Abstract
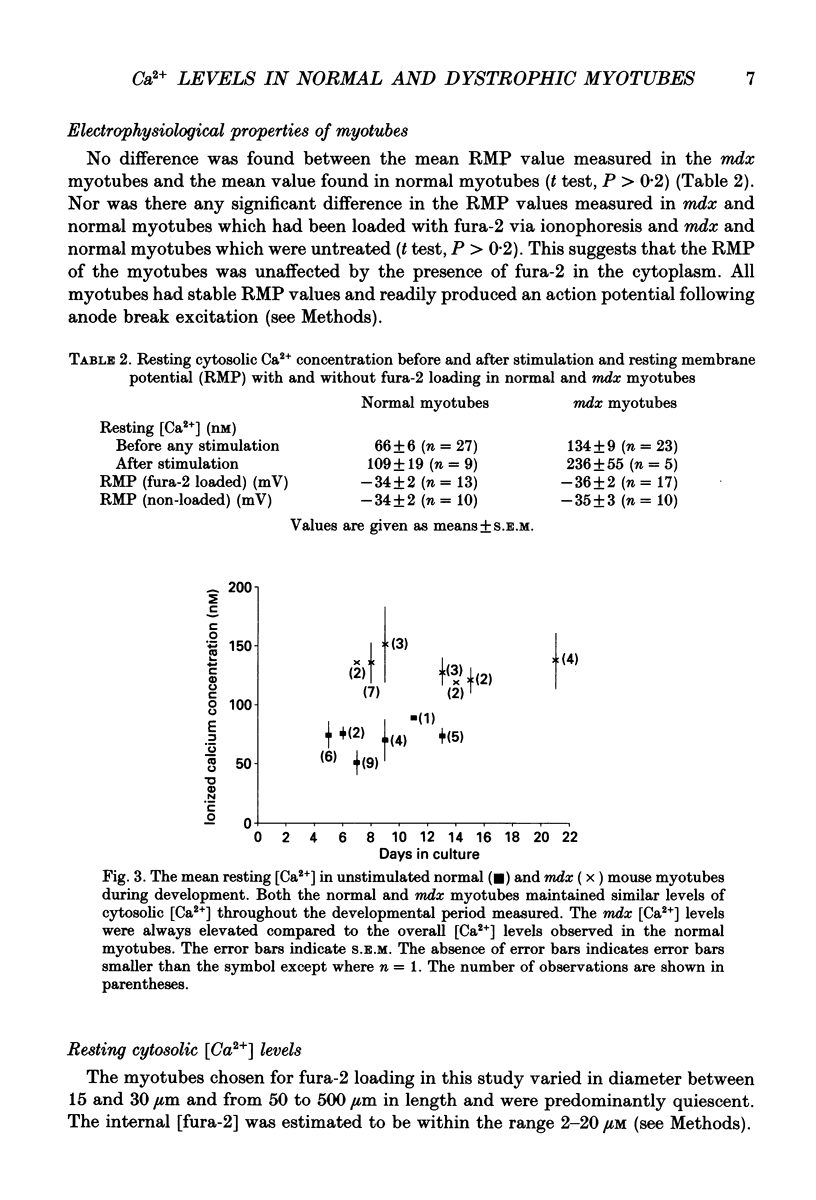
1. Myotubes were grown in culture from normal (C57BL/ScSn) and mdx mice and the cytosolic [Ca2+] was monitored through development (5-21 days in culture) using fura-2 loaded via ionophoresis. Simultaneous measurements of the membrane potential and cytosolic [Ca2+] were made in normal and mdx myotubes before, during and after stimulation by action potentials elicited following anode break excitation. All experiments were undertaken at 22 degrees C. All data are expressed as means +/- S.E.M. 2. A new method was developed which enabled accurate determination of the fluorescence characteristics of fura-2 in murine skeletal muscle fibres. In the under in vitro conditions by 14.60 +/- 0.05, 9.40 +/- 0.15 and 6.90 +/- 0.43% respectively. 3. The resting cytosolic [Ca2+] in the mdx myotubes was consistently higher than in the normal myotubes throughout the developmental period measured. Overall, the resting cytosolic [Ca2+] in mdx myotubes (134 +/- 9 nM, n = 22) was twofold higher than in normal myotubes (66 +/- 6 nM, n = 26). After stimulation (one to three action potentials) the cytosolic [Ca2+] of both mdx and normal myotubes remained elevated. The mdx myotubes (236 +/- 55 nM, n = 5) again had approximately double the cytosolic [Ca2+] of normal myotubes (109 +/- 19 nM, n = 9). 4. The time course and amplitude of the Ca2+ responses measured in the mdx and normal myotubes after action potential stimulation were variable. Two categories of Ca2+ response were observed in mdx and normal myotubes, the first consisted of a small, slow rise in [Ca2+] that remained elevated and the second consisted of a rapid (time to peak 7.4 +/- 1.5 ms) (n = 8) rise in [Ca2+] with amplitudes in the range 61-773 nM and a [Ca2+] decay rate constant of 4.35 +/- 1.57 s-1 (n = 8) (range 0.96-15 s-1). 5. In conclusion, the elevated cytosolic [Ca2+] reported here through development of cultured mdx myotubes suggests that this genetic disorder results in a defect which compromises the ability of the myotubes to strictly regulate cytosolic [Ca2+]. The results are consistent with the presence of functionally abnormal Ca2+ channels recently reported in mdx myotubes.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Amagai Y., Kasai S. Calcium action potential and prolonged afterhyperpolarization in developing myotubes of a mouse clonal myogenic cell line. Jpn J Physiol. 1989;39(1):33–42. doi: 10.2170/jjphysiol.39.33. