 mcg versus placebo
Rossi 2002 reported median rescue salbutamol use per day and median number of days without rescue salbutamol over the whole treatment period for both formoterol 12 mcg and 24 mcg doses. As this study included participants with both reversible and irreversible COPD and the results for this outcome was not stratified by reversibility it is not possible to include the relevant results in this review.
(d) Formoterol 18 mcg versus placebo
Wadbo 2002 reported that mean rescue short‐acting beta agonist use in the formoterol group was 3.6 daily inhalations compared with 4.7 daily inhalations in the placebo group in the last two months of treatment. No statistical tests of significance were applied as there was variation in the type of short‐acting beta agonist used and the data were not dose‐adjusted.
Discussion
This review aimed to determine the effectiveness of LABAs in COPD patients who demonstrate poor reversibility to short‐acting bronchodilators. The presence of increased resistance to airflow from airways obstruction is a characteristic of COPD. Although effective as an additive agent in asthma (Ni Chroinin 2005), salmeterol and formoterol are frequently advocated in the treatment of patients with COPD, where the acute response to bronchodilators in COPD is often minimal.
Respiratory Function
This review found statistically significant changes in favour of salmeterol for some lung function outcomes (change in FEV1, change in FEV1 (% predicted), change in PEF and end of study morning PEF). The clinical meaning of these differences, and their relationship with other variables such as quality of life and exacerbations, are yet to be established. The discrepancy between change and end of treatment FEV1 outcomes may be explained by the natural adjustment of imbalance between groups at baseline with change scores, and the superior statistical power derived from the larger number of studies (2026 versus 1468 participants). The effect of long‐acting beta‐agonists in COPD patients who have a reversible component for their airways disease to short acting beta agonists is outside the scope of this review. However, this review included studies with both groups of participants that were stratified by reversibility to short acting beta‐agonist. This included the Mahler 1999; Rennard 2001; Mahler 2002; Rossi 2002 and Hanania 2003 trials. These studies reported greater improvement in lung function in the subgroup with reversibility to short acting beta agonist versus the subgroup with poor reversibility.
The treatment period in the crossover studies should be of sufficient duration to detect improvements, but none were observed. The absence of demonstrable effect in these studies may be due to small sample sizes, which have less power to detect change.
In advanced COPD, exertional dyspnoea has been correlated with the level of dynamic lung hyperinflation (DH) (O'Donnell 1997). One study has demonstrated that Inspiratory Capacity (IC) correlates better with improvements in exercise tolerance and reduces dyspnoea than expiratory flow measurements (O'Donnell 1999). The improvements in IC and exercise tolerance (after ipratropium treatment) occurred in a proportion of participants (31%) who showed little or no improvement in FEV1 (< 10% predicted). Utilisation of FEV1 measurements in the studies in this review may therefore have underestimated the potential benefits of LABA therapy. However, the lack of improvement in exercise tolerance suggests this is not the case.
Exercise Tolerance, HRQoL and Symptom scores
Traditionally the efficacy of bronchodilator medication has been assessed in terms of changes in airflow obstruction using spirometry and peak flow measures. However, exercise tolerance, HRQL and symptom scores are important outcomes for people with COPD, and areas where one would hope to see significant improvements with LABAs. This review showed inconsistent effects of LABA across the reported outcomes.
The SGRQ was designed to allow direct comparisons of the health gain obtainable with therapies in asthma and COPD (Jones 1992). Being a disease specific HRQL instrument it is sensitive to treatment related improvements. The size of the treatment effects for change in total, activity and impact domains were small and the level of improvement did not exceed the four unit difference as a threshold for clinical significance in total SGRQ or its components (Jones 1991). Deselecting Boyd 1997 reduced the level of statistical heterogeneity indicating that a higher compliance rate with medication in this study or a more reversible group of participants, may have led to better outcomes with salmeterol. This study showed clinically significant improvements for the SGRQ total score and the impacts domain for the salmeterol 50 mcg group but not for the 100 mcg group suggesting that adverse effects participants experience at the higher dose may outweigh the benefits. Following the addition of new data, random‐effects modelling did not alter the direction of summary estimates in the domains of the SGRQ. The difference between subgroups when defined by the availability of reversibility criteria ('known to meet the review criteria' versus 'unclear') were significant according to the t test. However, the within subgroup I2 statistic for total SGRQ score was indicative of a heterogeneous subgroup of studies. Due to the unclear baseline reversibility thresholds, the influence of possible 'reversible' participants on the findings could vary between the unpublished studies.
The Chronic Respiratory Diseases Questionnaire (CRDQ) is a HRQL instrument COPD that measures both physical and emotional attributes. It is a seven point scale where a change of 0.5 units represents a clinically small change, 1.0 represents a moderate change and 1.5 represents a large change. A statistically significant and clinically large change in the fatigue and dyspnoea domains was detected in the salmeterol 50 mcg group. The reason behind the large statistical heterogeneity in the dyspnoea group is unclear.
The SF‐36 questionnaire is a generic HRQL instrument that assesses eight domains. The two studies investigating salmeterol 50 mcg presented SF‐36 scores differently, thus limiting opportunities for meta‐analysis. In general, the SF‐36 was unable to detect improvements in quality of life after salmeterol treatment, except in a few domains. Boyd 1997, which detected a clinically significant difference in the total SGRQ score for the salmeterol 50 mcg group, did not detect a difference in the SF‐36 questionnaire with the exception of the role physical domain. However, the SF‐36 may lack the disease specific sensitivity to detect changes in a population with COPD. Gupta 2002 reported that baseline scores for the placebo group tended to be higher than for the salmeterol group and improvement was shown in both the placebo and salmeterol groups. Given the treatment group had a lower baseline score, the improvement may have been heightened.
Exacerbations and withdrawal due to lack of efficacy
A reduction in exacerbations of COPD is one of the aims of all maintenance therapy (NICE/BTS 2004). Importantly, this review has demonstrated that the number of participants experiencing exacerbations was reduced with salmeterol 50 mcg treatment compared to placebo treatment (NNTB 21). This finding may be of significance given the relationship between exacerbations and deterioration in health related quality of life in COPD (Spencer 2004). The duration of treatment in these studies ranged from 12 to 52 weeks. This effect supports the finding on withdrawal due to lack of efficacy, although the NNTB was suggestive of a lower impact (NNTB 46). This may reflect differing surrogates of disease control and perception of benefit, but remains an important feature of clinical trial design in this population. Rossi 2002 (12 months duration) found no significant differences for exacerbation rates for formoterol 12 mcg and 24 mcg versus placebo. More trials with longer study duration are probably necessary to detect a change in exacerbation rate and the TORCH study, a three year study due for publication in 2006 will add important evidence regarding exacerbations and mortality.
Rescue short‐acting beta‐agonist use
There was a small but statistically significant reduction in number of daytime puffs of salbutamol in the salmeterol 50 mcg group compared with placebo. This is a predictable outcome given the long duration of action of these drugs which may improve medication compliance and reduce patient burden. Rescue medication use in the formoterol 18 mcg study (Wadbo 2002) showed the placebo group had one additional daily inhalation over the placebo group.
The quality of the evidence
The majority of studies were of good quality and included multi‐centre, multinational trials with varying numbers of participants. The search strategy was rigorous and included American Thoracic Society and European Respiratory Society abstracts. Authors and drug companies involved in trials were contacted in order to obtain any missing or unpublished data.
Limitations of the review
Meta‐analysis for a number of outcomes were limited. Several factors contributed to this problem: The small number of RCTs available meeting the criteria for inclusion in the review; inconsistent methods of reporting outcomes across studies; lack of provision of numerical data for outcome measures or standard deviations for mean effect sizes were not available. Imputation based on an average standard deviation is one possible countermeasure to censored reporting of data in clinical trials, but we were only able to undertake this in a limited number of outcomes (Table 1).
Publication bias is a known threat to the validity of any pooled estimate of efficacy (Sterne 2001) and we have identified a number of unpublished studies which are analysed in addition to the primary analyses on the published studies. Given the specific nature of COPD we analyse in this review, these studies may not have been conducted according to our entry criteria. However, their availability has enabled to us to assess whether the lack of a description of entry criteria could explain a response differential. The extent to which this was the case varied between the outcomes, and may in part reflect the different primary outcomes they selected, as well as indicating the mixed patient population hitherto excluded from this review.
There was variation in the duration of each of the studies and this may have had some effect on the results. We pooled studies of differing durations of exposure to treatment provided the treatment period was at least four weeks as it was felt that the treatment effect would be evident after four weeks of treatment. However, sensitivity analysis based on study duration was not required for most outcomes as indicated by the low I2 values where meta‐analysis was possible. Study duration did not resolve the heterogeneity for outcomes with I2 values greater than 20%.
Individual trials had different requirements for what additional medications were permitted for the participants to use. Some studies allowed patients to continue their non‐long acting beta‐agonist regular medication if maintained at a constant dose throughout (Grove 1996; Mahler 1999; Chapman 2002; Mahler 2002; Rossi 2002; Stockley 2002; Wadbo 2002; Celli 2003; Dal Negro 2003; 408DP‐03) permitted participants to continue with theophylline. This is important because the patient may have been taking additional drugs with similar mechanisms of action, the drugs may have additive or synergistic effects, or the benefits conferred by them may have underpowered the studies to find significant effects between long‐acting beta‐agonist.
We have not collected data on adverse events in this review. It was felt between the authors that the side‐effects associated with salmeterol may be affected by bronchodilation such that in a trial setting non‐responders may take an extra puff or two of study drugs to achieve some bronchodilation thus potentially increasing in systemic effects. Given that we have only been able to collect data for a subset of poorly reversible participants from many of the studies. This issue remains unclear.
The relevance of the evidence
This systematic review may differ slightly from other more recent COPD maintenance therapy reviews in that it was only concerned with those patients who have poor reversibility to short‐acting bronchodilators (Nannini 2004; Barr 2005). Recent consensus statements and guidelines have moved away from defining COPD in terms of bronchodilator response, and instead promote symptom history over spirometric findings in the definition and diagnosis of COPD. Airways obstruction is instead described as being not fully reversible (GOLD 2001; ATS/ERS 2004; NICE/BTS 2004). The findings of this review may not therefore apply directly to recent 'guideline' defined COPD. Although the validity of the reversible/non‐reversible dichotomy has been called in to question (Calverley 2003b), the progressive nature of the disease, and the mechanism of beta‐agonists justify selecting out those patients in whom these drugs are recommended, but whose initial response to beta‐agonist does not suggest that lung function will improve. The response of such people to maintenance beta‐agonist therapy has been explored in only a subset of trials. We defined poorly reversible COPD as an increase in FEV1 of < 200ml and < 15% of the baseline or percent predicted FEV1 after a short acting beta agonist. Studies in which the definition of reversibility were unclear were identified for sensitivity analysis, because the study population potentially includes a subset of participants who may have a larger reversible component of their disease. Whilst certain domains of the SGRQ gave more positive effects from the unclear studies (i.e. symptoms and total score), the effect on lung function, rescue bronchodilator usage, symptom scores, peak flow, and exacerbations did not reach statistical significance with data from studies applying unclear reversibility criteria.
Summary
This review has found evidence that long‐acting beta‐agonist treatment in people with poorly reversible COPD leads to statistically significant improvements in lung function, quality of life, symptom scores and significantly fewer exacerbations. The clinical relevance of these effects may be small, and should be borne in mind when deciding whether this treatment option is a viable maintenance therapy in people with COPD. Future trials are likely to recruit people with COPD defined more by symptom and smoking histories, and may therefore be less reliant on spirometry as a guide to assessing acute response to bronchodilator therapy. The role of long‐acting beta agonists in the treatment of COPD may become limited as other more potent therapies such as combination therapies show impressive effects over mono components, including salmeterol and formoterol (Nannini 2004).
Authors' conclusions
Implications for practice.
The evidence considered in this review only relates to those patients with poorly reversible COPD. In these patients who are treated with salmeterol 50 mcg there is a small statistically significant improvement in lung function. In the absence of evidence as to whether this difference is meaningful, this effect is of uncertain clinical significance. There were some benefits in quality of life measures and reduction in symptoms. Exacerbations and rescue salbutamol use were both reduced following treatment with salmeterol 50 mcg for at least four weeks. People who suffer frequent deterioration in symptoms prompting additional medication usage could benefit from this therapy. The strength of evidence for the use of salmeterol 100 mcg, formoterol 12 mcg, 18 mcg, 24 mcg was insufficient to provide clear guidance for practice. Clinicians should consider the trade‐offs between the possibly small clinical benefit of long‐acting beta‐agonists versus the side effect profile and financial cost for the individual patient.
Implications for research.
Longer term studies than those in this systematic review (for example TORCH) are needed to assess more fully the effect on exacerbations and health care utilisation, before more definite conclusions can be drawn. In addition to measures of symptoms, studies should include a measure of exercise tolerance and HRQL with perhaps less emphasis on FEV1 given emerging evidence. Cost‐benefit analysis is also an important outcome. Further studies are needed to clarify whether the positive responses observed (improved health status and reduced breathlessness) with LABA therapy are related to FEV1 reversibility status of participants/inclusion of asthmatics in these studies. This will require the incorporation of measurements of lung hyperinflation in spirometric assessments.
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2012 | Review declared as stable | This review is no longer being updated. A new review on LABA for COPD is being prepared (for protocol see Karner 2012). |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 1 August 2008 | Amended | Converted to new review format. |
| 23 March 2006 | New citation required and conclusions have changed | Addition of 18 published and unpublished studies. Whilst all of these studies contributed data, the reversibility criteria for some of the unpublished studies was unclear. They were therefore included as a subgroup analysis. The new studies are: 408DP‐03; Calverly 2003; Celli 2003; Chapman 2002; Dal Negro 2003; Dauletbaev 2001; Gupta 2002; Hanania 2003; Mahler 2002; Rennard 2001; Rossi 2002; SCO40030; SMS40298; SMS40314; SMS40315; SMS40318; Stockley 2002; Wadbo 2002 Changes to the review. The confidence intervals for change in FEV1 tightened; changes in health related quality of life measurements show significant differences in favour of salmeterol; there is now evidence that salmeterol reduces the odds of an exacerbation of COPD (NNT 21). |
| 31 August 2002 | New citation required and conclusions have changed | The updated review Quality of life data from Jones et al available now as change scores Review updated with four studies added to the review (Goodwin 1997; Mahler 1999; van Noord 2000; R‐van Molken 1999). Pooling of studies possible for some outcomes. SMD analysis of FEV1 outcome. |
Notes
This review is no longer being updated. A new review on LABA for COPD is being prepared (for protocol see Karner 2012).
Acknowledgements
We would like to thank Elizabeth Arnold, Veronica Stewart, Bettina Reuben, Karen Blackhall, Steve Milan, and Anna Bara for her help and assistance with data extraction, and Paul Jones and Peter Gibson, for advice. We would also like to thank Dr. Alison Grove, Dr Gerry Hagan and GSK who responded to our enquiries and requests for data.
