Abstract
Beetles, like other insects, depend on diuretic and antidiuretic hormones to control water balance. We have isolated, using head extracts from the beetle Tenebrio molitor, a peptide that strongly inhibits fluid secretion by the Malpighian tubules of this insect. This antidiuretic factor (ADF) appears to elicit its effect via cGMP as a second messenger but does not stimulate NO production. It has primary structure: Val-Val-Asn-Thr-Pro-Gly-His-Ala-Val-Ser-Tyr-His-Val-Tyr-OH. The ADF inhibits tubule secretion with high potency: the EC50 is around 10 fM. It bears no significant resemblance to other biologically active neuropeptides. To our knowledge this is the only endogenous insect ADF acting on Malpighian tubules to be sequenced, and the first coleopteran (beetle) antidiuretic factor fully characterized to date.
Keywords: cGMP‖water balance‖Tenebrio molitor‖Rhodnius prolixus‖Drosophila melanogaster
Unlike vertebrates, insects have low-pressure circulatory systems (1). Fluid homeostasis requires diuretic and antidiuretic hormones from the neuroendocrine organs to control active ion transport, which leads to fluid movement (2, 3). In most insects studied antidiuresis is primarily effected by fluid reabsorption in the hindgut and a few insect antidiuretic factors have been characterized that function in this manner (4–11). However, there have been reports of antidiuretic factors that act by a very different mechanism—i.e., inhibition of diuresis by the Malpighian tubules (12, 13). Two recent studies report attempted isolations of such factors from the ant Formica polyctena (14) and from the beetle Leptinotarsa decemlineata (15). Cardioactive peptide 2b (CAP2b), originally isolated as a myotropin from Manduca sexta (16), is the only insect peptide characterized to date that elicits cGMP production to affect Malpighian tubule fluid secretion. It is reported to elevate NO and thence cGMP to stimulate diuresis in Drosophila melanogaster (17). However, CAP2b has been characterized as antidiuretic in the bloodsucking insect Rhodnius prolixus; CAP2b seems to function through cGMP as a second messenger, independent of NO in this species, with an apparent EC50 between 3.5 and 10 nM (18).
We report the isolation to homogeneity and sequencing of the first endogenous insect antidiuretic factor (ADF) acting directly on Malpighian tubules. This factor, from Tenebrio molitor, is extremely potent, with an EC50 ≈ 107-fold lower than that of CAP2b on T. molitor tubules [85 nM (19)]. This ADF appears to use cGMP as a second messenger, like CAP2b.
Materials and Methods
Insects.
A colony of Tenebrio molitor was maintained at high population density at ≈27°C, with a 14 h light/10 h dark cycle. Pupae, adults, and larvae were separated from one another every second day; all insects were fed a diet of wheat bran and raw potatoes. Heads used for extraction of peptides were removed from pupae and frozen immediately in liquid N2. Combined heads were stored at −80°C before extraction. For bioassays of cGMP secretion, newly emerged (0–2 h) adult T. molitor were removed from the colony and Malpighian tubules dissected. For fluid secretion assays, last instar larvae were used.
Isolation (Second Messenger) Bioassay.
Throughout our purification, we followed ADF activity by measuring cGMP excreted from T. molitor tubules; secretion of nucleotide second messengers from insect Malpighian tubule cells is well established (20). cGMP-stimulating activity of aliquots from chromatographic fractions was measured with a competitive enzyme immunoassay (Cayman Chemicals, Ann Arbor, MI). Aliquots of extracts or chromatographic fractions to be assayed were dried down in the presence of 0.1 mg BSA in a Savant Speed Vac and resuspended in Nicolson's T. molitor saline (21) containing 100 μM Zaprinast, a selective inhibitor of cGMP phosphodiesterases. Malpighian tubules (six per animal) were dissected from newly emerged adult T. molitor and incubated singly in microtiter plate wells containing 150 μl of saline with 100 μM Zaprinast incubated for 1 h in a 30°C water bath. Each tubule was then carefully transferred to a well containing 150 μl of resolubilized sample (usually 2–5 head equivalents) and incubated for another hour. The first incubation gave the basal level of cGMP production, and the second showed any effects of the chromatographic fraction on cGMP levels. After each incubation, all saline was transferred to a 1.5-ml polypropylene tube and centrifuged for 10 min at 16,000 × g. Aliquots (50 μl) of the resulting supernatants were then removed from each tube for cGMP enzyme immunoassay (EIA).
cGMP Dose–Response (Second Messenger) Assay.
This variation of the assay method above was used with synthetic peptide and was intended to determine the effect of peptide on both intracellular and secreted cGMP levels. Malpighian tubules were dissected from newly emerged adult T. molitor. Two tubules were placed in a 5-ml polypropylene test tube containing 300 μl of either Nicolson's T. molitor saline or a defined concentration of peptide dissolved in saline; all tubes contained 100 μM Zaprinast. After a 1-h incubation at 30°C, all tubes were floated 5 min in boiling water, then allowed to cool another 2 min. Tubules in each test tube were homogenized with a Polytron, the homogenates transferred to 1.5-ml polypropylene tubes, and these centrifuged 10 min at 16,000 × g in a microcentrifuge. Fifty microliters of supernatant was then removed from each tube and assayed for cGMP by using EIA as described above. Three to six replicates were performed for each concentration.
cAMP Assay.
