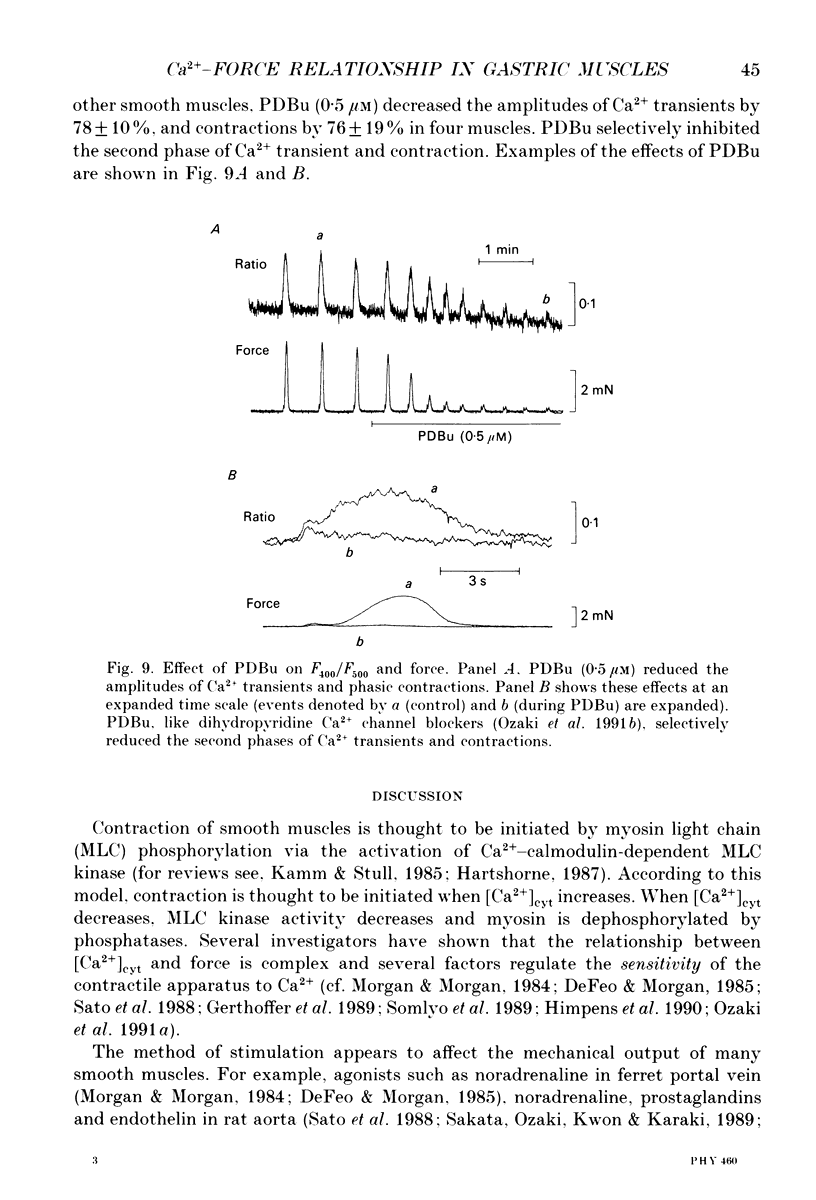
Abstract