Appendices
Appendix 1. Archive of previous methods for assessing study quality
Allocation concealment: Grade A: adequate Grade B: uncertain Grade C: clearly inadequate
Five point Jadad 1996: 1. The study was described as randomised (yes: 1, no: 0) 2. The method of randomisation was described and was appropriate (yes: 1, no: ‐1) 3. The study was described as double blind (yes: 1, no: 0) 4. The method of blinding was described and was appropriate (yes: 1, no: ‐1) 5. There was a description of withdrawals and drop outs (yes: 1, no: 0)
Appendix 2. Randomsiation procedures for GSK‐sponsored studies
The procedures for randomising GSK sponsored studies has been detailed in correspondence between Richard Follows and TL, the details of which are given below:
The randomisation software is a computer‐generated, centralised programme (RandAll). After verification that the randomisation sequence is suitable for the study design (crossover, block or stratification), Clinical Supplies then package the treatments according the randomisation list generated. Concealment of allocation is maintained by a third party, since the sites phone in and are allocated treatments on that basis. Alternatively a third party may dispense the drug at the sites. Unblinding of data for interim analyses can only be done through RandAll, and are restricted so that only those reviewing the data are unblinded to treatment group allocation.
Data and analyses
Comparison 1. Salmeterol (50 mcg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1(mls) from baseline to end of study (parallel study) | 8 | 2026 | mls (Fixed, 95% CI) | 51.00 [31.93, 70.07] |
| 1.1 FEV1 Reversibility to short acting beta‐agonist < 15 (% baseline) | 6 | 933 | mls (Fixed, 95% CI) | 49.63 [17.45, 81.82] |
| 1.2 FEV1 Reversiblity to short‐acting beta‐agonist < 15 (% predicted normal) | 2 | 1093 | mls (Fixed, 95% CI) | 51.73 [28.06, 75.41] |
| 2 Change in FEV1 (% predicted) from baseline to end of study (parallel study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pre‐bronchodilator FEV1(L) at end of study (parallel group studies) | 5 | 1468 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.18, 0.09] |
| 3.1 FEV1 Reversibility to short‐acting beta‐agonist < 15 (% baseline) | 3 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.23, 0.02] |
| 3.2 FEV1 Reversibility to short‐acting beta‐agonist < 15 (% predicted normal) | 1 | 733 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.04, 0.08] |
| 3.3 Unclear reversibility | 1 | 494 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FEV1 at end of study (crossover studies) | 2 | 158 | Litres (Fixed, 95% CI) | 0.00 [‐0.04, 0.04] |
| 5 PEF at end of study (crossover study) | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 5.1 Morning PEF | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Evening PEF | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF (L/min) at end of study (parallel study) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Morning PEF | 3 | 842 | Mean Difference (IV, Fixed, 95% CI) | 14.89 [10.86, 18.91] |
| 6.2 Evening PEF | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐11.13, 47.13] |
| 7 Change in am PEF (parallel studies) | 2 | 540 | L/min (Fixed, 95% CI) | 8.36 [2.88, 13.85] |
| 8 Change in am PEF (L/min ‐ parallel studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Six minute walk test, mean change (m) from baseline to end of study (crossover study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Six minute walk test, distance (m) at end of study (parallel group studies) | 4 | 659 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [‐10.73, 13.88] |
| 11 Six minute walk, Borg score (<3) for breathlessness at end of study (parallel study) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Borg Score for dyspnoea, pre‐walk, at end of study (parallel group studies) | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.38, 0.28] |
| 13 Borg Score for dyspnoea, post‐walk, at end of study (parallel group studies) | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.59, 0.27] |
| 14 Change in HRQL from baseline to end of study: SGRQ | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 symptoms | 4 | 1248 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐5.01, ‐0.60] |
| 14.2 activity | 4 | 1248 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐3.54, 0.12] |
| 14.3 impacts | 4 | 1248 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐5.53, 0.37] |
| 14.4 total | 4 | 1248 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐4.06, ‐0.45] |
| 15 Change in HRQL from baseline to end of study: SF‐36 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 role physical | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 general health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.7 role emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.8 mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Change in HRQL from baseline to end of study: CRDQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Dyspnoea | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Fatigue | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Emotion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Mastery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Total | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Total SGRQ score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18 HRQL at end of study: SF‐36 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Physical Functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Role Physical | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Vitality, Energy and Fatigue | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Social Functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Role limitation ‐ Emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Health Change | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.8 Mental Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 HRQL at end of study: CRDQ scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 overall score | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 5.05 [‐2.76, 12.87] |
| 19.2 Fatigue | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 1.86 [0.35, 3.37] |
| 19.3 Dyspneoa | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 2.21 [0.45, 3.97] |
| 19.4 Emotion | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 2.37 [‐0.19, 4.94] |
| 19.5 Mastery | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [‐0.41, 2.56] |
| 20 Symptom scores at end of study: Transition Dyspnoea Index (parallel studies) | 3 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.40, 0.72] |
| 21 Symptom Scores at end of study: Baseline Dyspnoea Index (parallel group study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22 Symptom Scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.1 Day time symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Night time symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Overall | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Symptom Scores‐daytime at end of study | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 shortness of breath | 3 | 941 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 23.2 cough | 3 | 941 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 23.3 chest tightness | 2 | 208 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.40, 0.15] |
| 23.4 Sputum production | 1 | 733 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.23, 0.06] |
| 24 Symptom Scores‐nighttime at end of study | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 shortness of breath | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.28, 0.23] |
| 24.2 cough | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.28, 0.24] |
| 24.3 chest tightness | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.11] |
| 25 Exacerbations, Number of subjects experiencing (parallel study) | 4 | 1741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.90] |
| 26 Exacerbations, Number of subjects experiencing (cross‐over study) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 27 Exacerbations per participant | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 28 Rescue medication use (number of day time puffs of salbutamol) | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.39, ‐0.60] |
| 29 Withdrawals due to lack of efficacy | 5 | 1581 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.54] |
| 30 Rescue medication use (% days with additional salbutamol use) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 31 Rescue medication use (% nights with additional salbutamol use) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 1 Change in FEV1(mls) from baseline to end of study (parallel study).
1.2. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 2 Change in FEV1 (% predicted) from baseline to end of study (parallel study).
1.3. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 3 Pre‐bronchodilator FEV1(L) at end of study (parallel group studies).
1.4. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 4 FEV1 at end of study (crossover studies).
1.5. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 5 PEF at end of study (crossover study).
1.6. Analysis.
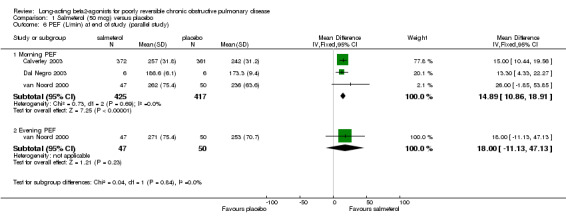
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 6 PEF (L/min) at end of study (parallel study).
1.7. Analysis.
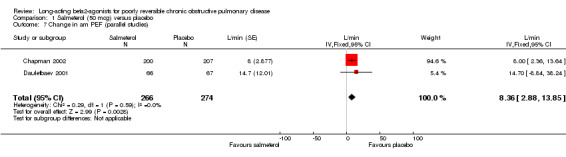
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 7 Change in am PEF (parallel studies).
1.8. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 8 Change in am PEF (L/min ‐ parallel studies).
1.9. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 9 Six minute walk test, mean change (m) from baseline to end of study (crossover study).
1.10. Analysis.
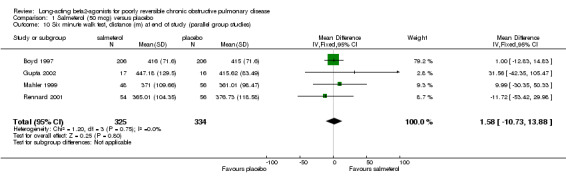
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 10 Six minute walk test, distance (m) at end of study (parallel group studies).
1.11. Analysis.
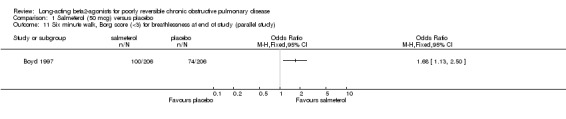
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 11 Six minute walk, Borg score (<3) for breathlessness at end of study (parallel study).
1.12. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 12 Borg Score for dyspnoea, pre‐walk, at end of study (parallel group studies).
1.13. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 13 Borg Score for dyspnoea, post‐walk, at end of study (parallel group studies).
1.14. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 14 Change in HRQL from baseline to end of study: SGRQ.
1.15. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 15 Change in HRQL from baseline to end of study: SF‐36.
1.16. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 16 Change in HRQL from baseline to end of study: CRDQ.
1.17. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 17 Total SGRQ score.
1.18. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 18 HRQL at end of study: SF‐36.
1.19. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 19 HRQL at end of study: CRDQ scores.
1.20. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 20 Symptom scores at end of study: Transition Dyspnoea Index (parallel studies).
1.21. Analysis.
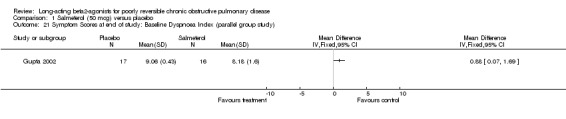
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 21 Symptom Scores at end of study: Baseline Dyspnoea Index (parallel group study).
1.22. Analysis.
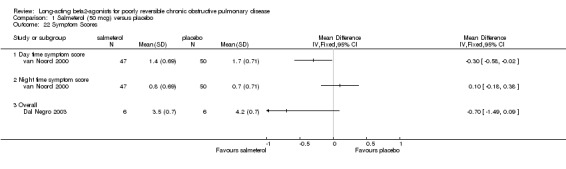
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 22 Symptom Scores.
1.23. Analysis.
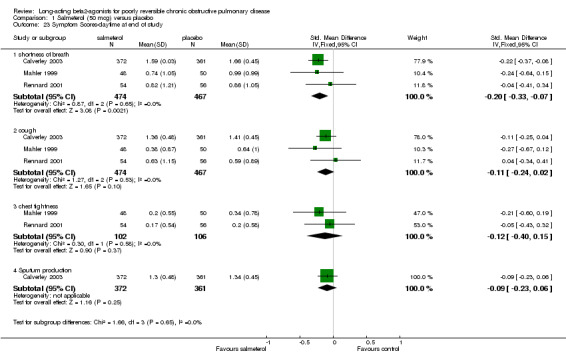
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 23 Symptom Scores‐daytime at end of study.
1.24. Analysis.
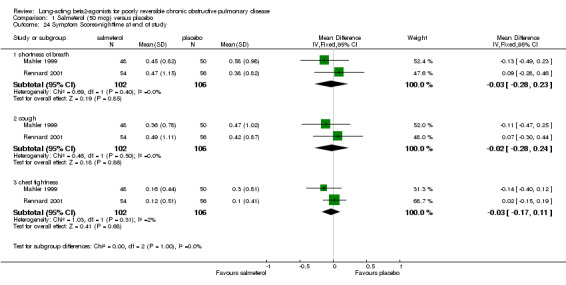
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 24 Symptom Scores‐nighttime at end of study.
1.25. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 25 Exacerbations, Number of subjects experiencing (parallel study).
1.26. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 26 Exacerbations, Number of subjects experiencing (cross‐over study).
1.27. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 27 Exacerbations per participant.
1.28. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 28 Rescue medication use (number of day time puffs of salbutamol).
1.29. Analysis.
Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 29 Withdrawals due to lack of efficacy.
1.30. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 30 Rescue medication use (% days with additional salbutamol use).
1.31. Analysis.

Comparison 1 Salmeterol (50 mcg) versus placebo, Outcome 31 Rescue medication use (% nights with additional salbutamol use).
Comparison 2. Salmeterol (100 mcg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1(L) from baseline to end of study (parallel study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Six minute walk test, Borg score (<3) for breathlessness at end of study (parallel study) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Change in HRQL from baseline to end of study: SGRQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 activity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 impacts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 total | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in HRQL from baseline to end of study: SF‐36 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 role physical | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 general health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 role emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations, number of subjects experiencing | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Salmeterol (100 mcg) versus placebo, Outcome 1 Change in FEV1(L) from baseline to end of study (parallel study).
2.2. Analysis.
Comparison 2 Salmeterol (100 mcg) versus placebo, Outcome 2 Six minute walk test, Borg score (<3) for breathlessness at end of study (parallel study).
2.3. Analysis.
Comparison 2 Salmeterol (100 mcg) versus placebo, Outcome 3 Change in HRQL from baseline to end of study: SGRQ.
2.4. Analysis.
Comparison 2 Salmeterol (100 mcg) versus placebo, Outcome 4 Change in HRQL from baseline to end of study: SF‐36.
2.5. Analysis.
Comparison 2 Salmeterol (100 mcg) versus placebo, Outcome 5 Exacerbations, number of subjects experiencing.
Comparison 3. Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1(mls) from baseline to end of study | 11 | 2644 | mls (Fixed, 95% CI) | 55.13 [38.16, 72.09] |
| 1.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 8 | 2026 | mls (Fixed, 95% CI) | 51.00 [31.93, 70.07] |
| 1.2 Unclear baseline reversibility data | 3 | 618 | mls (Fixed, 95% CI) | 70.77 [33.65, 107.88] |
| 2 Change in HRQL from baseline to end of study: SGRQ (total score) | 10 | 3607 | SGRQ units (Fixed, 95% CI) | ‐2.17 [‐2.88, ‐1.46] |
| 2.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 6 | 2111 | SGRQ units (Fixed, 95% CI) | ‐1.52 [‐2.43, ‐0.60] |
| 2.2 Unclear baseline reversibility data | 4 | 1496 | SGRQ units (Fixed, 95% CI) | ‐3.19 [‐4.33, ‐2.05] |
| 3 Change in HRQL from baseline to end of study: SGRQ (symptoms) | 7 | 2922 | SGRQ units (Fixed, 95% CI) | ‐2.17 [‐3.31, ‐1.02] |
| 3.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 5 | 1981 | SGRQ units (Fixed, 95% CI) | ‐1.04 [‐2.39, 0.30] |
| 3.2 Unclear baseline reversibility data | 2 | 941 | SGRQ units (Fixed, 95% CI) | ‐5.13 [‐7.32, ‐2.94] |
| 4 Change in HRQL from baseline to end of study: SGRQ (activity) | 8 | 3052 | SGRQ units (Fixed, 95% CI) | ‐2.13 [‐3.16, ‐1.10] |
| 4.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 6 | 2111 | SGRQ units (Fixed, 95% CI) | ‐1.61 [‐2.81, ‐0.40] |
| 4.2 Unclear baseline reversibility data | 2 | 941 | SGRQ units (Fixed, 95% CI) | ‐3.56 [‐5.55, ‐1.56] |
| 5 Change in HRQL from baseline to end of study: SGRQ (impact) | 7 | 2922 | SGRQ units (Fixed, 95% CI) | ‐2.13 [‐3.09, ‐1.17] |
| 5.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 5 | 1981 | SGRQ units (Fixed, 95% CI) | ‐2.03 [‐3.15, ‐0.91] |
| 5.2 Unclear baseline reversibility data | 2 | 941 | SGRQ units (Fixed, 95% CI) | ‐2.39 [‐4.24, ‐0.54] |
| 6 Exacerbations, Number of subjects experiencing (parallel study) | 6 | 2722 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.95] |
| 6.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 4 | 1741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.90] |
| 6.2 Unclear baseline reversibility data | 2 | 981 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.24] |
| 7 Symptom Scores ‐ cough at end of study | 4 | 1566 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.17, 0.02] |
| 7.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 3 | 941 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 7.2 Unclear baseline reversibility data | 1 | 625 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.18, 0.13] |
| 8 Rescue medication use (end of study) | 5 | 877 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.03, ‐0.53] |
| 8.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.39, ‐0.60] |
| 8.2 Unclear baseline reversibility data | 1 | 624 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.96, ‐0.32] |
| 9 Morning PEF (L/min) at end of study (parallel study) | 4 | 1467 | Mean Difference (IV, Fixed, 95% CI) | 15.81 [11.96, 19.67] |
| 9.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 3 | 842 | Mean Difference (IV, Fixed, 95% CI) | 14.89 [10.86, 18.91] |
| 9.2 Unclear baseline reversibility data | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 26.10 [12.69, 39.51] |
| 10 Change in clinic PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Unclear baseline reversibility data | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Symptom Scores ‐ breathlessness score at end of study | 4 | 1565 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.28, ‐0.08] |
| 11.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 3 | 941 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 11.2 Unclear baseline reversibility data | 1 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.30, 0.01] |
| 12 Symptom scores at end of study: Transition Dyspnoea Index (parallel studies) | 5 | 934 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.06, 0.81] |
| 12.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 3 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.40, 0.72] |
| 12.2 Unclear baseline reversibility data | 2 | 562 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.15, 1.15] |
| 13 Change in supplemental short‐acting beta agonist usage | 3 | 1222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.73, ‐0.36] |
| 13.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Unclear baseline reversibility data | 3 | 1222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.73, ‐0.36] |
| 14 Withdrawals due to lack of efficacy | 10 | 3363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.28, 0.68] |
| 14.1 Baseline reversibility to short acting beta‐agonist meet review criteria | 5 | 1581 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.54] |
| 14.2 Unclear baseline reversibility data | 5 | 1782 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.11] |