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol. 1986 Apr;373:513–525. doi: 10.1113/jphysiol.1986.sp016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong P. Y., Turner P. R., Denetclaw W. F., Steinhardt R. A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990 Nov 2;250(4981):673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990 Apr 12;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Huard J., Labrecque C., Dansereau G., Robitaille L., Tremblay J. P. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991 Feb;14(2):178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., McArdle A., Edwards R. H., Jones D. A. Muscle damage in mdx mice. Nature. 1991 Apr 25;350(6320):664–664. doi: 10.1038/350664b0. [DOI] [PubMed] [Google Scholar]
- Menke A., Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991 Jan 3;349(6304):69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Bonilla E., Martucci G., Moraes C. T., Hays A. P., Dimauro S. Immunocytochemical study of dystrophin in muscle cultures from patients with Duchenne muscular dystrophy and unaffected control patients. Am J Pathol. 1988 Sep;132(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Mokri B., Engel A. G. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975 Dec;25(12):1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Mongini T., Ghigo D., Doriguzzi C., Bussolino F., Pescarmona G., Pollo B., Schiffer D., Bosia A. Free cytoplasmic Ca++ at rest and after cholinergic stimulus is increased in cultured muscle cells from Duchenne muscular dystrophy patients. Neurology. 1988 Mar;38(3):476–480. doi: 10.1212/wnl.38.3.476. [DOI] [PubMed] [Google Scholar]
- Powell J. A., Fambrough D. M. Electrical properties of normal and dysgenic mouse skeletal muscle in culture. J Cell Physiol. 1973 Aug;82(1):21–38. doi: 10.1002/jcp.1040820104. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L. F., Duchen L. W. The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987 Apr;110(Pt 2):269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Vranesic I., Knöpfel T. Calculation of calcium dynamics from single wavelength fura-2 fluorescence recordings. Pflugers Arch. 1991 Mar;418(1-2):184–189. doi: 10.1007/BF00370469. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Head S. I., Bakker A. J., Stephenson D. G. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol. 1990 Sep;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]