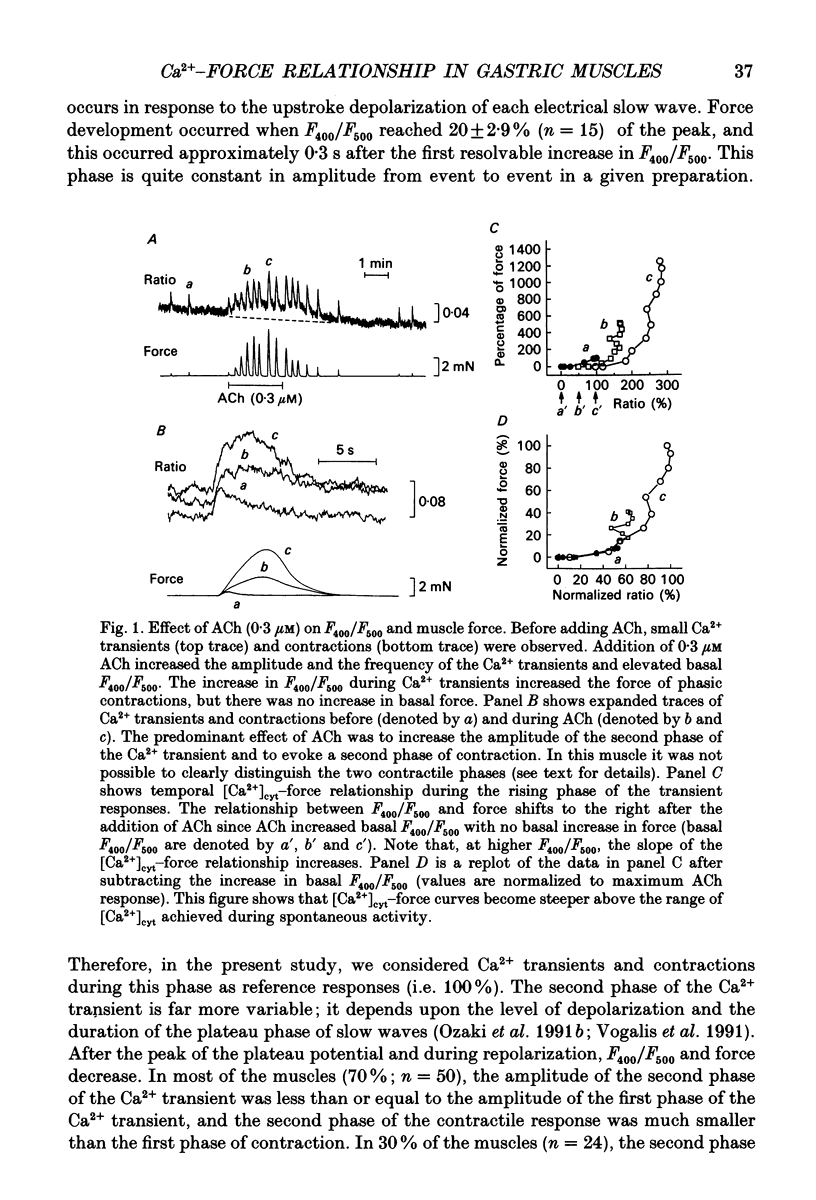
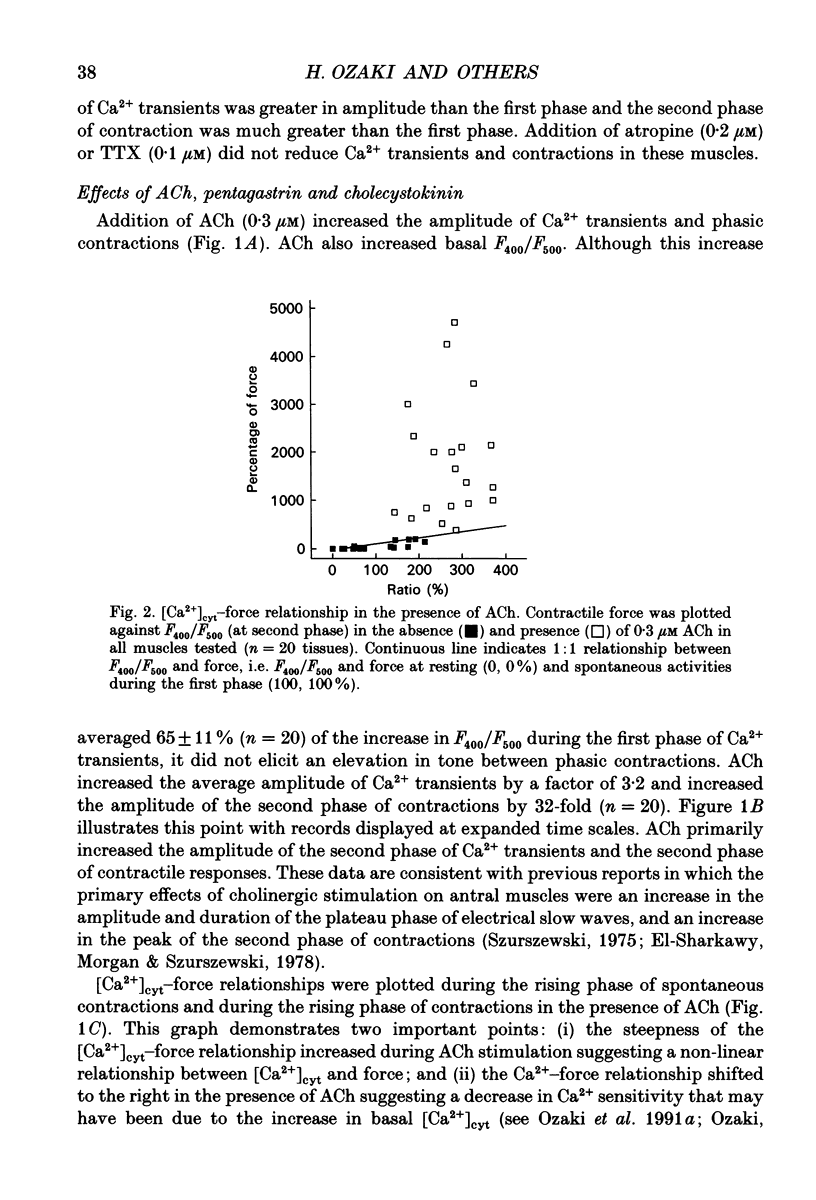