3.1. Analysis.
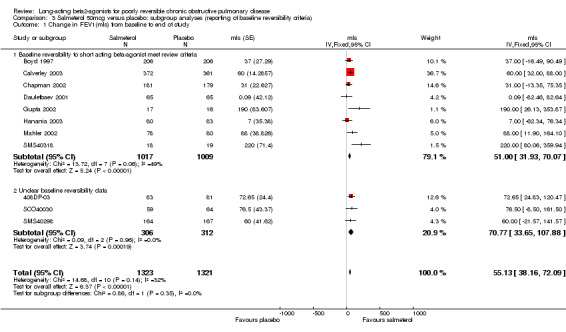
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 1 Change in FEV1(mls) from baseline to end of study.
3.2. Analysis.
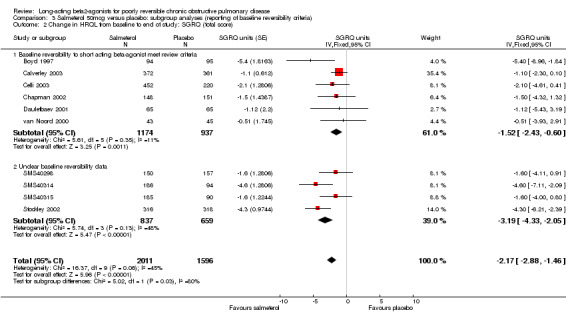
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 2 Change in HRQL from baseline to end of study: SGRQ (total score).
3.3. Analysis.
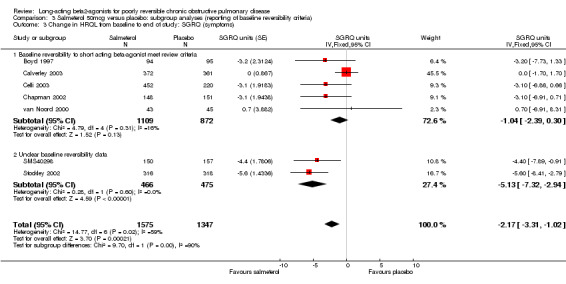
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 3 Change in HRQL from baseline to end of study: SGRQ (symptoms).
3.4. Analysis.
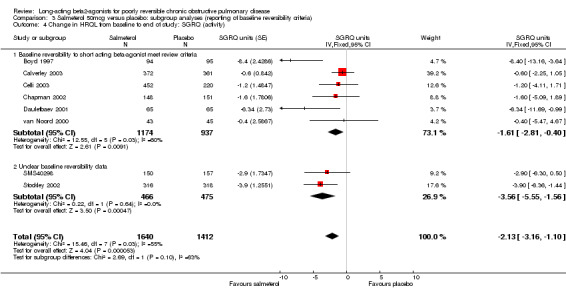
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 4 Change in HRQL from baseline to end of study: SGRQ (activity).
3.5. Analysis.
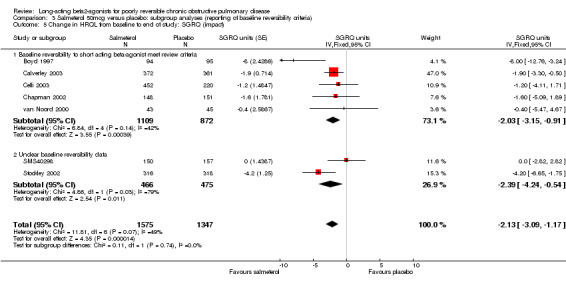
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 5 Change in HRQL from baseline to end of study: SGRQ (impact).
3.6. Analysis.
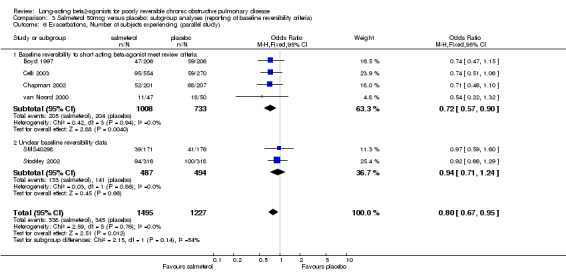
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 6 Exacerbations, Number of subjects experiencing (parallel study).
3.7. Analysis.
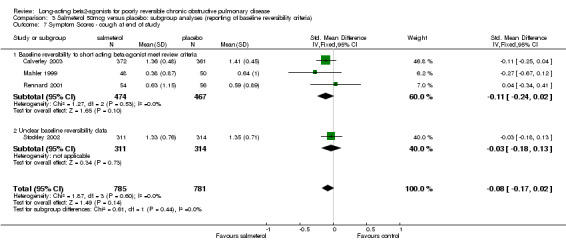
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 7 Symptom Scores ‐ cough at end of study.
3.8. Analysis.
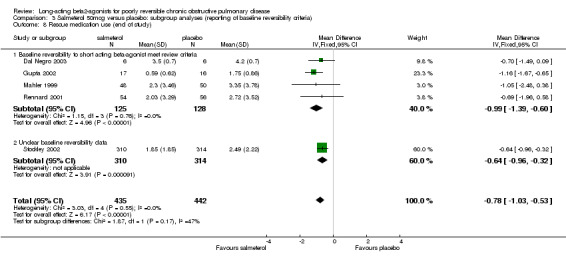
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 8 Rescue medication use (end of study).
3.9. Analysis.
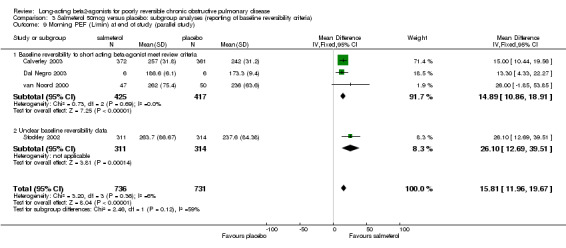
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 9 Morning PEF (L/min) at end of study (parallel study).
3.10. Analysis.
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 10 Change in clinic PEF (L/min).
3.11. Analysis.
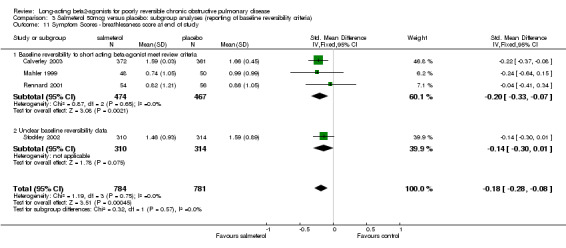
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 11 Symptom Scores ‐ breathlessness score at end of study.
3.12. Analysis.
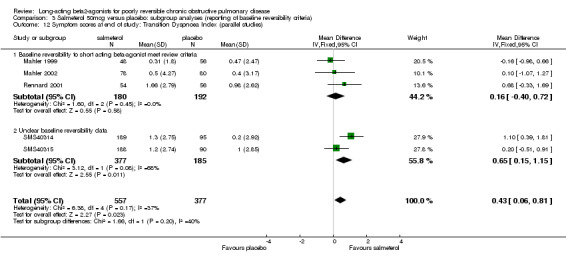
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 12 Symptom scores at end of study: Transition Dyspnoea Index (parallel studies).
3.13. Analysis.
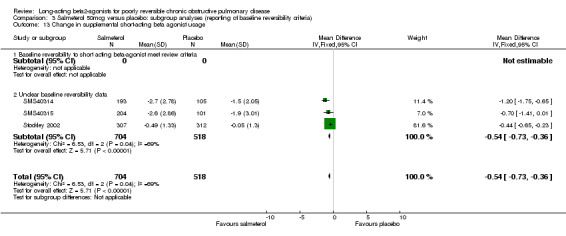
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 13 Change in supplemental short‐acting beta agonist usage.
3.14. Analysis.
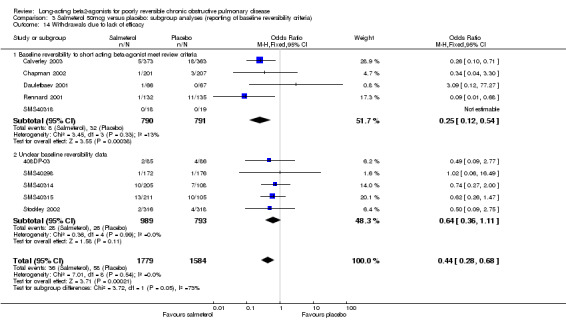
Comparison 3 Salmeterol 50mcg versus placebo: subgroup analyses (reporting of baseline reversibility criteria), Outcome 14 Withdrawals due to lack of efficacy.
Comparison 4. Formoterol (12mcg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐bronchodilator End of study FEV1(L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 12 months | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.08, 0.24] |
| 2 Pre‐bronchodilator end of study PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 12 months | 1 | 178 | Mean Difference (IV, Fixed, 95% CI) | 27.19 [‐1.39, 55.77] |
| 3 Number of subjects experiencing exacerbations over 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 number of subjects with at least one | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.45, 2.72] |
| 3.2 moderate: number of subjects requiring additional therapy | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.67] |
| 3.3 severe: requiring hospital admission | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.95] |
| 4 Quality of life: change from baseline to end of study SGRQ scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 symptoms | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐5.60 [‐11.29, 0.09] |
| 4.2 activity | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐5.76, 4.46] |
| 4.3 impacts | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐7.23, 3.69] |
| 4.4 total | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐6.70, 1.70] |
4.1. Analysis.
Comparison 4 Formoterol (12mcg) versus placebo, Outcome 1 Pre‐bronchodilator End of study FEV1(L).
4.2. Analysis.
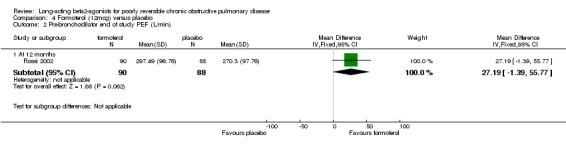
Comparison 4 Formoterol (12mcg) versus placebo, Outcome 2 Pre‐bronchodilator end of study PEF (L/min).
4.3. Analysis.
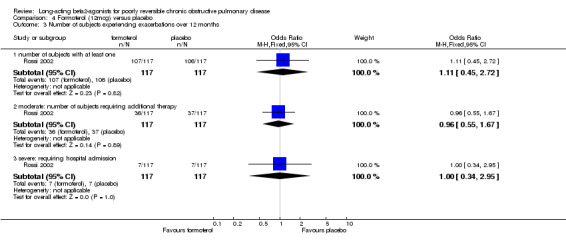
Comparison 4 Formoterol (12mcg) versus placebo, Outcome 3 Number of subjects experiencing exacerbations over 12 months.
4.4. Analysis.
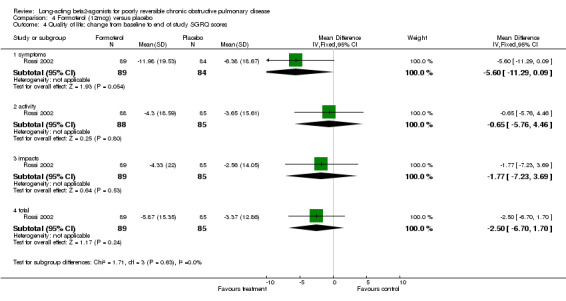
Comparison 4 Formoterol (12mcg) versus placebo, Outcome 4 Quality of life: change from baseline to end of study SGRQ scores.
Comparison 5. Formoterol (18mcg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1‐Mean change from baseline as % or % predicted | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FVC‐ Mean change from baseline | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in PEF (L/min), morning | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Shuttle Walking Test Distance (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Post shuttle walk test Borg dyspnea score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Rescue medication use | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Number of day time puffs of salbutamol | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Symptom scores at end of study: change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 shortness of breath at night | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 daytime shortness of breath | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 night‐time cough | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 daytime cough | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 sleep | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in quality of life (% units) at end of study: SGRQ | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 symptoms | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 activity | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 impacts | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 total | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.

Comparison 5 Formoterol (18mcg) versus placebo, Outcome 1 FEV1‐Mean change from baseline as % or % predicted.
5.2. Analysis.

Comparison 5 Formoterol (18mcg) versus placebo, Outcome 2 FVC‐ Mean change from baseline.
5.3. Analysis.

Comparison 5 Formoterol (18mcg) versus placebo, Outcome 3 Change in PEF (L/min), morning.
5.4. Analysis.

Comparison 5 Formoterol (18mcg) versus placebo, Outcome 4 Shuttle Walking Test Distance (change from baseline).
5.5. Analysis.

Comparison 5 Formoterol (18mcg) versus placebo, Outcome 5 Post shuttle walk test Borg dyspnea score.
5.6. Analysis.
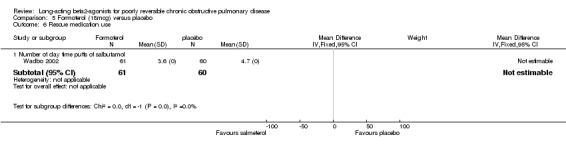
Comparison 5 Formoterol (18mcg) versus placebo, Outcome 6 Rescue medication use.
5.7. Analysis.
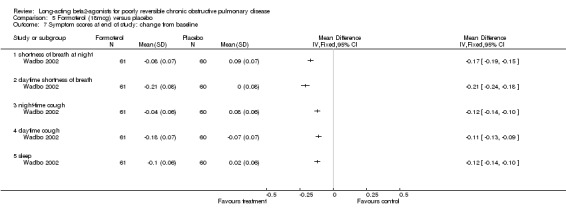
Comparison 5 Formoterol (18mcg) versus placebo, Outcome 7 Symptom scores at end of study: change from baseline.