A competitive cAMP EIA was used to measure the effect of T. molitor ADF on cAMP produced by Malpighian tubules. One-hour incubations were done with 300 μl of T. molitor saline alone, 300 μl of T. molitor saline plus 10 nM T. molitor DH37, or the same plus either 1 pM or 1 nM T. molitor ADF in 5-ml tubes containing two Malpighian tubules per tube (always from different animals). After incubation was complete, 3-isobutyl-1-methylxanthine was added to a concentration of 1 mM, to prevent further hydrolysis by phosphodiesterases. Tubules were then boiled for 5 min, homogenized, and the homogenate transferred to polypropylene tubes, which were centrifuged 10 min at 16,000 × g. Twenty-five microliters were removed from each tube and used for cAMP immunoassay. cAMP was quantified by using an EIA procedure adapted from ref. 22. Briefly, 27.5 μl of goat anti-rabbit IgG (American Qualex, La Mirada, CA) was diluted in 10 ml of PBS (0.02 M sodium phosphate/0.15 M NaCl, pH 7.4), and 90 μl of this solution was added to the wells of an immunoabsorbent plate (Nunc, #442404) and incubated for 4–5 h. The plate contents were discarded and wells blocked by adding 315 μl of blocking buffer [40 ml of EIA buffer (22) and 3 ml of normal goat serum (Colorado Serum, Denver)]. The plate was incubated for 1 h, its contents then discarded, and the wells washed twice with 150 μl of wash buffer (PBS with 0.05% Tween 20). Rabbit anti-cAMP (American Qualex) was diluted with EIA buffer, 75 μl was added to each well, and 25 μl of standards/samples were added (cAMP standards ranged from 1,000 pmol/25 μl to 0.01 pmol/25 μl). The cAMP-HRP conjugate (prepared as in ref. 23, but modified as in ref. 22), diluted with EIA buffer, was added to each well in 25-μl aliquots. The plate was covered, placed on a shaker for 5 min, and left overnight at 4°C. Following four rinses with wash buffer, 100 μl of 3,3′,5,5′-tetramethylbenzidine microwell peroxidase substrate (Kirkegaard & Perry Laboratories) was added to each well. The plate was covered and kept for 10–15 min. The enzymatic reaction was then quenched by adding 100 μl of 1 M H3PO4 and absorbance read at 450 nm by using a Molecular Devices Vmax Microplate Reader and SOFTPROMAX 3.0 software. All reactions were performed at 22° ± 1°C unless otherwise noted. The resulting data were analyzed by using PRISM 2.0b Mac software.
Fluid Secretion Assay.
A modified Ramsay assay (21) was used for measurement of fluid secretion by larval T. molitor Malpighian tubules at various concentrations of the synthetic peptide. Secretion from tubules was measured in control solution (Ringer's), which was then replaced with either Ringer's solution or ADF plus Ringer's solution. Antidiuretic activity was calculated as the difference in fluid secretion rates (nl/min) measured before (maximal basal fluid secretion, control) and after the addition of antidiuretic factor, expressed as percent inhibition of secretion. Each tubule served as its own control, with 5–8 replicates done for each experiment. BSA was maintained at a constant concentration of 0.5 mg/ml throughout all assays to prevent loss of peptide.
Effect of NO Donors and NO Synthase Inhibitors On Malpighian Tubules.
We conducted experiments to study the involvement of NO by using a crude extract of peptides as a stimulant, because T. molitor ADF had not yet been identified. Two thousand T. molitor heads were collected and stored as described above. Heads were homogenized as described below for the isolation, except that the material soluble in 90% methanol/10% acetic acid was used for testing. The entire solution was aliquoted into 1.5-ml polypropylene tubes at ten T. molitor head equivalents per tube; 0.1 mg BSA was then added to each tube, and the solutions were dried down on a vacuum centrifuge. Before utilization in bioassays, the peptide extract was redissolved in T. molitor saline, 75 μl of saline per head equivalent. Two head equivalents were used in each assay, including positive controls. The cGMP assay described as “isolation assay” was used for these studies; we had not yet developed the more reproducible assay used for cGMP dose–response measurements. We had also not discovered that neutral extraction of the pellet after acidic methanol extraction gave a larger amount of ADF than acidic extraction.
We tested the effects of two NO donors, the nonspecific donor sodium nitroprusside at 10 μM and 1 mM and the selective donor S-nitroso-L-cysteine at 1 μM and 0.25 mM, for their effect on the amount of cGMP secreted by T. molitor Malpighian tubules. S-nitroso-L-cysteine was synthesized, a few hours before each experiment because of its instability, by reaction of sodium nitrite with L-cysteine as described (24). We also attempted to block the cGMP stimulation caused by two head equivalents of semipurified T. molitor ADF with the NO synthase inhibitors NG-monomethyl-L-Arg (L-NMMA) at 1 μM, 10 μM, and 10 mM. Similar experiments were performed with another NO synthase inhibitor, S-methylisothiourea, at 10 μM and 5 mM. The amount of cGMP produced was compared with that produced by a two head equivalent positive control lacking inhibitor.