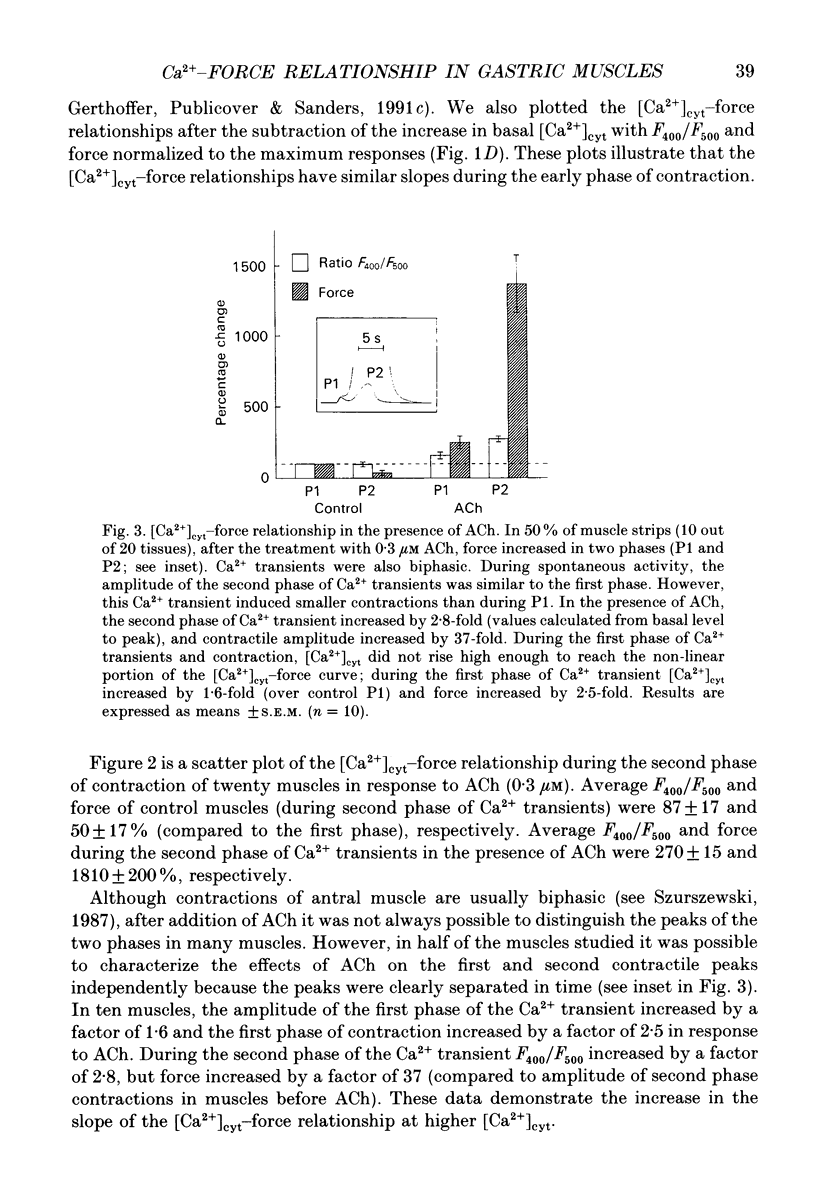