5.8. Analysis.
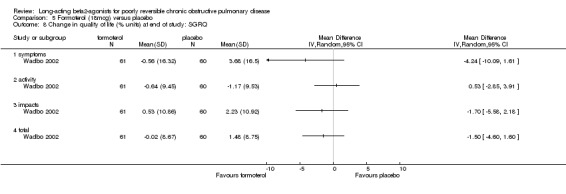
Comparison 5 Formoterol (18mcg) versus placebo, Outcome 8 Change in quality of life (% units) at end of study: SGRQ.
Comparison 6. Formoterol (24mcg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of study pre‐bronchodilator FEV1(L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 12 months | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.15, 0.17] |
| 2 Daily morning PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 12 months | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 22.73 [‐7.70, 53.16] |
| 3 number of subjects experiencing exacrbations over 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 mild: percentage of bad days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of subjects experiencing exacerbations over duration of study | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 number of subjects with at least one exacerbation | 1 | 214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.34, 1.96] |
| 4.2 moderate: number of subjects requiring additional therapy | 1 | 214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.49, 1.57] |
| 4.3 severe:requiring hospital admission | 1 | 214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.06, 1.05] |
| 5 Quality of life at 12 months: Change form baseline SGRQ scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 symptoms | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐7.19, 4.69] |
| 5.2 activity | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐7.31, 2.91] |
| 5.3 impacts | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐3.66 [‐8.26, 0.94] |
| 5.4 total | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐2.92 [‐7.05, 1.21] |
6.1. Analysis.
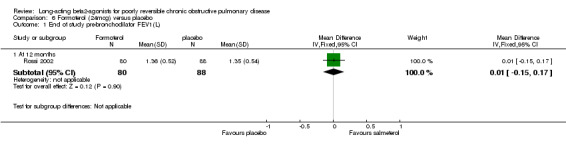
Comparison 6 Formoterol (24mcg) versus placebo, Outcome 1 End of study pre‐bronchodilator FEV1(L).
6.2. Analysis.
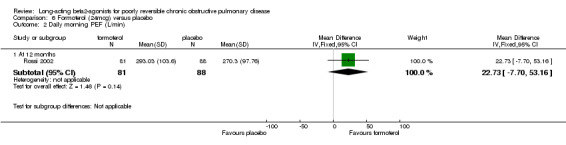
Comparison 6 Formoterol (24mcg) versus placebo, Outcome 2 Daily morning PEF (L/min).
6.3. Analysis.

Comparison 6 Formoterol (24mcg) versus placebo, Outcome 3 number of subjects experiencing exacrbations over 12 months.
6.4. Analysis.
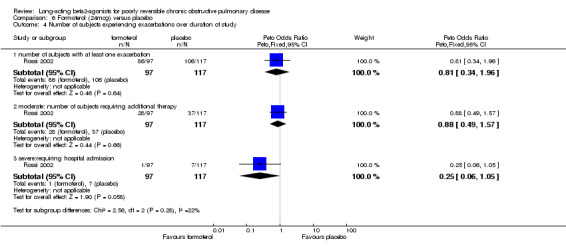
Comparison 6 Formoterol (24mcg) versus placebo, Outcome 4 Number of subjects experiencing exacerbations over duration of study.
6.5. Analysis.
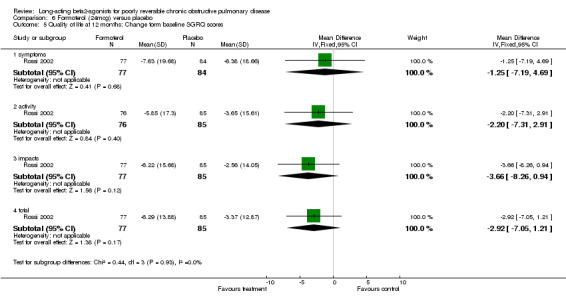
Comparison 6 Formoterol (24mcg) versus placebo, Outcome 5 Quality of life at 12 months: Change form baseline SGRQ scores.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
408DP‐03.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 12 week treatment period; 12 week extension phase. Baseline characteristics: comparable. Power calculation: Not described. Intention to treat analysis stated. Jadad Score: 3 |
|
| Participants | 1) Setting: 29 centres in Japan 2) Participants randomised: 191 (salmeterol: 85; placebo: 86) 3) Baseline characteristics: Mean age: 71.5 years; lung function not reported 4) Inclusion Criteria: >/= 40 years with diagnosis of COPD for 6 months prior to screening; FEV1/FVC ratio < 70%; < 300ml and 20% reversibility to SABA; >/= 10 pack year history; use of xanthines/anticholinergics for 12 weeks prior to screening. 5) Exclusion Criteria: O2 therapy; unable to have PFT; significant untreated co‐morbidity; co‐existing lung disease that could interfere with efficacy of treatment; moderate or severe exacerbation during run‐in (requiring antibiotics, oral steroids or hospitalisation); |
|
| Interventions | 1) Salmeterol 50mcg two puffs BID 2) Placebo Participants allowed concomitant therapy with xanthines or anticholinergic |
|
| Outcomes | 1) Change in FEV1 (M) | |
| Notes | Unpublished study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices |
Boyd 1997.
| Methods | Parallel group design. Excluded: not described. Withdrawals: numbers but not reasons described. Trial duration: 2‐week run in period, 16 weeks treatment, 2‐weeks follow up. Baseline characteristics: comparable. Power calculation: 420 evaluable participants required to give 90% power to detect a difference of one in the five point symptom score. Intention to treat analysis stated. Jadad Score:4 |
|
| Participants | 1) Setting: Multi‐centre (75 centres), multi‐national (18 countries) study. 2) Participants randomised n = 674 in 3 participant arms. Placebo n = 227; Salmeterol 50 mcg n = 229; salmeterol 100 mcg n = 218. 3) Baseline characteristics: Mean age 62 years; mean FEV1: 1.28 L 4) Inclusion Criteria: smokers/ex‐smokers, FEV1 <70% predicted, FEV1/FVC =< 60% predicted, and 5‐15 % reversibility of FEV1 following salbutamol, symptom score of at least 2 on at least 4 of 7 days prior to randomisation. 5) Exclusion Criteria: evidence of serious uncontrolled systemic disease, respiratory disorders other than COPD, pregnancy and lactation, hospitalisation for COPD, treatment for acute respiratory infection, medication changes in the 4 weeks prior to start of study, oxygen therapy, inability to complete 6 minute walk, hypersensitivity to beta‐2 agonists, use of beta‐blocker therapy, other research medication. |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Salmeterol 100 mcg bd Medication delivered by MDI. 3) Placebo Participants allowed to be on medications if prescribed prior to 4 weeks before study entry. |
|
| Outcomes | 1) Lung function: Change in FEV1 (M) 2) Exercise tolerance: distance walked in 6 min (D), Borg score (< 3) for breathlessness post 6 minute walk (M) 3) Quality of life: Change in SGRQ for symptoms, activity, impacts and total domains (M); Change in SF‐36 scores for physical functioning, role physical, pain, general health, vitality, social functioning, role emotional and mental health domains (M) 4) Symptom scores: Breathlessness (Borg scores), Day and night symptom scores recorded on diary cards. (D) 5) Exacerbations: No. participants experiencing at least one exacerbation (M) 6) Rescue salbutamol use: Yes (D) |
|
| Notes | FEV1 reversibility (% baseline FEV1): </= 5%: 8% of participants </= 10%: 42% of participants > 10%, </= 15%: 50% of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Calverley 2003.
| Methods | Parallel group design. Excluded: Described. Withdrawals: Described. Trial duration: 2 week run‐in period, 52 weeks treatment, 2‐week follow‐up. Baseline characteristics: Comparable. Power calculation: Yes Intention to treat analysis: stated. Jadad Score: 5 |
|
| Participants | 1) Setting: Multi‐national (25 countries), Multicentre (196 hospitals) study. 2) Participants randomised n = 1465 in 4 study arms; Placebo n = 361; Salmeterol 50 mcg n = 372. 3) Baseline characteristics: Mean age: 63 years; mean FEV1 = 1.26L (44% predicted). 4) Inclusion criteria: Baseline FEV1 25 ‐ 75% predicted; FEV1/ FVC ratio </= 70%; Poor reversibility: < 10% increase of predicted FEV1 30 minutes after inhaling 400 mcg salbutamol; at least 10 pack years smoking history; history of exacerbations (at least 1 in the last year) requiring OCS and/or antibiotics. At least one episode of acute COPD per year in the previous 3 years. 5) Exclusion criteria: respiratory disorders other than COPD. Oxygen treatment, systemic corticosteroids, high doses of inhaled corticosteroids (> 1000 mcg daily beclomethasone dipropionate, budesonide or flunisolide or > 500 mcg daily fluticasone) or antibiotics in the four weeks before the 2 week run‐in period. |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Fluticasone 500 mcg bid 3) Salmeterol and Fluticasone combination 50 mcg/500 mcg bid 4) Placebo All treatments delivered via identically packaged inhalers Participants not on other maintenance therapies |
|
| Outcomes | 1) Lung Function: Prebronchodilator end of study FEV (M) 2) PEF at end of study (M) 3) Change in FEV1 (D) 4) Exercise tolerance (M) 5) Quality of life: SGRQ (Total scores: (M); Dyspnoea and symptoms: symptom score for shortness of breath, cough and sputum production (M); Impacts (M); Activity (M)) 6) Exacerbations (D) 7) Rescue Salbutamol use (M) |
|
| Notes | FEV1 reversibility (% predicted normal) Mean Reversibility (% predicted) = 3.8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Celli 2003.
| Methods | Parallel group design. Excluded: described. Withdrawals: described. Trial duration: 24 weeks with 2 week baseline assessment period and 4 week follow‐up period. Baseline characteristics: comparable Power calculation: Yes Intention to treat analysis: stated Jadad score: 3 |
|
| Participants | 1) Setting: Multi‐national (15 countries), Multicentre (189 hospitals) study. 2) Participants randomised n = 1368 in 3 study arms; Placebo n = 270; Salmeterol 50 mcg n = 554. 3) Baseline characteristics: Mean age: 64 years; mean FEV1: 1.32L (42% predicted). 4) Inclusion criteria: COPD symptoms > 2 years; Baseline FEV1 20 ‐ 70% predicted normal; Baseline FEV1/FVC < 65%; FEV1 reversibility to 400 mcg Salbutamol < 15% baseline or < 200ml; Age 40 ‐ 80 years, Smoking history > 15 pack years; > 1 exacerbation 2 ‐ 12 months prior to involvement in study requiring corticosteroids or antibiotics 5) Exclusion criteria: Concurrent respiratory disease other than COPD, onset of disease under age 35 years, need for regular nebulised therapy or domiciliary oxygen. COPD exacerbation in 6 weeks before the trial. Concurrent malignancy, CVD, other clinically significant disease/ abnormality. |
|
| Interventions | 1) Salmeterol 50 mcg bd plus placebo tds 2) Sibenet 500 mcg tds plus placebo bd 3) Placebo bId plus placebo tds All treatments were delivered via pressurised metered‐dose inhaler. Participants permitted concomitant therapy with oral or inhaled steroids |
|
| Outcomes | 1) Lung function: Pre‐bronchodilator FEV1 (D); FVC (D); Slow vital capacity (SVC) (D) 2) Exercise tolerance: ‐ 3) Quality of life: SGRQ for symptoms, activity, impacts, total domains (M); EuroQoL‐5D (EQ‐5D) (D) 4) Dyspnoea and symptoms: Breathlessness, cough and sputum scale (BCSS) (D) 5) Exacerbations: No. participants experiencing at least 1 exacerbation (M) 6) Rescue Salbutamol use: Yes (D) |
|
| Notes | FEV1 reversibility < 15% or < 200ml 99% patients not reversible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutated blocks and uneven scheme where patients were randomised on a 2:2:1 ratio favouring treatment groups over placebo |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy design |
Chapman 2002.
| Methods | Parallel group design. Excluded: described Withdrawals: described Trial duration: 24 weeks with 4 week run‐in period and 2 week follow‐up period. Baseline characteristics: comparable Power calculation: Yes Intention to treat analysis: stated Jadad score: 5 |
|
| Participants | 1) Setting: Multi‐national (6 countries), Multicentre (52 centres). 2) Participants randomised n = 408 in 2 study arms; Placebo n = 207; Salmeterol 50 mcg n = 201. 3) Baseline characteristics: Mean FEV1: 1.24L (45% predicted). 4) Inclusion criteria: Baseline FEV1 </= 85% predicted normal; FEV1/FVC ratio </= 70% predicted normal; FEV1 reversibility to Salbutamol 5 ‐ 15% predicted normal value; >/= Patients taking anti‐cholinergic agents alone or in combination therapy for at least four weeks prior to the study, 10 pack year smoking; sputum production most days on 3 consecutive months for 2 consecutive years; symptoms on at least 7/14 previous day/night periods of the run‐in phase 5) Exclusion criteria: Respiratory infection requiring prescribed medication or COPD hospitalisation in the 4 weeks before run‐in, concurrent respiratory disorders, pregnant or lactating women |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Placebo Medication delivered via diskus/accuhaler inhaler Participants were using anticholinergic agents during the study. |
|
| Outcomes | 1) Lung function: Change in FEV1 from baseline to end of study (M); Change in PEF (M) 2) Exercise tolerance: ‐ 3) Quality of life: Change in SGRQ from baseline to end of study for symptoms, activities, impacts and total domains (M) 4) Dyspnoea and symptoms: Symptom scores (D) 5) Exacerbations: Yes (M) 6) Rescue salbutamol use: Yes |
|
| Notes | FEV1 reversibility 5‐15% ( predicted normal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Dal Negro 2003.
| Methods | Parallel group design. Excluded: nil Withdrawals: nil. Trial duration: 12 months with 2 week run‐in period. Baseline characteristics: comparable Power calculation: No Intention to treat analysis: Not stated Jadad score: 5 |
|
| Participants | 1) Setting: pilot study 2) Participants randomised n = 18 in 3 study arms. Placebo n = 6; Salmeterol 50 mcg n = 6. 3) Baseline characteristics: Age range: 50‐78, mean FEV1 = 1.45L (49% predicted) 4) Inclusion criteria: FEV1 < 80% predicted normal, but > 800ml; FEV1/FVC ratio < 70% predicted; FEV1 reversibility to 400 mcg Salbutamol < 12% predicted normal, regular treatment with oral theophylline 200mg bid and short acting beta2‐agonist prn for a period of 6 months, smoking history of >/= 10 pack years. 5) Exclusion criteria: Asthma, respiratory disease other than COPD; regular use inhaled corticosteroids; exacerbations requiring oral/parental corticosteroids, changes in COPD medication or lower respiratory tract infection 4 weeks prior to study; unstable angina or unstable arrhythmias, recent MI or heart failure; Type I diabetes; Neuropsychiatric disorders; concurrent use of interacting medications (beta‐blockers, interact with methylxanthine products) Evidence of alcohol abuse |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Salmeterol/Fluticasone 50/250 mcg combination bid 3) Placebo All treatments were delivered via diskus inhaler. Participants with xanthines included in the study. |
|
| Outcomes | 1) Lung function: % Change in FEV1 over baseline pre‐treatment value, morning PEF at end of study (M) 2) Exercise tolerance: ‐ 3) Quality of life: ‐ 4) Dyspnoea and symptoms: Symptom scores using ATS system, overall (M) 5) Exacerbations: Yes, mean number per year (M) 6) Rescue salbutamol use: No. daytime puffs salbutamol (M) |
|
| Notes | FEV1 reversibility (< 12% predicted normal) Mean reversibility 3.08% (of basal value) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Dauletbaev 2001.