Extraction from Heads.
T. molitor heads were collected from pupae and stored at −80°C before usage. Heads were delipidated by homogenizing, using a Polytron with PTA 20TS saw teeth (Brinkmann), first in cold (0–4°C) isooctane (400 ml), then 3 times in cold (0–4°C) CH2Cl2 (400 ml each). The solid pellet was extracted using 90% methanol/10% acetic acid (400 ml) with 0.1% (vol/vol) 2-(methylthio)-ethanol to minimize oxidation. After re-extraction with the same solution, the remaining pellet was extracted with 20 mM pH 4 Tris-acetate buffer (400 ml) containing protease inhibitors: 10 μM leupeptin, 1 μM pepstatin, and 0.2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride. This solution also contained 0.1% (vol/vol) 2-(methylthio)-ethanol and 1 mg BSA. After each extraction, the homogenate was centrifuged at 15,000 × g. The major cGMP-stimulating activity extracted into the pH 4 buffer; in all previous steps it had remained in the pellet. The centrifuged supernatant was vacuum filtered using a 20-μm polyethylene frit in a 75-ml polypropylene syringe reservoir to remove particulates before the first chromatographic step.
Peptide Isolation.
One thousand five hundred head equivalents of filtered extract in buffer were loaded on CM650 M Toyopearl cation exchange support (1.5 ml), contained in a 5-ml polypropylene syringe reservoir with polyethylene frit, then washed with pH 4 Tris-acetate buffer. Peptides were subsequently eluted with 20 mM Tris, pH 7 (20 ml), after which trifluoroacetic acid (TFA) was added to 0.1% (vol/vol) before the first reversed-phase liquid chromatography (RPLC) step. All RPLC steps were done on a Hewlett–Packard model 1050 pumping system and detector, with Rheodyne 7125 loop injector. Five hundred head equivalents (a one-third aliquot from the prior step) were loaded at 0.2 ml/min onto a C8 Zorbax (2.1 × 150 mm; 30 nm) reversed-phase column, which was equilibrated with 0.1% aqueous TFA before and after loading of samples. The bound fractions were eluted with a 60-min 0–57% CH3CN in 0.1% TFA gradient at 0.2 ml/min, at ambient temperature. The detector was set to 220 nm at 0.05 absorbance units full scale (AUFS). Fractions were collected manually as individual peaks when possible based on the peak shapes on the strip chart recorder tracing. The most active ADF fraction eluted at ≈17% CH3CN. This chromatography step was repeated twice, each time with 500 head equivalent aliquots from the previous (cation exchange) step; active fractions from the three runs were pooled and diluted 5-fold with 0.1% aqueous TFA before the next step. These pooled fractions were then loaded onto a C4 Vydac (1.0 × 150 mm; 30 nm) reversed-phase column at 60 μl/min, at ambient temperature, and equilibrated as before with 0.1% TFA. The detector was again set to 220 nm at 0.05 AUFS. The bound fractions were eluted with the same gradient, with the most active ADF fraction eluting from the column at 13.5% CH3CN. This fraction was again diluted 5-fold with 0.1% aqueous TFA, then loaded onto a Zorbax SB cyano (Michrom 1.0 × 150 mm, 30 nm) reversed-phase column at 60 μl/min and equilibrated as before with 0.1% aqueous TFA. The bound fractions were eluted as in the preceding two steps (same gradient; same temperature, detector conditions), with the most active fraction eluting at 19% CH3CN. All RPLC fractions were collected in 1.5-ml lubricated (Corning Costar) tubes to prevent sticking; no BSA was added to RPLC fractions.
Peptide Microanalysis and Synthesis.
Mass spectral analysis of the purified peptide was performed on a Bruker Proflex Plus matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) instrument. Sequence analysis of the purified ADF was performed using automated Edman degradation on an Applied Biosystems Procise 492 sequencer. ADF peptides were synthesized with Nα-9-fluorenylmethoxycarbonyl (Fmoc) chemistry by using an Applied Biosystems 431A synthesizer using 1-hydroxybenzotriazole in 1-methyl-2-pyrrolidinone in the presence of dicyclohexylcarbodiimide for Fmoc-amino acid activation. Extended 2-h single coupling cycles with 10-fold molar excess of acylating species were used. Both acid and amide C-terminal forms of the peptide were synthesized simultaneously by using a combination of 50 μmol each of p-hydroxymethyl-phenoxy (Wang resin) and Rink MBHA amide resins. An excellent separation of the two forms was achieved by purification on a 20 × 250 mm C8 YMC column on a Thermo Separations (San Jose, CA) P4000 preparative liquid chromatograph. The gradient used was 0–57% ethanol in 60 min with 0.1% TFA maintained throughout. After separation, the synthetic amidated and free-acid forms of the peptide were analyzed by MALDI-TOF to confirm their identity.