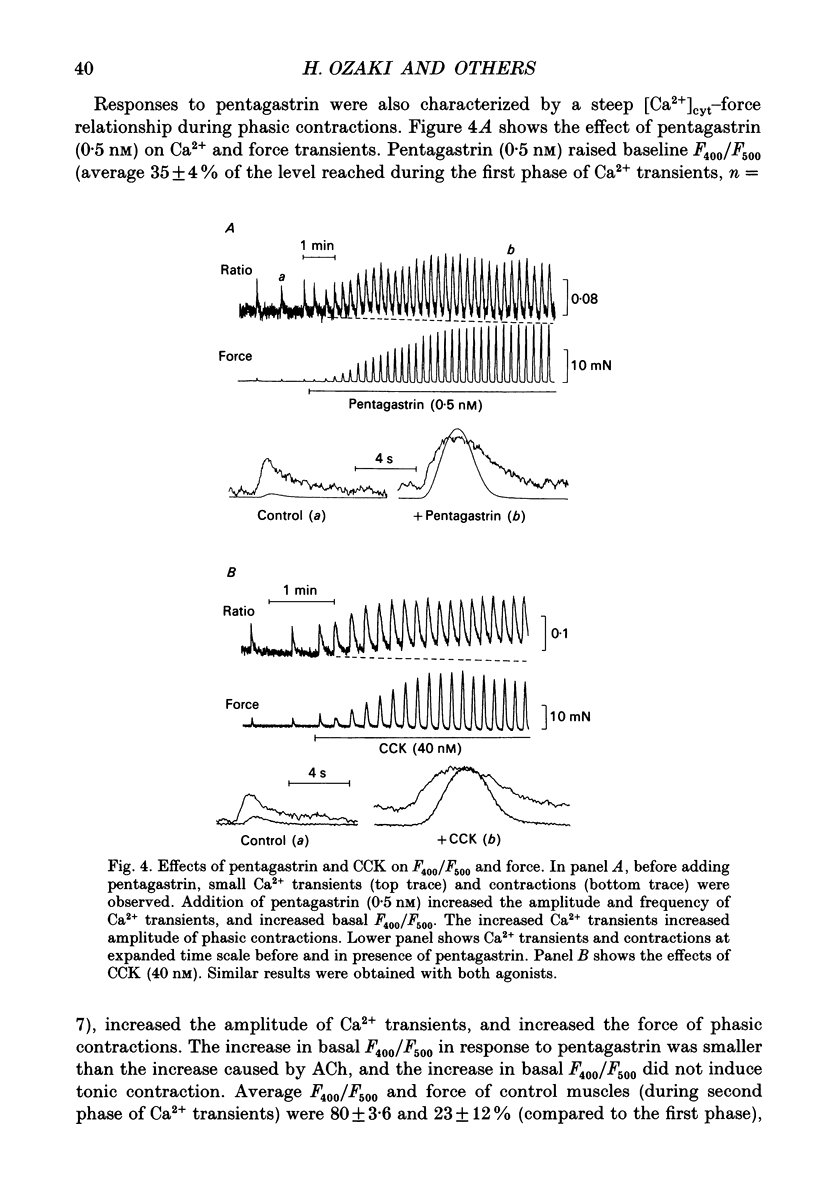