| Methods | Parallel group design. Excluded: not described Withdrawals: described. Trial duration: 8 weeks. Baseline characteristics: comparable. Power calculation: not described. Jadad Score: 4 |
|
| Participants | 1) Setting: 30 centres in Germany 2) Participants randomised: SAL: 66; PLA: 67 3) Baseline characteristics: Mean age: 59.8 years; Mean FEV1: 1.75L. 4) Inclusion criteria: M/F >/= 40 years; mild‐moderate COPD (ATS criteria: FEV1 40‐80%, predicted & reversibility <= 10%), symptom score >/= 1 on > 7 days during run‐in) 5) Exclusion criteria: long acting beta agonists, xanthine or anticholinergic therapy in 2 weeks prior to study entry; corticosteroids 4 weeks prior to study entry |
|
| Interventions | 1) Salmeterol 50mcg bid 2) Placebo Participants not treated with concomitant maintenance therapies. |
|
| Outcomes | 1) Quality of life (SGRQ) (M) 2) Withdrawals (M) 3) FEV1 (M) 4) am PEF (M) 5) symptoms (D) 6) adverse events: ‐ |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Grove 1996.
| Methods | Crossover study. Excluded: described Withdrawals: described. Trial duration: 4 weeks with one week run‐in period one week washout period between treatments Baseline characteristics: comparable. Power calculation: not given. Intention to treat analysis stated. Jadad Score:4 |
|
| Participants | 1) Setting: Scotland, respiratory outpatient clinic. 2) Patients randomised n = 29 in 2 crossover study arms. Placebo followed by Salmeterol n = 14; Salmeterol followed by placebo n = 15. 3) Baseline characteristics: Mean age 64 yrs, Mean FEV1 1.17 L (42% predicted). 4) Inclusion criteria: stable COPD, FEV1 of 25‐75% predicted, 5‐15 % reversibility following salbutamol. 5) Exclusion criteria: Other significant systemic or musculoskeletal disease, maintenance therapy with oral steroids. |
|
| Interventions | 1) Salmeterol 50 mcg bd followed by Placebo bid. 2) Placebo bd/ followed by salmeterol 50mcg bid Medication delivered via MDI. Participants permitted to continue with maintenance therapy including ICS and xanthines. |
|
| Outcomes | 1) Lung function: End of treatment FEV1 (M) 2) Exercise tolerance: 6 minute walk test (M), cycle ergometry (D) 3) Quality of life 4) Dyspnoea and symptoms 5) Exacerbations 6) Rescue salbutamol use | |
| Notes | FEV1 reversibility (% baseline FEV1) mean (SD): 12.5 (7.0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Gupta 2002.
| Methods | Parallel group study. Excluded: not described. Withdrawals: not described. Trial duration: 8 weeks with 2‐week run‐in period Baseline characteristics: lung function and HRQL measures in salmeterol group lower baseline than salmeterol group. Power calculation/intention to treat analysis: not stated. Jadad score: 4 |
|
| Participants | 1) Setting: Outpatients 2) Patients randomised n = 33 in 2 treatment arms. Placebo n = 16; Salmeterol 50 mcg n = 17. 3) Baseline characteristics: All patients were male; mean age: 58 years; mean FEV1: Placebo 1.44L, Salmeterol 0.93L. 4) Inclusion criteria: COPD as per BTS criteria; FEV1 < 60% predicted, FEV1/FVC ratio < 70%, reversibility post salbutamol < 12% and 200ml. 20 pack year smoking history, productive cough and exertional dyspnoea. 5) Exclusion criteria: history of asthma or other respiratory disorder, recent (4 weeks) hospitalisation for COPD or respiratory tract infection. |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Placebo Treatments delivered via MDI |
|
| Outcomes | 1) Lung function: End of treatment FEV1 (M); Change in FEV1 mls (D) 2) Exercise tolerance: 6 minute walk distance (M). 3) Quality of life: End of study SF‐36 for physical functioning, role limitation physical, pain, general health, vitality energy and fatigue, social functioning, role limitation emotional, health change, mental health domains (M) 4) Dyspnoea and symptoms: Baseline dyspnoea index, patient self assessment (M) 5) Exacerbations: ‐ 6) Rescue salbutamol use: No. daytime puffs (M) |
|
| Notes | FEV1 reversibility (% baseline FEV1) Patients on existing regiment of ipratropium bromide and beclomethasone dipropionate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Hanania 2003.
| Methods | Parallel group study. Excluded: described. Withdrawals: described. Trial duration: 24 weeks with 2‐week run‐in period. Baseline characteristics: comparable. Power calculation: given. Intention to treat analysis: not stated. Jadad score: 4 |
|
| Participants | 1) Setting: USA, Multi‐centre (76 hospitals) 2) Patients randomised n = 723 in 4 study arms. Only patients with non‐reversible obstruction included in review. Placebo n = 185; Salmeterol 50 mcg n = 177. 3) Baseline characteristics: Mean age: 64 years; mean FEV1: 1.27L (42% predicted) 4) Inclusion criteria: stable COPD, FEV1 40 ‐ 65% predicted, FEV1/FVC < 70% predicted, symptoms of chronic bronchitis and moderate dyspnoea 5) Exclusion criteria: current diagnosis of asthma, use of oral steroids in past 6 weeks, abnormal ECG, LTOT, moderate ‐ severe exacerbation in run in. Other significant medical disorder. |
|
| Interventions | 1) Salmeterol 50 mcg bid 2) Fluticasone propionate 250 mcg 3) Salmeterol/Fluticasone propionate 50/250 mcg bid 4) Placebo Participants not on other maintenance therapies. |
|
| Outcomes | 1) Lung function: Change in FEV1 from baseline to end of study (M). PEF data not stratified by reversibility. 2) Exercise tolerance: N/A 3) Quality of life: CRDQ, CBSQ not stratified by reversibility 4) Dyspnoea and symptoms: Transitional dyspnoea index, Baseline dyspnoea index not stratified by reversibility 5) Exacerbations: Results not stratified by reversibility 6) Rescue salbutamol use: Data not stratified by reversibility |
|
| Notes | FEV1 reversibility < 12% or 200ml (of baseline FEV1) Reversibility stratified data. Mean % increase non‐reversible patients = 8.8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Mahler 1999.
| Methods | Parallel group study. Excluded: not described Withdrawals: described Trial duration: 12 week study with 14 ‐ 21 day run‐in period. Baseline characteristics: comparable. Power calculation: not given. Intention to treat analysis: stated. Jadad Score:5 |
|
| Participants | 1) Setting: USA, multi‐centre (27 centres) study. 2) Patients randomised n = 411 in 3 treatment arms. Only participants with non‐reversible obstruction included in review. Placebo n = 50; salmeterol n = 48. 3) Baseline characteristics: Mean age: 63 years; male:female (%): 74/26; mean FEV1: 40% predicted. 4) Inclusion criteria: stable participants with COPD defined by ATS criteria, >10 year pack history of smoking, > 35 years of age, FEV1 < 65% predicted, FEV1/FVC <70 %, SOB on mild exertion at baseline using modified MRC Dyspnoea scale. 5) Exclusion criteria: history of asthma, other respiratory disease, significant concurrent disease, change in medication or unstable respiratory status within 4 weeks prior to screening, oxygen therapy other than nocturnal use. |
|
| Interventions | 1) Salmeterol, 42 mcg bd +placebo ipratropium bromide 2) Ipratropium, 36 mcg qd + placebo salmeterol 3) Placebo for 1) & 2) All medications delivered via metered dose inhalers. Participants allowed prior maintenance therapy. |
|
| Outcomes | 1) Lung function: End of study FEV1 (M) 2) Exercise tolerance: six minute walk distance at week 10 (M), with post walk Borg scores for dyspnoea (M) 3) Quality of life: End of study CRDQ scores for overall, fatigue, dyspnoea, emotion and mastery domains (M) 4) Dyspnoea and symptoms: Transitional Dyspnoea Index (TDI). Day and night time symptom scores for shortness of breath, cough, chest tightness (M); Borg Scores (M) 5) Exacerbations: % patients with at least one exacerbation (D); time to first COPD exacerbation (D) 6) Rescue salbutamol use: No. daytime puffs (M) |
|
| Notes | FEV1 reversibility (% baseline FEV1) mean (SD): salmeterol:9.4(9.0) placebo:12.5(7.8) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
Mahler 2002.
| Methods | Parallel group study. Excluded: described. Withdrawals: described. Trial duration: 24 weeks. Baseline characteristics: comparable Power calculation: Yes Intention to treat analysis: Jadad score: 3 |
|
| Participants | 1) Setting: Multi‐centre study (65 centres) 2) Patients randomised n = 691 in 4 treatment arms. Data from 46 patients could not be evaluated. Only patients with non‐reversible obstruction included in review. Placebo n = 80; Salmeterol 50 mcg n = 78; 3) Baseline characteristics: Mean age: 64; FEV1 Litres: 1.25. BD% response: 20 . 4) Inclusion criteria: Participants with COPD according to ATS guidelines. Baseline pre‐bronchodilation FEV1 < 65% predicted and > 0.70L. Baseline pre‐bronchodilation FEV1/FVC < 70% predicted. Age > 40, 20 pack‐year history smoking, day or night symptoms present on 4 out of last 7 days during run‐in period. 5) Exclusion criteria: history of asthma, corticosteroid use in last 6 weeks, abnormal ECG, oxygen therapy, moderate or severe exacerbation during run‐in, significant concurrent disease. |
|
| Interventions | 1) Salmeterol 50 mcg bid. 2) Fluticasone Proprionate 500 mcg bid 3) Fluticasone Proprionate + Salmeterol 500/50 mcg bid 4) Placebo Medication delivered via Diskus. Participants not on other maintenance therapies |
|
| Outcomes | 1) Lung function: Change in FEV1 from baseline to end of study (M). 2) Exercise tolerance: ‐ 3) Quality of life: ‐ 4) Dyspnoea and symptoms: End of study dyspnoea (TDI) (M) CBSQ, CRDQ: results not stratified by reversibility 5) Exacerbations: Yes, results not stratified by reversibility 6) Rescue salbutamol use: Yes, results not stratified by reversibility |
|
| Notes | COPD participants reversible and non‐reversible, < 15% (baseline) improvement in FEV1 to salbutamol. Reversibility stratified data. Mean FEV1 reversibility 11.0% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Rennard 2001.
| Methods | Parallel group study. Excluded: not described Withdrawals: described Trial duration: 12 weeks with 2‐week run‐in period Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis. Jadad Score:5 |
|
| Participants | 1) Setting: USA, multi‐centre (27 centres) study. 2) Patients randomised n = 405 in 3 study arms. Only participants with non‐reversible obstruction included in review. Placebo n = 56; salmeterol n = 54. 3) Baseline characteristics: Mean age: 63 years; mean FEV1: 1.18 L; mean FEV1 % predicted:41%. 4) Inclusion criteria: stable participants with COPD defined by ATS criteria. >10 year pack history of smoking, > 35 years of age, FEV1 < 65% predicted, FEV1/FVC <70 %, SOB on mild exertion at baseline using modified MRC Dyspnoea scale. 5) Exclusion criteria: history of asthma, other respiratory disease, significant concurrent disease, change in medication or unstable respiratory status within 4 weeks prior to screening, oxygen therapy other than nocturnal use. |
|
| Interventions | 1) Salmeterol 42 mcg bd + placebo ipratropium bromide 2) Ipratropium, 36 mcg qd + placebo salmeterol 3) Placebo devices for 1) & 2) All medications delivered via metered dose inhalers Participants not on other maintenance therapies |
|
| Outcomes | 1) Lung function: End of study FEV1 (M) 2) Exercise tolerance: six minute walk test distance (M); post walk Borg scores for dyspnoea (M) 3) Health related quality of life: End of study CRDQ scores for overall, fatigue, dyspnoea, emotion and mastery domains (M) 4) Dyspnoea and symptoms: Transitional Dyspnoea Index (TDI). Day and night time symptom scores for SOB, cough and chest tightness (M) 5) Exacerbation: % patients with at least one exacerbation (D); time to first COPD exacerbation (D) 6) Rescue salbutamol use: No. daytime puffs (M) |
|
| Notes | Unpublished Glaxo‐Wellcome clinical trial data: SLGA4004. FEV reversibility (% baseline FEV1) mean (SD): salmeterol: 9.80%(7.6) placebo:11.22%(7.1) Reversibility stratified data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
Rossi 2002.