Nerve Cord Extraction and Partial Purification.
Brains and nerve cords were separately dissected from pupae; the nerve cords consisted of the first thoracic to last abdominal ganglion. All tissues were rinsed in T. molitor saline before extraction. Nerve cords from ten T. molitor pupae were extracted using a scaled-down version of the isolation protocol; brains were extracted separately. A total volume of 1 ml of solvent in 1.5-ml polypropylene tubes was used for each extraction; homogenization was done with a teflon pestle fitted to the 1.5-ml tubes. The defatted nerve-cord extract was loaded onto a CM650 M Toyopearl cation exchange support (0.5 ml) contained in a 1.5-ml polypropylene syringe reservoir with polyethylene frit, then washed with pH 4 20 mM Tris-acetate buffer. Peptides were eluted with 4 ml of 20 mM Tris, pH 7, after which TFA was added to 0.1% (vol/vol) before RPLC separation. The solution was loaded on a 1-mm C4 Vydac reversed-phase column, and eluted as described in the isolation protocol. The strongest cGMP-stimulating zone was then diluted five times with 0.1% aqueous TFA and separated on a 1-mm Zorbax cyano RPLC column, again using the same conditions as described above. Brains were separated using only the Vydac C4 column.
Results
Isolation and Identification of ADF.
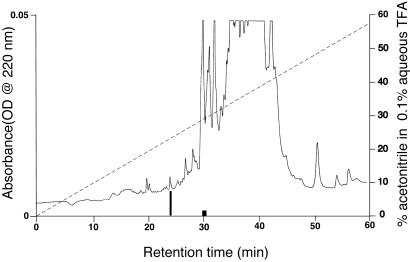
From an initial extraction of 10,000 frozen T. molitor heads, 1,500 head equivalents were purified by ion exchange and reversed-phase chromatography, yielding ≈50 pmol of pure ADF peptide. This single 15% aliquot of the entire sample sufficed for mass spectral and microsequence analysis. There was a single additional active fraction in the first HPLC separation (C8 column, Fig. 1), which had a somewhat greater retention time on the reversed-phase column than the more active fraction. This second factor appears very significantly less active and is still under investigation.
Figure 1.
Reversed-phase separation of 500 head equivalents of T. molitor extract on a C8 Zorbax (2.1 × 150 mm) column, using the conditions described in Materials and Methods. The taller blackened box shows a fraction containing the T. molitor ADF, whereas the shorter darkened box is a fraction corresponding to a second, less active entity. Fractions were collected based on peak morphology.
Edman sequence analysis of the purified ADF indicated a primary structure of VVNTPGHAVSYHVY, with a theoretical monoisotopic Mr of 1,541.7; MALDI-TOF analysis of the natural peptide indicated a mass of 1,541.58, which agrees well with the theoretical Mr. Peptides having this sequence were synthesized in both amidated and free-acid carboxyl terminus forms and purified by RPLC. The native ADF was coinjected separately with amide and acid synthetic ADF on a 1-mm C4 Vydac (Hesperia, CA) reversed-phase column; the native peptide coelutes with the free-acid form of synthetic ADF, confirming the sequence analysis and demonstrating a complete structure of VVNTPGHAVSYHVY-OH. The free-acid structure was also confirmed by analysis of mixed native and synthetic peptide by MALDI-TOF mass spectral analysis, yielding a single ion at the correct Mr.
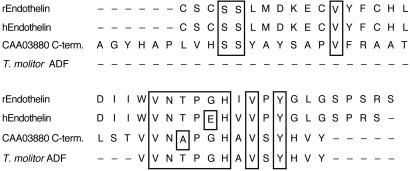
T. molitor ADF has significant resemblance to only one known neuroendocrine peptide; it is similar to a portion of rabbit big endothelin I (Fig. 2): 57% identity and an additional 14% similarity. However, the similarity is to the inactive portion of big endothelin I, which is removed in the conversion to active endothelin I; the similarity begins exactly at the site of cleavage of big endothelin (Fig. 2). Interestingly, T. molitor ADF also has near identity to the C terminus of a known T. molitor cuticle protein, differing only by a threonine (peptide) for alanine (protein) substitution 11 residues from the carboxyl terminus of the protein (25).
Figure 2.
Sequence alignment of T. molitor ADF, T. molitor cuticle protein CAA03880 (40 C-terminal residues only of 293 residue protein), and rabbit (r) and human (h) big endothelin I, aligned using clustal w. v. 1.8 on sequences from the Swiss-Prot database. Identical residues are boxed. Endothelin I is cleaved by endothelin converting enzyme at the W–V bond; the homology to T. molitor ADF occurs in the inactive part of big endothelin I, on the C-terminal side of the cleavage site.
Biological and cGMP Second Messenger Assays.