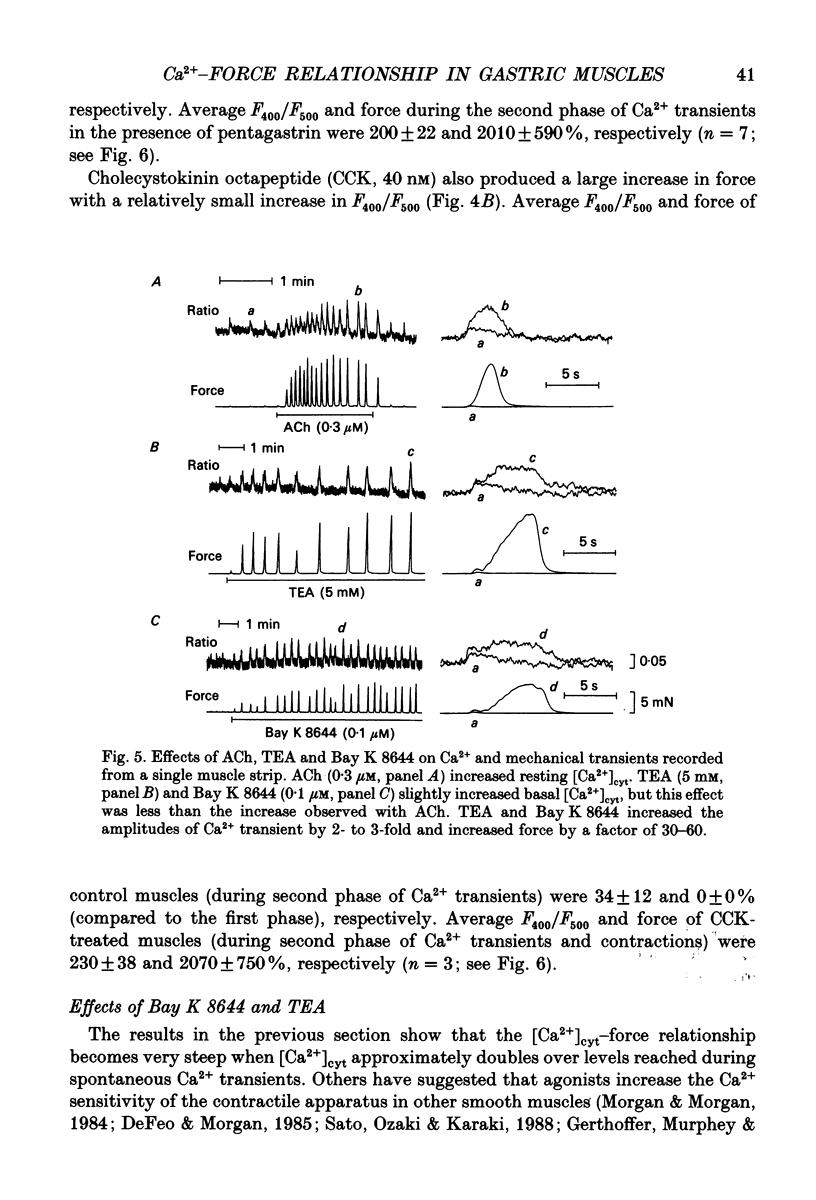
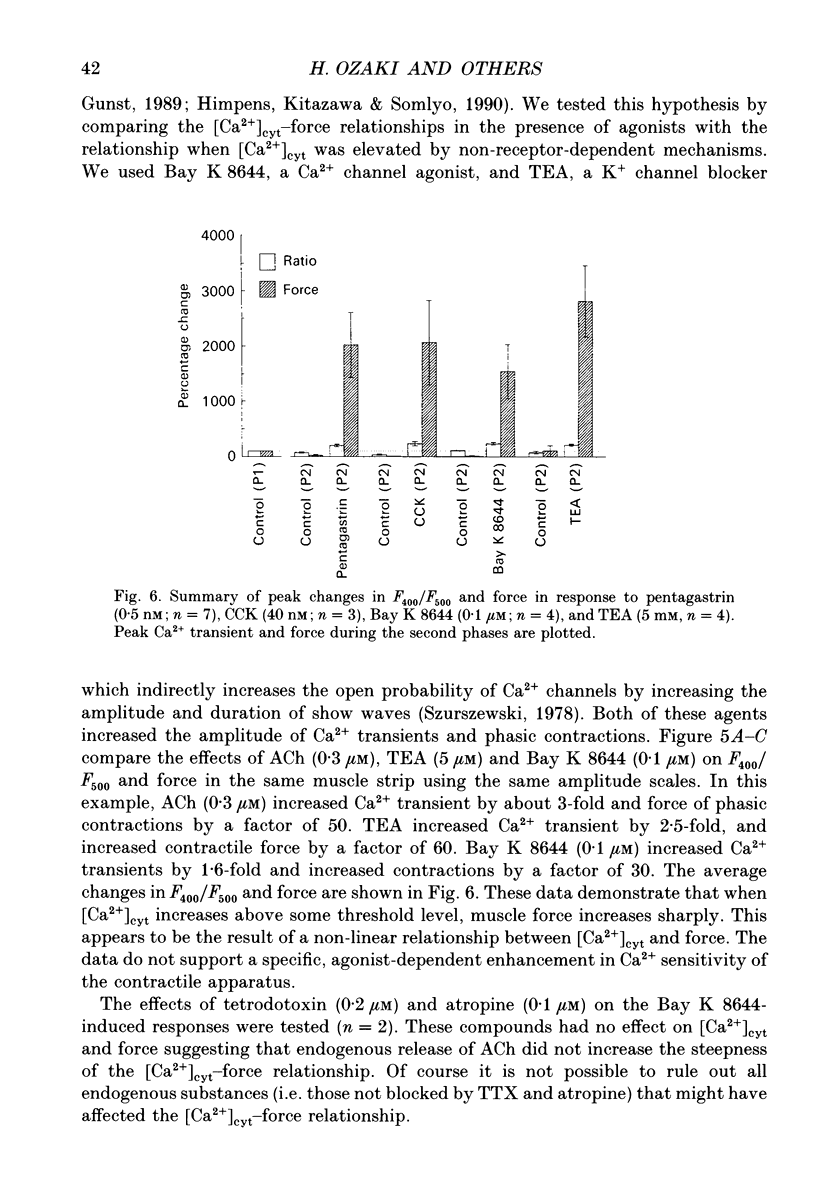