| Methods | Parallel group study. Excluded: not described Withdrawals: fully described. Trial duration: 12 months with 10 ‐21 day run‐in period. Baseline characteristics: comparable. Power calculation: given. Intention to treat analysis: stated Jadad Score: 4 |
|
| Participants | 1) Setting: Worldwide multi‐centre (81) study. 2) Patients randomised n = 854 in 4 study arms. Only participants with non‐reversible obstruction included in review. Placebo n = 109; Formoterol 12 mcg n = 113; Formoterol 24 mcg n = 92. 3) Baseline characteristics: Mean age: 63; FEV1 Litres: 1.38; male/female (%): 83/17. 4) Inclusion criteria: Patients with COPD as defined by ATS, age >= 40, current or ex‐smoker, FEV1 < 70% pred, >= 0.75 litres, FEV1/FVC <88% pred for men, <89% pred females. Day &/or night time symptoms present 4 of 7 days of run‐in. 5) Exclusion criteria: current or past diagnosis of asthma, respiratory tract infection in previous month, LTOT, QTc > 0.46s, change in ICS use, oral or parenteral use of CS in previous month, current theophylline treatment, oral or inhaled anticholinergics and LABA. |
|
| Interventions | 1) Dry powder formoterol (Foradil) 12mcg, bid via Aeroliser. 2) Dry powder formoterol (Foradil) 24mcg, bid via Aeroliser. 3) Theophylline, oral slow release. 4) Placebo via Aerolizer. Some participants treated with ICS as continuation of prior medication regimen. |
|
| Outcomes | 1) Lung function: End of study pre‐bronchodilator FEV1 (M) and PEF (M) 2) Exercise tolerance: ‐ 3) Quality of life: End of study SGRQ for symptoms, activity, impact and total domains (M) 4) Dyspnoea and symptoms: daily total symptom score: (Ability to perform ADL, SOB over prev 24 hrs, waking at night with symptoms, SOB on rising, cough, sputum. [0(none)‐3(worst)] Max score 18/day) (D) 5) Exacerbations: frequency of COPD exacerbations: (i.e. Mild: days with > 1 symptom >= 2 &/or PEF reduced 20%. ii. Moderate: additional therapy, CS, antibiotics, O2. iii. Severe: hospitalisation). 6) Rescue salbutamol use: N/A |
|
| Notes | Participants tested for reversibility to salbutamol, and then stratified according to reversibility was <15% (baseline). Patients previously on inhaled corticosteroids were kept on treatment throughout study Short courses of antibiotics, oral corticosteroids and/or oxygen were permitted in case of exacerbations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices |
SCO40030.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 8 weeks. Baseline characteristics: comparable. Power calculation: 50 participants per treatment group 90% power to detect difference of 400ml between combination and placebo. Intention to treat analysis stated. Jadad Score: 3. |
|
| Participants | 1) Setting: 22 centres in North America 2) Participants randomised: 185 (three groups: FP/SAL combination: 62; placebo: 64; salmeterol: 59) 3) Baseline characteristics: 65 years; FEV1: 1.12L 4) Inclusion criteria: M/F >/= 40 years of age; diagnosis of COPD; >/= 10 pack year; baseline Borg dyspnoea index <7; FEV1 <70% predicted; FRC >/= 120% predicted 5) Exclusion criteria: Current diagnosis of asthma; use of xanthines/LABAs/OCS/ICS |
|
| Interventions | 1) Salmeterol 50mcg bid 2) Fluticasone/salmeterol combination 500/50mcg bid 3) Placebo bid |
|
| Outcomes | 1) Change in FEV1 (M) 2) Withdrawals (M) 3) exercise time (D) |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
SMS40298.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 16 weeks. Baseline characteristics: comparable. Power calculation: 145 participants per treatment group 80% power to detect difference of 4 units of the SGRQ between SAL and placebo. Intention to treat analysis stated. Jadad Score: 3 |
|
| Participants | 1) Setting: 27 centres in Canada 2) Participants randomised: SAL: 172; PLA: 176 3) Baseline characteristics: 67 years; FEV1: 1.16L 4) Inclusion criteria: M/F 40‐79 years of age; diagnosis of COPD (ATS definition); >/= 10 pack year; receiving BD therapy; FEV1 </= 85% predicted; FEV1/FVC ratio: 70%; Reversibility <10% predicted within 12 months of assessment 1 5) Exclusion criteria: Not stated |
|
| Interventions | 1) Salmeterol 50mcg BID 2) Placebo |
|
| Outcomes | 1) Change FEV1(M) 2) Change in clinic PEF (M) 3) Quality of life (SGRQ) (M) 4) Withdrawals (M) 5) Symptoms (D) 6) Supplemental medication usage (D) 7) Exacerbations (M) |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
SMS40314.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 8 weeks. Baseline characteristics: comparable. Power calculation: 200 participants in active treatment groups to detect a significant difference of 1.2 L‐hours in FEV1 AUC. Jadad Score: 3 |
|
| Participants | 1) Setting: 55 centres in USA 2) Participants: Sal: 205; Placebo: 108; Sal/IpB: 213; IpB: 205 3) Baseline characteristics: Mean age: 64‐65 years FEV1: 1.25‐1.33 (42% predicted) 4) Inclusion criteria: M/F >/= 40 years; diagnosis of COPD; >/= 20 pack years; FEV1/FVC ratio of <0.7; FEV1 >/= 0.7L & </= 65% predicted; 5) Exclusion criteria: concurrent use of long acting beta agonists, anti‐leukotrienes, xanthine or anticholinergic therapy; corticosteroids > 10 mcg per day |
|
| Interventions | 1) Salmeterol 42mcg BID + placebo IpB 2) Salmeterol 42mcg BID + IpB 18mcg 4 x daily 3) Placebo salmeterol + IpB 18mcg 4 x daily 4) Placebo salmeterol + placebo IpB |
|
| Outcomes | 1) FEV1 AUC: ‐ 2) SGRQ (M) 3) TDI (M) 4) Symptoms (D) 5) Withdrawals (M) |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
SMS40315.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 8 weeks. Baseline characteristics: comparable. Power calculation: 200 participants in active treatment groups to detect a significant difference of 1.2 L‐hours in FEV1 AUC. Jadad Score: 3 |
|
| Participants | 1) Setting: 56 centres in USA 2) Participants randomised: SAL: 211; PLA: 105 3) Baseline characteristics: 63.5 years; FEV1: 1.33L (43% predicted) 4) Inclusion criteria: M/F >/= 40 years; diagnosis of COPD; >/= 20 pack years; FEV1/FVC ratio of <0.7; FEV1 >/= 0.7L & </= 65% predicted; 5) Exclusion criteria: concurrent use of long acting beta agonists, anti‐leukotrienes, xanthine or anticholinergic therapy; corticosteroids > 10 mcg per day |
|
| Interventions | 1) Salmeterol 42mcg BID + placebo IpB 2) Salmeterol 42mcg BID + IpB 18mcg 4 x daily 3) Placebo salmeterol + IpB 18mcg 4 x daily 4) Placebo salmeterol + placebo IpB |
|
| Outcomes | 1) FEV1 AUC: ‐ 2) Quality of life (SGRQ) (M) 3) Supplemental medication usage (M) 4) Withdrawals (M) 5) Symptoms (D) |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
SMS40318.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 4 weeks. Baseline characteristics: comparable. Power calculation: Not stated. Jadad Score: 3 |
|
| Participants | 1) Setting: three centres in Germany 2) Participants: Sal: 18; Placebo: 19 3) Baseline characteristics: Mean age: 59‐60 years 4) Inclusion criteria: M/F >/= 40 years; diagnosis of COPD (GOLD guidelines); >/= 10 pack years; increase in FEV1 <12% baseline or 200ml 5) Exclusion criteria: LTOT; LABA or CS treatment; change in medication/hospitalisation in preceding 4 weeks |
|
| Interventions | 1) Salmeterol (2 x 50mcg/d) 2) Placebo |
|
| Outcomes | 1) Inspiratory capacity (D) 2) Borg (D) 3) Withdrawals (M) |
|
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Stockley 2002.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 52 weeks. Baseline characteristics: comparable. Power calculation: 282 participants per group had 90% to detect a difference of 0.5% exacerbations at 5% level. Jadad Score: 3 |
|
| Participants | 1) Setting: 84 centres in Austria, Belgium, Holland, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, France, Germany, Hungary, Latvia, Poland, Slovakia, Slovenia, Spain, Ukraine, UK & Ireland 2) Participants: Salmeterol: 316; Placebo: 318 3) Baseline characteristics: Mean age: 62 years; 1.3 L; FEV1/FVC ratio: 54% 4) Inclusion criteria: M/F >/= 40 years; diagnosis of COPD; two or more exacerbations in previous year (none in 4 weeks prior to study entry) >/= 10 pack years; FEV1 <70% predicted 5) Exclusion criteria: asthma/eczema or allergic rhinitis; high dose BDP (> 2000mcg/d); requirement for O2 therapy |
|
| Interventions | 1) Salmeterol 50mcg BID 2) Placebo Participants were allowed to continue with their usual medications (including ICS) |
|
| Outcomes | 1) FEV1 (M); FVC; MMEF; Inspiratory capacity; FEV1/FVC ratio; morning PEF (M) 2) change in SGRQ (M) 3) Exacerbations (M) 4) Symptoms (M) 5) Withdrawals (M) 6) Rescue medication usage (M) |
|
| Notes | Unpublished study ‐ study appeared in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
Ulrik 1995.
| Methods | Crossover study. Excluded: described. Withdrawals: described. Trial duration: 8 weeks with 2‐week run‐in period. Baseline characteristics: Power calculation: not given. Intention to treat analysis: not stated. Jadad Score: 4. |
|
| Participants | 1) Setting: Denmark 2) Patients randomised n = 63 in 2 crossover arms. Salmeterol/ Placebo n = 31; Placebo/ Salmeterol n = 32. 3) Baseline characteristics: Mean age 65 years, mean pre‐bronchodilator FEV1 = 1.2 L (45.4% predicted). 4) Inclusion criteria: current smokers, > 40 years if age, FEV1< 60% predicted, FEV1/fvc <60%, < 15% FEV1 reversibility after salbutamol, day and night symptoms for at least one short period during 5/7 days during run‐in. 5) Exclusion criteria: history of asthma, allergic rhinitis, hospital admission for COPD in the month prior to entry into study, serious uncontrolled other disease and treatment with inhaled steroids within previous 6 months. |
|
| Interventions | 1) Placebo bd followed by Salmeterol 50 mcg bd 2) Salmeterol 50 mcg bd followed by Placebo bd. Medication delivered by MDI. | |
| Outcomes | 1) Lung function: FEV1 (M) Morning and evening PEF (M) 2) Exercise tolerance: ‐ 3) Quality of life: ‐ 4) Dyspnoea and symptoms: respiratory symptom scores (D) 5) Exacerbations: ‐ 6) Rescue salbutamol use: Yes (D) | |
| Notes | FEV1 reversibility mean pre‐bronchodilator FEV1(litres)‐mean post‐bronchodilator FEV1(litres) : 0.155 litres = approximately 12.6 % reversibility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
van Noord 2000.
| Methods | Parallel group study. Excluded: not described Withdrawals: described Trial duration: 12 weeks with 2 week follow‐up period. Baseline characteristics: comparable Power calculation: 63% power to detect a 4% difference (MCD) in SGRQ Total score between groups. 89% to detect a mean difference in the CRQ Total domain score of 0.5 Intention to treat analysis: not stated Jadad Score:5 |
|
| Participants | 1) Setting: 3 Dutch outpatient clinics. 2) Patients randomised n = 144 in three treatment arms. Salmeterol + placebo n = 47 placebo + placebo n = 50. 3) Baseline characteristics: mean age: 63.8 years male: female = 88%: 12% mean baseline FEV1: 1.33 Litres mean FEV1: 44% predicted. 4) Inclusion criteria: participants with COPD defined by ATS criteria, current/ex‐smokers, > 40, <75 years if age, FEV1 > 40%, < 65% predicted after inhalation of salbutamol, symptoms on mild exertion on > 4/7 days during run‐in period. Ex‐smokers stopped smoking > 6 months prior to run‐in. 5) Exclusion criteria: history of asthma, allergic rhinitis, atopy, other respiratory disease, significant concurrent disease, respiratory tract infection or change in medication within 6 weeks commencement of study, oxygen therapy. |
|
| Interventions | 1) Placebo matched to ipratropium, qid plus placebo matched to salmeterol bd. 2) Salmeterol, 50 mcg bd plus placebo matched to ipratropium, qid. 3) Salmeterol, 50 mcg bd plus ipratropium 40 mcg qid. All medications delivered via metered dose inhalers. |
|
| Outcomes | 1) Lung function: End of study pre‐bronchodilator FEV1 (M); morning and evening Peak Expiratory Flow (M) 2) Exercise tolerance: ‐ 3) Quality of life: a) Change in CRDQ for dyspnoea, fatigue, emotion, mastery, total domains (M). b) Change in SGRQ for symptoms, activity, impacts, total domains (M) 4) Dyspnoea and symptoms: day and night time symptom scores (M) 5) Exacerbations: no. participants experiencing (M) 6) Rescue salbutamol use: Yes, % days with additional salbutamol use (M); % nights with additional salbutamol use (M) |
|
| Notes | FEV1 reversibility (% baseline FEV1) mean (SD): salmeterol: 12.9%(9.5) placebo:13.8%(7.3) FEV1 reversibility (% predicted FEV1) mean (SE): salmeterol:5.7%(4.2) placebo:5.8(3.1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 2 |
| Allocation concealment (selection bias) | Low risk | See Appendix 2 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
Wadbo 2002.
| Methods | Parallel group design. Excluded: not described. Withdrawals: described. Trial duration: 12 weeks with a 2‐week run‐in period. Baseline characteristics: Power calculation: Yes Intention to treat analysis stated. Jadad score: 3 |
|
| Participants | 1) Setting: Multicentre (14 centres in Sweden) study. 2) Patients randomised n = 183 in 3 treatment arms. Placebo n = 16; Formoterol 18 mcg n = 17. 3) Baseline characteristics: Mean age = 64, FEV1 = 0.85L (33% predicted) 4) Inclusion criteria: Baseline pre‐bronchodilation FEV1 < 60% predicted normal, baseline pre‐bronchodilation FEV1/FVC < 70% predicted normal. Reversibility to Ipratropium bromide 120 mcg or Formoterol 27 mcg 45 min after inhalation < 12% of predicted normal. Outpatients 40 ‐ 75, reduced exercise capacity due to dyspnoea, >/= 10 pack year history smoking, oxygen tension in arterial blood at rest > 7.3 kPa 5) Exclusion criteria: Asthma, long term oxygen therapy. |
|
| Interventions | 1) Placebo 2) Formoterol 18 mcg bid 3) Ipratropium bromide 80 mcg tid [not of interest] All treatments delivered with pMDI. Participants permitted to continue with maintenance therapies. |
|
| Outcomes | 1) Lung function: Change in FEV1 from baseline to end of study as % predicted (M), Mean change from baseline FVC (M), Change in morning PEF (D) 2) Exercise tolerance: Shuttle walking test distance (M); Post shuttle Borg dyspnoea score (M). 3) Quality of life: Change in SGRQ for symptoms, activity, impacts and total domains (M) 4) Dyspnoea and symptoms: Morning and evening symptom scores: shortness of breath at night (M); daytime shortness of breath (M); Night cough (M); Daytime cough (M); Sleep (M) 5) Exacerbations: ‐ 6) Rescue salbutamol use: Yes (M) |
|
| Notes | FEV1 reversibility to formoterol < 12% FEV1 (predicted normal) Mean reversibility 6% (predicted normal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'In order to have comparable groups, stratification was performed according to the walking distance achieved at the randomisation visit. Patients with a walking distance <300 metres were sequentially assigned the lowest randomisation number and patients with a walking distance >300 metres the highest available randomisation number.' |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhalers |
(M): included in meta‐analysis, (D): included as description. ATS: American Thoracic Society; bd: twice daily; BDI: Baseline dyspnoea index; BCSS: Breathlessness, Cough and Sputum Scale; CBSQ: Chronic Bronchitis Symptom Questionnaire; COPD: Chronic Obstructive Pulmonary Disease; CRDQ: Chronic respiratory disease questionnaire; EQ‐5D: European Quality of Life Questionnaire; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; ICS: LABA: OCS: pMDI: pressurised meter dose inhaler; PFT: qd: four times daily; PLA: SAL: SF‐36: Short Form 36‐health survey questionnaire; SGRQ: St George's Respiratory Questionnaire; SVC: Slow vital capacity; td: three times daily; TDI: Transitional dyspnoea index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aalbers 2002 | Study included subjects with reversible COPD. Request for subgroup data from Astra Zenica denied |
| Anderson 1997 | Study included subjects with reversible COPD. No response from authors for clarification. |
| Bailey 1997 | Outcomes not presented in this abstract in a usable form. No response to request for data from authors. |
| Barnes 1993 | Review article. |
| Boulet 1992 | Review article. |
| Brogden 1992 | Review article. |
| Brusasco 2003 | Study included subjects with reversible COPD. Authors did not conduct reversibility test. |
| Calverley 2003a | Study included patients with reversible COPD. |
| Cazzola 1994 | Inclusion criteria not met. Subjects had >15% reversibility. The study was only 8 days in duration. |
| Cazzola 1995 | Inclusion criteria not met. Subjects had >15% reversibility. The study was a time course experiment over 12 hours only. |
| Chazan 1995 | Study was not a RCT. |
| D'Urzo 2001 | Study compared formoterol with salbutamol in combination with ipratropium bromide. |
| Dahl 2001 | Study included patients with reversible COPD. |
| Del Torre 1992 | This study was not a RCT. The study duration was only twelve hours. |
| Donohue 2002 | Study included subjects with reversible COPD. No response from authors for clarification. |
| Germouty 1992 | This study was not a RCT and asthmatics were included. |
| Howder 1993 | Review article. |
| Joseph 1994 | Review article. |
| Kotaniemi 1994 | Study included subjects with asthma and was not a RCT. |
| Mahler 1997 | Subjects with reversible airways disease enrolled. No response to requests for clarification of results from authors. |
| Maltais 1995 | Review article. |
| Matera 1995 | Short duration, 5 day study. |
| Matera 1996 | Short duration, 5 day study. |
| Melani 1996 | Treatment protocol unclear. |
| Newman 1996 | No outcomes presented in this abstract in a form that could be used. Authors responded but help refused. |
| Patakas 1995 | Study of exercise tolerance on three separate days. |
| Pingleton 1996 | Review article. |
| Ramirez‐Venegas 1997 | Dose response study over 4 hours. |
| Schultze‐Wern. 1996 | Review article. |
| Schultze‐Wern.1990 | Not a RCT and patients have reversible airways disease. |
| SLMF 4010 | Patients had reversible airways disease |
| Szafranski 2003 | Patients with reversible COPD included in review |
| Vollmer 1995a | Study was of too short a duration. |
| Vollmer 1995b | Time course study over 12 hours. |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
TORCH.