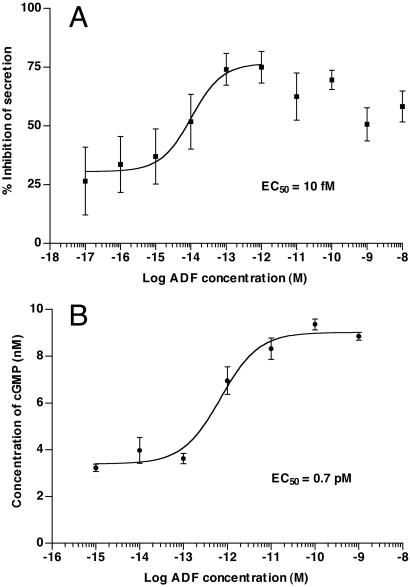
Because we used a cGMP assay to confirm the identity of our antidiuretic factor, we sought also to demonstrate dose-dependent biological activity in a fluid secretion assay. Using a modified Ramsay assay, we demonstrated that the ADF caused a reversible and dose-dependent decrease in fluid secretion by T. molitor tubules (Fig. 3A) with an exceptionally low EC50 (10 fM; 95% CL = 2.8–38 fM). Significant desensitization of maximal response occurs at higher concentrations of ADF, indicating possible receptor desensitization or internalization. Thus, the data in Fig. 3A were fitted to nonlinear regression analysis (PRISM 3.0a) only from 10−17 to 10−12 M, because of the inability of the algorithm to account for decreases at higher concentrations. Although data up to 10−8 M are shown, these were not included in the nonlinear regression. T. molitor ADF causes a dose-dependent increase of cGMP production in the Malpighian tubules of the insect; the EC50 for the second messenger is 0.7 pM (Fig. 3B). [CAP2b also elevates cGMP in T. molitor tubules, but with far lower potency (R.A.E., unpublished data)]. In a related publication (19), we have demonstrated that exogenous cGMP inhibits fluid secretion by T. molitor tubules, and that both exogenous cGMP and T. molitor ADF cause reversal of previous stimulation by cAMP or the T. molitor diuretic hormone DH37 (26), respectively.
Figure 3.
(A) Dose–response curve for T. molitor ADF with Malpighian tubules of larval T. molitor. Fluid secretion was measured at various concentrations of hormone and the percent inhibition determined. The data are given as % inhibition of secretion ± standard error, and were fitted by nonlinear regression analysis using PRISM 3.0, fitting only data from 10−17 to 10−12 M because of apparent desensitization at higher concentrations. The EC50 is 10 fM (95% CL = 2.8–38 fM). Basal secretion was determined on Malpighian tubules initially in control solution (Ringer's saline); subsequently this was replaced with more Ringer's solution or Ringer's solution containing antidiuretic factor. All fractions contained 0.5 mg/ml BSA. Some loss of basal secretion is always observed after fluid replacement; this accounts for the response never reaching zero inhibition. (B) Dose–response curve for T. molitor ADF, but measuring elevation of cGMP in adult Malpighian tubules rather than fluid secretion. The EC50 is 0.7 pM (95% CL = 0.36–1.4 pM), 70 times higher than the EC50 for fluid secretion.
Biochemical Evaluation of Peptide Source.
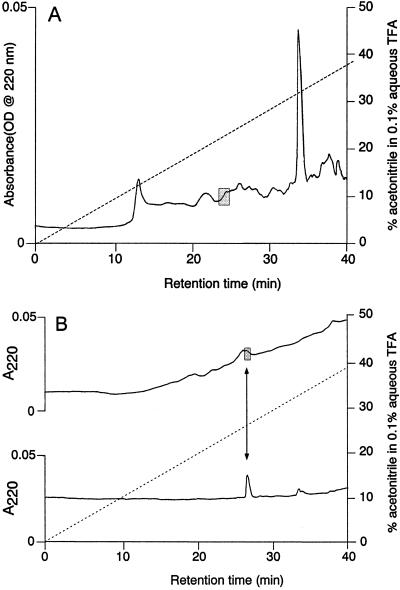
Because of the near identity of T. molitor ADF to a cuticle protein, we extracted and partially purified peptides from T. molitor tissues containing no cuticle: the nerve cord and brain. We used a protocol that was scaled down, but otherwise identical to that used for extraction and initial purification of the ADF peptide. The region showing cGMP-stimulating activity after purification on a 1-mm Vydac C4 column (Fig. 4A) eluted at approximately the same retention time as did ADF in previous steps. To verify this similarity of elution, the active region was rechromatographed on a 1-mm Zorbax (Michrom, Auburn, CA) cyano column and synthetic (free acid) peptide was subsequently run using identical conditions. Fig. 4B shows the agreement in retention times for the two peptides. We also demonstrated that biological activity from partially purified brain extract has retention time similar to that of the synthetic ADF on the Vydac C4 column (data not shown). This result indicates the likelihood that this peptide is not a randomly proteolyzed fragment of cuticle protein, but a genuine neuroendocrine molecule.