| Trial name or title | The TORCH (TOwards a Revolution in COPD Health) survival study |
| Methods | |
| Participants | Setting: Multi‐centre, multi‐national, study. Participants randomised n = 6200 in 4 participant arms. Placebo n = 1,510; Salmeterol 50 mcg n = 1,510. Baseline characteristics: Inclusion criteria: Male or female aged 40 ‐ 80 years; > 10 pack year smoker or ex‐smoker; FEV1 < 60% predicted, < 10% reversibility in predicted FEV1; FEV1/FVC ratio < 70%; Established history of COPD Exclusion criteria: Asthma or other respiratory disorder; lung volume reduction or transplant surgery; > 12 hours LTOT treatment; oral corticosteroid therapy; serious uncontrolled disease likely to cause death within 3 year period or interfere with study |
| Interventions | 1) Placebo bd 2) Salmeterol 50mcg bd 3) Fluticasone propionate 500mcg bd [Not of Interest] 4) Salmeterol/ Fluticasone Propionate 50/500mcg combination bd [Not of interest] Medication delivered via Accuhaler (TM)/ Diskus (R) |
| Outcomes | 1) Lung Function: FEV1 2) Exercise Tolerance: unknown 3) Quality of life: SGRQ, EQ‐5D 4) Symptom scores: unknown 5) Exacerbations: Yes 6) Rescue salbutamol use: unknown 7) Other: All cause mortality |
| Starting date | |
| Contact information | J. Vestbo North West Lung Research Centre Wythenshawe Hospital Manchester M23 9LT UK Fax: 44 1612912832 E‐mail: jvestbo@man.ac.uk |
| Notes | Results available 2007 |
Contributions of authors
SLA: Assessment of studies for inclusion, study quality assessment, data extraction and data entry, first draft and revisions to manuscript. Update of review with new studies. PP: Statistical advice re update. Editing/review of updated version of review. BS: Assessment of studies for inclusion, study quality assessment and manuscript review. AV: Study quality assessment and manuscript review. AB: Data extraction and data entry. MMKC: 2005 update review with new studies including assessment of studies for inclusion, study quality assessment, data extraction and data entry. Revisions to manuscript. TJL: Update 2006; data entry & analysis; revision to manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Garfield Weston Foundation, UK.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
408DP‐03 {published data only}
- 408D03. A multi‐center, randomized, double‐blind, parallel‐group, comparison of salmeterol xinafoate inhalation Rotadisk versus placebo in subjects with chronic obstructive pulmonary disease treated with current medications. GlaxoSmithKline Clinical Trial Register (http:ctr.gsk.co.uk) 2005.
Boyd 1997 {published and unpublished data}
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1997;10(4):815‐21. [PubMed] [Google Scholar]
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory & Critical Care Medicine 1997;155:1283‐9. [DOI] [PubMed] [Google Scholar]
- SLGT28. A multicentre, randomised, double‐blind, parallel group study to compare the efficacy and safety of inhaled salmeterol xinafoate 50µg bd and inhaled salmeterol xinafoate 100µg bd with placebo, all administered via the metered‐dose inhaler, in the treatment of patients with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Calverley 2003 {published and unpublished data}
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet 2003;361(9356):449‐56. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. http://www.abstracts2view.com. 2003.
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/Fluticasone propionate combination for one year provides greater clinical benefit than its individual components [A98] [Poster: 306]. Proceedings of the 98th International American Thoracic Society Conference (http://www.abstracts‐on‐line.com/abstracts/ATS). 2002.
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s. [Google Scholar]
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 503.
- Hunjan MK, Williams DT. Costs of avoiding exacerbations in patients with chronic obstructive pulmonary disease (COPD) treated with salmeterol/ fluticasone propionate combination (seretide) and salmeterol. European Respiratory Journal 2004;24(Suppl 48):291s. [Google Scholar]
- Hunjan MK, Williams DT. Salmeterol/ fluticasone propionate combination is clinically effective in avoiding exacerbations in patients with moderate/severe COPD. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
- Jones PW, Edin HM, Anderson J. Salmeterol/fluticasone propionate combination improves health status in COPD patients [A39] [Poster K39]. Proceedings of the 98th International American Thoracic Society Conference (http://www.abstracts‐on‐line.com/abstracts/ATS). 2002.
- Jones PW, Ståhl E. Budesonide /formoterol sustains clinically relevant improvements in health status in COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1352. [Google Scholar]
- Jones PW, Vestbo J, Pauwels RA, Calverley PMA, Anderson JA, Spencer MD. Informative drop out in COPD studies. Investigation of health status of withdrawals in the TRISTAN study. 13th ERS Annual Congress, 27 Spetember 2003, Vienna. 2003:P1593.
- Nitschmann S. Inhalational combination therapy in chronic obstructive lung disease. Tristan study. German Internist 2004;45(6):727‐8. [DOI] [PubMed] [Google Scholar]
- Pauwels R, Vestbo J, Calverley PM, Jones PW, Pride NB, Gulsvik A. Characterization of Exacerbations in the TRISTAN study of salmeterol/fluticasone propionate (SFC) combination in moderate to severe COPD. http://www.abstracts2view.com. 2003.
- Pauwels RA, Calverly PMA, Vestbo J, Jones PW, Pride N, Gulsvik A, et al. Reduction of exacerbations with salmeterol/fluticasone combination 50/500 mcg bd in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):240S. [Google Scholar]
- SFCB3024. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of the salmeterol/FP combination product at a strength of 50/500mcg bd with salmeterol 50mcg bd alone and FP 500mcg bd alone, delivered via the DISKUS™/ACCUHALER™, in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 12 months. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23(6):619‐37. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal 2004;24(Suppl 48):290s. [Google Scholar]
- Vestbo J, Calverley PMA, Pauwels R, Jones P, Pride N, Gulsvik A, et al. Absence of gender susceptibility to the combination of salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):240. [Google Scholar]
- Vestbo J, Pauwels R, Anderson JA, Jones P, Calverley P. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax 2005;60(4):301‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Pauwels RS, Calverley PMA, Jones PW, Pride NB, Gulsvik A. Salmeterol / fluticasone propionate combination produces improvement in lung function detectable within 24 hours in moderate to severe COPD. http://www.abstracts2view.com. 2003.
- Vestbo J, Soriano JB, Anderson JA, Calverley P, Pauwels R, Jones P. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respiratory Medicine 2004;98(11):1045‐50. [DOI] [PubMed] [Google Scholar]
Celli 2003 {published data only}
- Celli B, Halpin D, Hepburn R, Byrne N, Keating E, Goldman M. Symptoms are an important outcome in chronic obstructive pulmonary disease clinical trials: results of a 3‐month comparative study using the Breathlessness, Cough and Sputum Scale (BCSS). Respiratory Medicine 2003;97:S35‐S43. [PubMed] [Google Scholar]
Chapman 2002 {published and unpublished data}
- Chapman K, Arvidsson P, Chuchalin A, Dhillon D, Faurschou P, Goldstein R, et al. The addition of salmeterol 50 mcg bid to anticholinergic treatment in patients with COPD: a randomised placebo controlled trial. Canadian Respiratory Journal 2002;9(3):178‐85. [DOI] [PubMed] [Google Scholar]
- Chapman K, James MH, Kuipers AF, Goldstein R. Addition of salmeterol 50 mcg bid to anticholinergic treatment in COPD. American Journal of Respiratory & Critical Care Medicine 1999;159(3):A523. [Google Scholar]
- SLGF53. A multi‐centre, double‐blind, parallel group study to evaluate the efficacy of serevent 50mg bid versus placebo bid all administered via the multi dose powder inhaler (diskus/accuhaler) in terms of symptoms in the treatment of patients with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Dal Negro 2003 {published and unpublished data}
- Dal Negro R, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 microg/250 microg bid in combination provides a better long‐term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology & Therapeutics 2003;16:241‐6. [DOI] [PubMed] [Google Scholar]
Dauletbaev 2001 {published data only}
- Dauletbaev N, Viel K, Bargon J. Salmeterol or ipratropium bromide/fenoterol in stable mild‐moderate COPD. European Respiratory Journal 2001;18(Suppl 33):426s. [Google Scholar]
- SMS40308. A randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, multicenter study to compare efficacy and tolerability of salmeterol (50 µg b.i.d., Serevent® Diskus®) and a combination of ipratropium/fenoterol (40/100 µg q.i.d., Berodual® MDI) in patients with mild‐to‐moderate chronic obstructive lung disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Grove 1996 {published and unpublished data}
- Grove A, Lipworth BJ, Reid P, Smith RP, Ramage L, Ingram CG, et al. Effect of regular salmeterol on lung function and exercise capacity in patients with chronic obstructive airways disease. Thorax 1996;51:689‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2002 {published data only}
- Gupta R, Chhabra S. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease. The Indian Journal of Chest Diseases & Allied Sciences 2002;44:165‐72. [PubMed] [Google Scholar]
Hanania 2003 {published data only}
- Hanania N, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 ug)/salmeterol (50 ug) combined in the Diskus Inhaler for the treatment of COPD. Chest 2003;124:834‐43. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory & Critical Care Medicine 2001;163(5 Suppl):A279. [Google Scholar]
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination. European Respiratory Journal 2003;22(Suppl 45):50s. [Google Scholar]
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:Abstract no: 1081.
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P429. [Google Scholar]
- SFCA3007. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the diskus formulations of salmeterol (sal) 50mcg bid and fluticasone propionate (fp) 250mcg bid individually and in combination as salmeterol 50mcg/fluticasone propionate 250mcg bid (sfc 50/250) compared to placebo in copd subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22(Suppl 45):51s. [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal 2004;24(Suppl 48):290s. [Google Scholar]
Mahler 1999 {published and unpublished data}
- Goodwin B, Cox F, Anderson W, Wisniewski M, Rickard K. Comparison of salmeterol 42ug BID (SLG) versus ipratropium bromide 36 ug q.i.d. vs placebo on disease specific quality of life in COPD patients: reversible or non‐reversible to ventolin. 1997‐1999 ALA/ATS Abstracts‐on‐disk.. 1997.
- Mahler DA, Donohue JF, Barbee RA, Goldman M, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115:957‐65. [DOI] [PubMed] [Google Scholar]
- SLGA4005. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (prn ventolin®) in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Mahler 2002 {published data only}
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting Lung Function Responses to Combination Therapy in Chronic Obstructive Pulmonary Disease (COPD) Predicting Lung Function Responses to Combination Therapy in Chronic Obstructive Pulmonary Disease (COPD). http://www.abstracts2view.com. 2003.
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2002;166:1084‐91. [DOI] [PubMed] [Google Scholar]
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the diskus formulations of salmeterol (sal) 50mcg bid and fluticasone propionate (fp) 500mcg bid individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg bid (sfc 50/500) compared to placebo in COPD subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22(Suppl 45):51s. [Google Scholar]
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93, Poster: 308.
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal 2004;24(Suppl 48):290s. [Google Scholar]
Rennard 2001 {published and unpublished data}
- Goodwin B, Cox F, Anderson W, Wisniewski M, Rickard K. Evaluation of quality of life in reversible and non‐reversible patients with COPD receiving salmeterol xinafoate 42mcg BID (SLG) versus ipratropium bromide 36mcg QID (IP) versus placebo (PL). American Journal of Respiratory & Critical Care Medicine. 1997; Vol. 155:A724.
- Rennard S, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled Beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critcal Care Medicine 2001;163(5):1087‐92. [DOI] [PubMed] [Google Scholar]
- SLGA4004. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (prn ventolin) in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Rossi 2002 {published and unpublished data}
- Rossi A, Thomson M, Della Cioppa G. Comparison of the efficacy, tolerability and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121:1058‐69. [DOI] [PubMed] [Google Scholar]
SCO40030 {unpublished data only}
- SCO40030. A randomized, double‐blind, placebo‐controlled, parallel group clinical trial evaluating the effect of the fluticasone propionate/salmeterol combination product 250/50mcg bid via diskus and salmeterol 50mcg bid via diskus on lung hyperinflation in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40298 {published data only}
- SMS40298. A multi‐centre, randomized, double‐blind, parallel group study to evaluate the impact on Quality of Life (QOL) of adding Serevent 50ug bid via MDI to patients’ existing therapy in patients with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40314 {published data only}
- SMS40314. A multicenter, randomized, double‐blind, double‐dummy, parallel‐group, 8‐week comparison of salmeterol xinafoate versus ipratropium bromide versus salmeterol xinafoate plus ipratropium bromide versus placebo in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40315 {published data only}
- SMS40315. A multicenter, randomized, double‐blind, double‐dummy, parallel group, 8‐week comparison of salmeterol xinafoate versus ipratropium bromide versus salmeterol xinafoate plus ipratropium bromide versus placebo in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40318 {published data only}
- SMS40318. Randomized, double blind, placebo‐controlled, parallel‐group trial over 4 weeks to evaluate the effect of salmeterol (2x50 µg/d by Diskus®) on the lung volumes at rest and during sub‐maximal exercise in subjects with moderate chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Stockley 2002 {unpublished data only}
- SMS40026. A multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study to investigate the effect of 12 months treatment with salmeterol (50mcg bd), delivered via the diskus* inhaler, on the incidence of moderate and severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD) when added to their usual treatment regimen. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
- Stockley R, Chopra N. Salmeterol added to usual therapy, is an effective bronchodilator over 12 months of treatment in chronic obstructive pulmonary disease [abstract]. European Respiratory Society Annual Congress. 2002:P1568.
- Stockley R, Rice L, Chopra N. Salmeterol added to usual therapy gives a rapid improvement in lung function that is sustained at 6 and 12 months in patients with COPD [abstract]. European Respiratory Society Annual Congress. 2002:P1567.
- Stockley R, Rice L, Chopra N. Salmeterol added to usual therapy significantly reduces the frequency of moderate/severe exacerbations in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:A108, Poster C51.
Ulrik 1995 {published data only}
- SLGL17. A single centre, randomised, double‐blind, cross‐over study to compare the efficacy of inhaled salmeterol xinafoate dry powder 50mg bid via the diskhaler with placebo dry powder bid via the diskhaler in the treatment of non‐reversible COPD patients and to provoke with histamine and methacholine before and after treatment to investigate whether salmeterol can reduce the bronchial hyperreactivity. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
- Ulrik CS. Efficacy of inhaled salmeterol in the management of smokers with chronic obstructive pulmonary disease: a single centre randomised, double blind, placebo controlled, crossover study. Thorax 1995;50:750‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Noord 2000 {published data only}
- Rutten‐van Molken M, Roos B, Noord JA. An empirical comparison of the St George's Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial. Thorax 1999;54:995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noord JA, Munck DR, Bantje TA, Hop WCJ, Bommer AM. Long‐term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. European Respiratory Journal 2000;15(5):878‐85. [DOI] [PubMed] [Google Scholar]
Wadbo 2002 {published and unpublished data}
- Stahl E, Wadbo M, Bengtsson T, Strom K, Lofdahl C‐G. Health‐related quality of life, symptoms, exercise capacity and lung function during treatment for moderate to severe COPD. Journal of drug assessment 2002;5:81‐94. [Google Scholar]
- Stahl E, Wadbo M, Bengtsson T, Strom K, Lofdahl CG. Health‐related quality of life, symptoms, exercise capacity and lung function during treatment for moderate to severe COPD. Journal of Outcomes Research 2001;5:11‐24. [Google Scholar]
- Wadbo M, Lofdahl CG, Larsson K, Skoogh BE, Tornling G, Arwestrom E, et al. Effects of formoterol and ipratropium bromide in COPD: a 3‐month placebo‐controlled study. European Respiratory Journal 2002;20:1138‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aalbers 2002 {published data only}
- Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3‐month trial. European Respiratory Journal 2002;19:936‐43. [DOI] [PubMed] [Google Scholar]
Anderson 1997 {published data only}
- Anderson WH, Wisniewski M, Anzueto A, Jenkinson S, Goldman M, Ramsdell J, et al. The safety and efficacy of salmeterol in patients with COPD: Results from SLGA4005, a multicentre trial. Chest 1997;112(3 Suppl):79s‐80s. [Google Scholar]
Bailey 1997 {published data only}
- Bailey W, Yancey S, Rickard K. Peak flow and symptom monitoring in COPD: a 12 week comparison of placebo, Atrovent and Serevent. American Journal or Respiratory & Critical Care Medicine 1997;155(4):A278. [Google Scholar]
Barnes 1993 {published data only}
- Barnes NC. New developments in the treatment of asthma and chronic obstructive pulmonary disease. Respiratory Medicine 1993;87(Suppl B):53‐6. [DOI] [PubMed] [Google Scholar]
Boulet 1992 {published data only}
- Boulet LP. Bronchodilator effect of salmeterol and inhibition of bronchial hyperreactivity. Revue des Maladies Respiratoires 1992;9(Suppl 1):R11‐13. [PubMed] [Google Scholar]
Brogden 1992 {published data only}
- Brogden RN, Faulds D. Salmeterol xinafoate: Aareview of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 1991;42(5):895‐912. [DOI] [PubMed] [Google Scholar]
Brusasco 2003 {published data only}
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calverley 2003a {published and unpublished data}
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22, Poster 505.
- Calverley PMA, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol ina single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024, Poster 418.
- Calverley PMA, Peterson S. Combining budesonide/formoterol in a single inhale reduces exacerbation frequency in COPD [abstract]. American Thoracic Society 99th International Conference. 2003:D092, Poster 211.
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435. [Google Scholar]
- Calverly PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22:912‐9. [DOI] [PubMed] [Google Scholar]
- Calverly PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journa 2003;22(Suppl 45):P436. [Google Scholar]
- Calverly PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P1587. [Google Scholar]
- Halpin D, Stahl E, Lundback B, Anderson F, Peterson S. Treatment costs and number needed to treat (NNT) with budesonide/formoterol to avoid one exacerbation of COPD [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22, Poster 525.
- Jones PW, Stahl E. Budesonide/formoterol in a single inhaler improves health status in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024, Poster 419.
- Jones PW, Ståhl E. Reducing exacerbations leads to a better health‐related quality of life in patients with COPD. 13th ERS Annual Congress, 27th September, 2003, Vienna. 2003:P1586.
- Lofdahl CG. Reducing the impact of COPD exacerbations: clinical efficacy of budesonide/formoterol. European Respiratory Review 2004;13(88):14‐21. [Google Scholar]
- Lofdahl CG, Andreasson E, Svensson K, Ericsson A. Budesonide/formoterol in a single inhaler improves health status in patients with COPD without increasing healthcare costs [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P433. [Google Scholar]
Cazzola 1994 {published data only}
- Cazzola M, Santangelo G, Piccolo A, Salzillo A, Matera MG, D'Amato G, et al. Effects of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1994;7:103‐7. [DOI] [PubMed] [Google Scholar]
Cazzola 1995 {published data only}
- Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose‐response study. Respiratory Medicine 1995;89:357‐62. [DOI] [PubMed] [Google Scholar]
Chazan 1995 {published data only}
- Chazan R, Jaworski A, Grubek‐Jaworska H, Droszcz W. Effect of 16 week use of salmeterol on ECP levels, pulmonary function tests and bronchial hyperreactivity in patients with chronic obstructive pulmonary disease. Polski Tygodnik Lekarski 1995;50(40‐44):48‐49. [PubMed] [Google Scholar]
D'Urzo 2001 {published data only}
- D'Urzo AD, Salvo MC, Ramirez‐Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottakis J, FOR‐INT‐03 Study Group. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium : a 3‐week, randomized, double‐blind, within‐patient, multicenter study. Chest 2001;119(5):1347‐56. [DOI] [PubMed] [Google Scholar]
Dahl 2001 {published data only}
- Dahl R, Greefhorst L, Nowak D, Nonikov V, Byrne A, Thomson M, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2001;164:778‐84. [DOI] [PubMed] [Google Scholar]
Del Torre 1992 {published data only}
- Torre L, Melica EV, Torre M. Effectiveness of salmeterol in patients with emphysema. Current Therapeutic Research 1992;52(6):888‐98. [Google Scholar]
Donohue 2002 {published data only}
- Donohue J, Bateman E, Lee A, Kesten S. A 6‐month, placebo‐controlled study comparing lung function and health status changes in copd patients treated with tiotropium or salmeterol. Chest 2002;122:47‐55. [DOI] [PubMed] [Google Scholar]
Germouty 1992 {published data only}
- Germouty J, Aubert J, Clavier J, Paramelle B, Voisin C. Tolérance à longe terme du Formotérol chez des bronchopathes chroniques obstructifs. Allergie et Immunologie 1992;24(9):342‐7. [PubMed] [Google Scholar]
Howder 1993 {published data only}
- Howder CL. Anti‐muscarinic and Beta2 adrenoceptor bronchodilators in obstructive airways disease. Respiratory Care 1993;38(12):1364‐88. [Google Scholar]
Joseph 1994 {published data only}
- Joseph JC. Chronic obstructive pulmonary disease U.S. Pharmacist 1994;19(12):H3‐14. [Google Scholar]
Kotaniemi 1994 {published data only}
- Kotaniemi JT. Salmeterol in asthma and COPD: a long term follow‐up. American Journal of Critical Care Medicine 1994;149(4):A207. [Google Scholar]
Mahler 1997 {published data only}
- Mahler D, ZuWallach R, Rickard K, Yancey S, Wisniewski M, Anderson W. Effects of salmeterol and ipratropium bromide on dyspnea as measured by the six minute walk and baseline dyspnea index/transitional dyspnea index (BDI/TDI). American Journal of Respiratory & Critical Care Medicine 1997;155:A278. [Google Scholar]
Maltais 1995 {published data only}
- Maltais F, Bourbeau J. Medical management of emphysema. Chest Surgery Clinics of North America 1995;5(4):673‐89. [PubMed] [Google Scholar]
Matera 1995 {published data only}
- Matera MG, Cazzola M, Vinciguerraaa A, Perna F, Calderaro F, Caputi M, et al. Comparison of hte bronchodilating effects of salmeterol, salbutamol and ipratropium bromide in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1995;8:267‐71. [DOI] [PubMed] [Google Scholar]
Matera 1996 {published data only}
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90:497‐9. [DOI] [PubMed] [Google Scholar]
Melani 1996 {published data only}
- Melani AS, Pirelli M, Gregorio A. Effects of inhaled salmeterol and orally dose‐titrated theophylline on exercise capaity of stable COPD patients. European Respiratory Journal 1996;9:391s. [Google Scholar]
Newman 1996 {published data only}
- Newman AM, Wiggins J, Smith M, Taak NK, Barnes P. Salmeterol xinafoate (100 mcg BD) in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1996;9:183s. [Google Scholar]
Patakas 1995 {published data only}
- Patakas D, Andreadis D, Mavrofridis E, Argyropoulou P, Sarigiannidis A. Comparison of the effect of salmeterol and ipratropium bromide, on exercise performance and breathlessness in patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1995;8(Suppl 19):94s. [DOI] [PubMed] [Google Scholar]
Pingleton 1996 {published data only}
- Pingleton S. Pulmonary Medicine. JAMA 1996;275(23):1849‐50. [PubMed] [Google Scholar]
Ramirez‐Venegas 1997 {published data only}
- Ramirez‐Venegas A, Ward J, Lentine T, Mahler D. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest 1997;112:336‐40. [DOI] [PubMed] [Google Scholar]
Schultze‐Wern.1990 {published data only}
- Schultze‐Werninghaus G. Multicentre 1‐year trial on Formoterol, a new long acting Beta2‐agonist, in chronic obstructive airways disease. Lung 1990;168(Suppl):83‐9. [DOI] [PubMed] [Google Scholar]
Schultze‐Wern. 1996 {published data only}
- Schultze‐Werninghaus G. Salmeterol, a long‐acting beta2‐agonist in airways obstruction. Effects, side effects and therapeutic assessment [Der langwirksame inhalative Beta2‐agonist Salmeterol bei obstrucktiven Atemwegserkrankungen. Wirkungen, Nebenwirkungen und Stellenwert]. Allegro Journal 1996;5(8):453‐60. [Google Scholar]
SLMF 4010 {published data only}
- SLMF 4010. Multicentre, randomised, parallel group, placebo‐controlled, double‐blind, study, stratified on tobacco status at enrollment, evaluating during 6 months the efficacy of salmeterol powder for inhalation, 50 µg two times per day for the reduction of thoracic distension in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Szafranski 2003 {published data only}
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2003;21:74‐81. [DOI] [PubMed] [Google Scholar]
Vollmer 1995a {published data only}
- Vollmer M, Schmidt EW, Ulmer WT. Effect duration and treatment effectiveness of salmeterol, fenoterol and salbutamol in severe forms of respiratory tract obstruction. Pneumologie 1995;49(10):528‐34. [PubMed] [Google Scholar]
Vollmer 1995b {published data only}
- Vollmer M, Schmidt EW, Ulmer WT. Responder and non‐responder in the bronchodilator test? Variability of short‐term responses to beta2‐adrenoceptor agonists in chronic airways obstruction. Pneumologie 1995;49(11):584‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Della Cioppa 2001 {unpublished data only}
- Della Cioppa G, Byrne A, Till D, Wood R. Formoterol demonstrates bronchodilator efficacy in patients with reversible or poorly reversible chronic obstructive pulmonary disease, regardless of concomitant corticosteroid use [abstract]. European Respiratory Journal 2001;18(Suppl 33):177s. [Google Scholar]
Greefhorst 2000 {published data only}
- Greefhorst AP, Dahi R, Nowak D, Nonikov V, Byrne A, Colcchio C, et al. Effect of inhaled formoterol and ipratropium bromide on quality of life, "bad days" and exacerbations in patients with COPD. American Thoracic Society 2000 Toronto (Abstracts‐On‐Disk). 2000.
Kristufek 2001 {unpublished data only}
- Kristufek P, Levine B, Till D, Byrne A. Inhaled formoterol (Foradil(r)) improves lung function in patients with both reversible and poorly reversible COPD [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A280. [Google Scholar]
Littner 2001 {unpublished data only}
- Littner M, Yates J, Fischer T, Horstman D, Wire P. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following 24 weeks of treatment with the salmeterol/fluticasone propionate combination 50/500 BID via the Diskus Inhaler [abstract]. European Respiratory Journal 2001;18(Suppl 33):176s. [Google Scholar]
Sybrecht 1999 {published data only}
- Sybrecht GW. Inhaled formoterol was an effective and safe treatment in COPD patients. European Respiratory Society Annual Congress (Abstracts‐on‐Disk). 1999:P2510.
References to ongoing studies
TORCH {published data only}
- The TORCH Study Group. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. European Respiratory Journal 2004;24:206‐10. [DOI] [PubMed] [Google Scholar]
Additional references
ATS/ERS 2004
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23:932‐46. [DOI] [PubMed] [Google Scholar]
Barr 2005
- Barr RG, Bourbeau J, Camargo CA, Ram FSF. Tiotropium for stable chronic obstructive pulmonary disease (Cochrane review). Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calverley 2003b
- Calverley P, Burge P, Spencer S, Anderson J, Jones P. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003;58:659‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. Bio Med Central 2002;BMC Medical Research Methodology 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2001
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine 2001;163:1256–76. [DOI] [PubMed] [Google Scholar]
Guyatt 1988
- Guyatt G, Townsend M, Nogradi S, Pugsley S, Keller J, Newhouse M. Acute response to bronchodilator. An imperfect guide for bronchodilator therapy in chronic airflow limitation. Archives of Internal Medicine 1988;148:1949‐52. [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85:25‐31. [DOI] [PubMed] [Google Scholar]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation. American Review of Respiratory Diseases 1992;145:1321‐7. [DOI] [PubMed] [Google Scholar]
Karner 2012
- Karner C, Stovold E. Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nannini 2004
- Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta‐agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, Ducharme FM. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. The Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI] [PubMed] [Google Scholar]
NICE/BTS 2004
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59(Suppl 1):1‐232. [PMC free article] [PubMed] [Google Scholar]
O'Donnell 1997
- O'Donnell D, Bertley J, Chau L, Webb K. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiological mechanisms. American Journal of Respiratory & Critical Care Medicine 1997;155:109‐15. [DOI] [PubMed] [Google Scholar]
O'Donnell 1999
- O'Donnell D, Lam M, Webb K. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;160:542‐9. [DOI] [PubMed] [Google Scholar]
Reidelmeier 1996
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. Journal of Clinical Epidemiology 1996;49(11):1215‐19. [DOI] [PubMed] [Google Scholar]
Spencer 2004
- Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23(5):698‐702. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, et al. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews Health Care. Meta‐analysis in context. BMJ Books, 2001:189‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tashkin 2003
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123:1441‐9. [DOI] [PubMed] [Google Scholar]
Verberne 1998
- Verberne AA, Fuller R. An overview of nine clinical trials of salmeterol in an asthmatic population. Respiratory Medicine 1998;92:777‐82. [DOI] [PubMed] [Google Scholar]
Wilding 1997
- Wilding P, Clark M, Thompson Coon J, Lewis S, Rushton L, Bennett J, et al. Effect of long term treatment with salmeterol on asthma control: a double blind, randomised crossover study. BMJ 1997;314:1441‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