Figure 4.
(A) T. molitor nerve cord extract separated by RPLC; 1 × 150 mm C4 Vydac column, eluted at 50 μl/min by using a gradient of 0–57% acetonitrile in 60 min with 0.1% TFA maintained throughout. Detector was set to 220 nm and 0.05 absorbance units full scale (AUFS). Shaded area indicates the fraction containing cGMP stimulating activity, which has retention time similar to the T. molitor ADF standard (not shown). (B) Upper chromatogram: Fractionation of cGMP-stimulating zone from above figure on 1 × 150 mm Zorbax cyano column, eluted with the same gradient and conditions except full scale shown is 0.02 AUFS. A fraction with cGMP stimulating activity is shown by a shaded fraction on the chromatogram. Lower chromatogram: 20 pmol T. molitor ADF run on the same column with same conditions, but after the biological sample to avoid contamination.
Other Chemical Messengers.
In some biological systems, NO acts as a second messenger for peptide hormones (27) by stimulation of a soluble guanylyl cyclase, whose heme group binds NO. The myotropic peptide CAP2b, originally isolated from Manduca sexta (16), causes diuresis in D. melanogaster by elevation of NO and thence cGMP (17), yet in R. prolixus CAP2b causes antidiuresis without the involvement of NO (18). Therefore, early in this project we tested the ability of the nonspecific NO donor sodium nitroprusside at 10 μM and 1 mM concentrations to stimulate cGMP elevation. We used as a negative control T. molitor saline and tubule without NO donor. Sodium nitroprusside failed to cause a significant elevation of cGMP at both concentrations. The specific NO donor S-nitroso-L-cysteine also failed to significantly stimulate cGMP elevation at both 1 μM and 0.25 mM. We also tested the ability of two NO synthase inhibitors, NG-monomethyl-L-Arg (L-NMMA) and S-methylisothiourea, to block the cGMP elevation induced by crude soluble extract. For S-methylisothiourea, a two head equivalent positive control showed no significant difference in cGMP production in the presence or absence of either 10 μM or 5 mM S-methylisothiourea. L-NMMA showed no effect at 1 μM and 10 μM. There was a significant decrease in cGMP production when the ADF sample was assayed with 10 mM L-NMMA, but at such high concentrations of Arg derivatives, nonspecific effects have been reported (28). These results together suggest that NO does not act in signal transduction in T. molitor Malpighian tubules. It is likely that this peptide works through a membrane-bound guanylyl cyclase/receptor, as does atrial natriuretic factor (29).
Using cAMP EIA, we also confirmed that synthetic ADF causes a striking reduction in cAMP production, when added to Malpighian tubules stimulated by 10 nM T. molitor DH37. T. molitor ADF at 1 pM was much more effective than 1 nM ADF for lowering cAMP (Fig. 5), again indicative of receptor desensitization at high concentrations of ADF (the term “hormesis” (30) has been coined for the general phenomenon of a decrease in response resulting from an overstimulation of receptor by ligand; this phenomenon appears to constitute an organismal response to restore homeostasis).
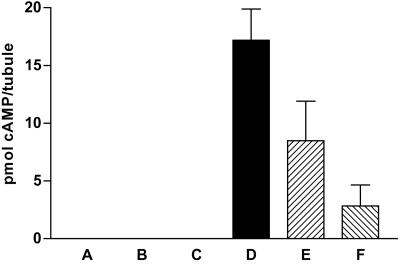
Figure 5.
The effects of various treatments on cAMP levels determined by EIA in 300 μl incubations containing two Malpighian tubules and lacking any phosphodiesterase inhibitor. Results are shown as pmol per tubule of cAMP ± SEM; columns indicated as zero had an amount of cAMP lower than the limit of detection (0.6 pmol per incubation) of the EIA. A, control; B, 1 nM ADF; C, 1 pM ADF; D, 10 nM T. molitor DH37; E, 10 nM T. molitor DH37 + 1 nM ADF; F, 10 nM T. molitor DH37 + 1 pM ADF.
T. molitor DH37 is a potent diuretic hormone (DH) acting through cAMP (26) with an EC50 of 2.6 nM; its EC50 in a fluid secretion assay in this species is 0.12 nM (19), or about 20-fold lower. Interestingly, the EC50 for the ADF is also much lower in the fluid secretion assay (70-fold) than in the cGMP second messenger assay, consistent with the notion that considerably less than a half-maximal production of second messenger is required to give a maximal increase in biological effect. However, some of this difference is likely attributable to use of larval tubules for the fluid secretion assays (Pretoria) and adult tubules for the cGMP assays (Reno); the two colonies are of different origin and rearing conditions differ. Opposing effects for the two cyclic nucleotides are documented in R. prolixus (31) and now also in T. molitor (19).
Discussion
In this paper, we describe the isolation and characterization of an extremely potent antidiuretic peptide from T. molitor. This peptide exerts its effects in the unusual manner of stimulating cGMP and thereby inhibits fluid production by the Malpighian tubules through decreasing cAMP concentration. It is interesting that we were able to identify this highly potent peptide by using only a 15% aliquot of the 10,000 heads extracted. This yield suggests it is more abundant than Tenmo-DH37 or -DH47; isolation of the former was successful from 8,400 T. molitor pupal heads (26), but isolation of the latter required more than 28,000 heads for successful sequencing (32). The combination of an apparent higher abundance of ADF compared with the DH, versus its very high potency, is surprising.
The structure of T. molitor ADF is unique among neuropeptides, with its only known resemblance to the portion of rabbit big endothelin I, which is removed in the conversion to active endothelin I. T. molitor ADF also has near identity to the C terminus of a known T. molitor cuticle protein, differing only by a threonine (peptide) for alanine (protein) substitution 11 residues from the carboxyl terminus of the protein (25). It is possible that these sequences are identical. The sequence of the cuticle protein was deduced from cDNA sequence data; a single base error in sequencing would account for the observed difference in protein sequence. Virtually all neuropeptide precursors have paired dibasic residues immediately preceding and immediately following the peptide sequence; the dibasic residues are a consensus site for cleavage by Kex-2, furin, or their homologues (33). The cuticle protein has a Ser-Thr sequence just “upstream” of the ADF-like sequence, rather than a dibasic cleavage site, suggesting the protein is not likely to be a conventional neuropeptide precursor. It is interesting to recall that both the cuticle and the brain plus nervous tissue are of common ectodermal origin in the embryo. T. molitor ADF could have evolved by an interesting gene duplication event. Gene duplication events appear to explain (34) the existence of two CRF-like DH in each of three insect species (32, 34, 35), the smaller of the three CRF-like DH form a paralogous family as a result of gene duplication: a situation analogous to that observed in the CRF-sauvagine-urotensin I family of peptides (36).
Recently Lavigne et al. (15) and Laenen et al. (14) have reported attempted isolation of antidiuretic factors from the beetle Leptinotarsa decemlineata and the ant Formica polyctena, respectively. Both studies used fluid secretion assays, with no characterization of the second messenger involved. The properties of the factor from L. decemlineata are similar to those of T. molitor ADF; the factor from L. decemlineata elutes from a C18 solid phase extraction cartridge with less than 25% CH3CN, and it partially permeates a 3,000-Da cut-off membrane. From these data the authors inferred it was a peptide of 25–50 residues (ref. 15; such size estimates from membrane permeability are extremely approximate). In contrast, the factor from F. polyctena is not eluted by 40% CH3CN; it requires 60% CH3CN to elute from a C18 solid phase extraction cartridge. It is thus far more hydrophobic than the ADF from either beetle. Nevertheless, the ant ADF is reported to be active on Malpighian tubules of T. molitor (14).
Because of the exceptional potency of the antidiuretic factor, we feel confident that it plays a role as an endogenous antidiuretic neurohormone (ADH) and represents a potential target for insect control. Antagonists of this peptide could impair the ability of T. molitor to survive in dry environments, such as grain silos, where it can do significant damage. If this ADH is evolutionarily conserved among the Insecta, it could be used to develop weapons against a number of pests that depend on antidiuretic factors for their ability to survive dry conditions.
Acknowledgments
We thank Vincent C. Lombardi (University of Nevada, Reno) for the reagents for cAMP EIA. We gratefully acknowledge financial support from the National Science Foundation (IBN-9602148), the Nevada Agricultural Experiment Station, and the South African National Research Foundation.
Abbreviations
- ADF
antidiuretic factor
- EIA
enzyme immunoassay
- RPLC
reversed-phase liquid chromatography
- TFA
trifluoroacetic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the Swiss-Prot Database (accession no. P82965).
References
- 1.Phillips J E. Am J Physiol. 1981;241:R241–R257. doi: 10.1152/ajpregu.1981.241.5.R241. [DOI] [PubMed] [Google Scholar]
- 2.Phillips J E, Hanrahan J, Chamberlin M, Thomson B. Adv Insect Physiol. 1986;19:329–422. [Google Scholar]
- 3.Coast G M. Peptides. 1996;17:327–336. doi: 10.1016/0196-9781(95)02096-9. [DOI] [PubMed] [Google Scholar]
- 4.Proux B H, Proux J P, Phillips J E. J Exp Biol. 1984;113:409–421. [Google Scholar]
- 5.Audsley N, McIntosh C, Phillips J E. J Exp Biol. 1992;173:261–274. doi: 10.1242/jeb.173.1.261. [DOI] [PubMed] [Google Scholar]
- 6.Audsley N, McIntosh C, Phillips J E, Schooley D A, Coast G M. In: Perspectives in Comparative Endocrinology. Davey K G, Peter R E, Tobe S S, editors. Ottawa: National Research Council of Canada; 1994. pp. 74–80. [Google Scholar]
- 7.Fournier B, Girardie J. J Insect Physiol. 1988;34:309–313. [Google Scholar]
- 8.Girardie J, Girardie A, Huet J-C, Pernollet J-C. FEBS Lett. 1989;248:4–8. doi: 10.1016/0014-5793(89)80179-6. [DOI] [PubMed] [Google Scholar]
- 9.King D S, Meredith J, Wang Y J, Phillips J E. Insect Biochem Mol Biol. 1999;29:11–18. doi: 10.1016/s0965-1748(98)00098-8. [DOI] [PubMed] [Google Scholar]
- 10.Liao S, Audsley N, Schooley D A. J Exp Biol. 2000;203:605–615. doi: 10.1242/jeb.203.3.605. [DOI] [PubMed] [Google Scholar]
- 11.Phillips J E, Wiens C, Audsley N, Jeffs L, Bilgen T, Meredith J. J Exp Zool. 1996;275:292–299. doi: 10.1002/(SICI)1097-010X(19960701)275:4<292::AID-JEZ7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Spring J H, Morgan A M, Hazelton S R. Science. 1988;241:1096–1098. doi: 10.1126/science.241.4869.1096. [DOI] [PubMed] [Google Scholar]
- 13.De Decker N, Hayes T K, Van Kerkhove E, Steels P. J Insect Physiol. 1994;40:1025–1036. [Google Scholar]
- 14.Laenen B, De Decker N, Steels P, Van Kerkhove E, Nicolson S. J Insect Physiol. 2001;47:185–193. doi: 10.1016/s0022-1910(00)00104-9. [DOI] [PubMed] [Google Scholar]
- 15.Lavigne C, Embleton J, Audy P, King R R, Pelletier Y. Insect Biochem Mol Biol. 2001;31:339–347. doi: 10.1016/s0965-1748(00)00126-0. [DOI] [PubMed] [Google Scholar]
- 16.Huesmann G R, Cheung C C, Loi P K, Lee T D, Swiderek K M, Tublitz N J. FEBS Lett. 1995;371:311–314. doi: 10.1016/0014-5793(95)00929-4. [DOI] [PubMed] [Google Scholar]
- 17.Davies S A, Huesmann G R, Maddrell S H P, O'Donnell M J, Skaer N J V, Dow J A T, Tublitz N J. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1321–R1326. doi: 10.1152/ajpregu.1995.269.6.R1321. [DOI] [PubMed] [Google Scholar]
- 18.Quinlan M C, Tublitz N J, O'Donnell M J. J Exp Biol. 1997;200:2363–2367. doi: 10.1242/jeb.200.17.2363. [DOI] [PubMed] [Google Scholar]
- 19.Wiehart, U. I. M., Nicolson, S. W., Eigenheer, R. A. & Schooley, D. A. (2001) J. Exp. Biol., in press. [DOI] [PubMed]
- 20.Rafaeli A, Pines M, Stern P S, Applebaum S W. Gen Comp Endocrinol. 1984;54:35–42. doi: 10.1016/0016-6480(84)90195-3. [DOI] [PubMed] [Google Scholar]
- 21.Nicolson S W. J Insect Physiol. 1992;38:139–146. [Google Scholar]
- 22.Kingan T G, Gray W, Zitnan D, Adams M E. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- 23.Horton J K, Martin R C, Kalinka S, Cushing A, Kitcher J P, O'Sullivan M J, Baxendale P M. J Immunol Methods. 1992;155:31–40. doi: 10.1016/0022-1759(92)90268-x. [DOI] [PubMed] [Google Scholar]
- 24.Gibson A, Babbedge R, Brave S R, Hart S L, Hobbs A J, Tucker J F, Wallace P, Moore P K. Br J Pharmacol. 1992;107:715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathelin J, Quennedey B, Bouhin H, Delachambre J. Gene. 1998;211:351–359. doi: 10.1016/s0378-1119(98)00125-5. [DOI] [PubMed] [Google Scholar]
- 26.Furuya K, Schegg K M, Wang H, King D S, Schooley D A. Proc Natl Acad Sci USA. 1995;92:12323–12327. doi: 10.1073/pnas.92.26.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt H H, Lohmann S M, Walter U. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 28.Buxton I L, Cheek D J, Eckman D, Westfall D P, Sanders K M, Keef K D. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig A, Seidman C E. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese E J, Baldwin L A. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan M C, O'Donnell M J. J Insect Physiol. 1998;44:561–568. doi: 10.1016/s0022-1910(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 32.Furuya K, Schegg K M, Schooley D A. Peptides. 1998;19:619–626. doi: 10.1016/s0196-9781(97)00475-0. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama K. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldwin D, Schegg K M, Furuya K, Lehmberg E, Schooley D A. Peptides. 2001;22:147–152. doi: 10.1016/s0196-9781(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn M B, Kingan T G, Bodnar W, Shabanowitz J, Hunt D F, Kempe T, Wagner R M, Raina A K, Schnee M E, Ma M C. Biochem Biophys Res Commun. 1991;181:927–932. doi: 10.1016/0006-291x(91)92025-f. [DOI] [PubMed] [Google Scholar]
- 36.Lovejoy D A, Balment R J. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]