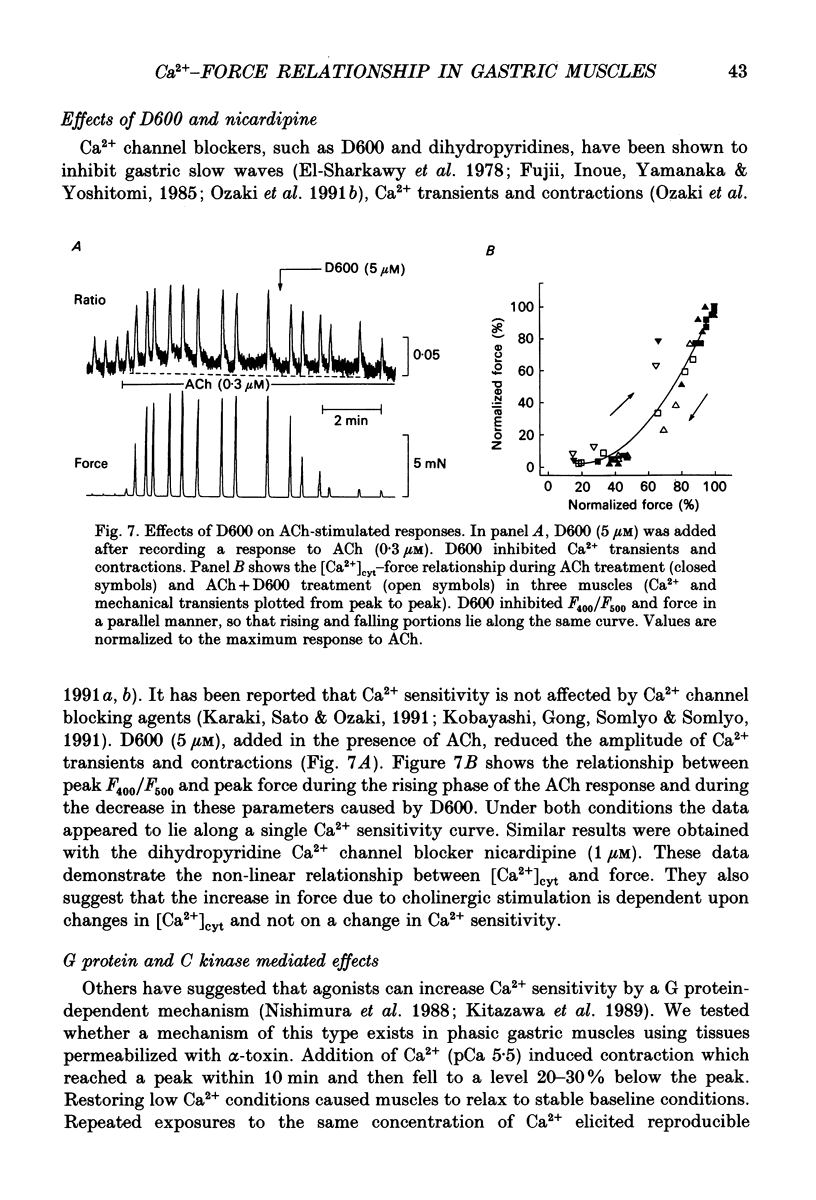
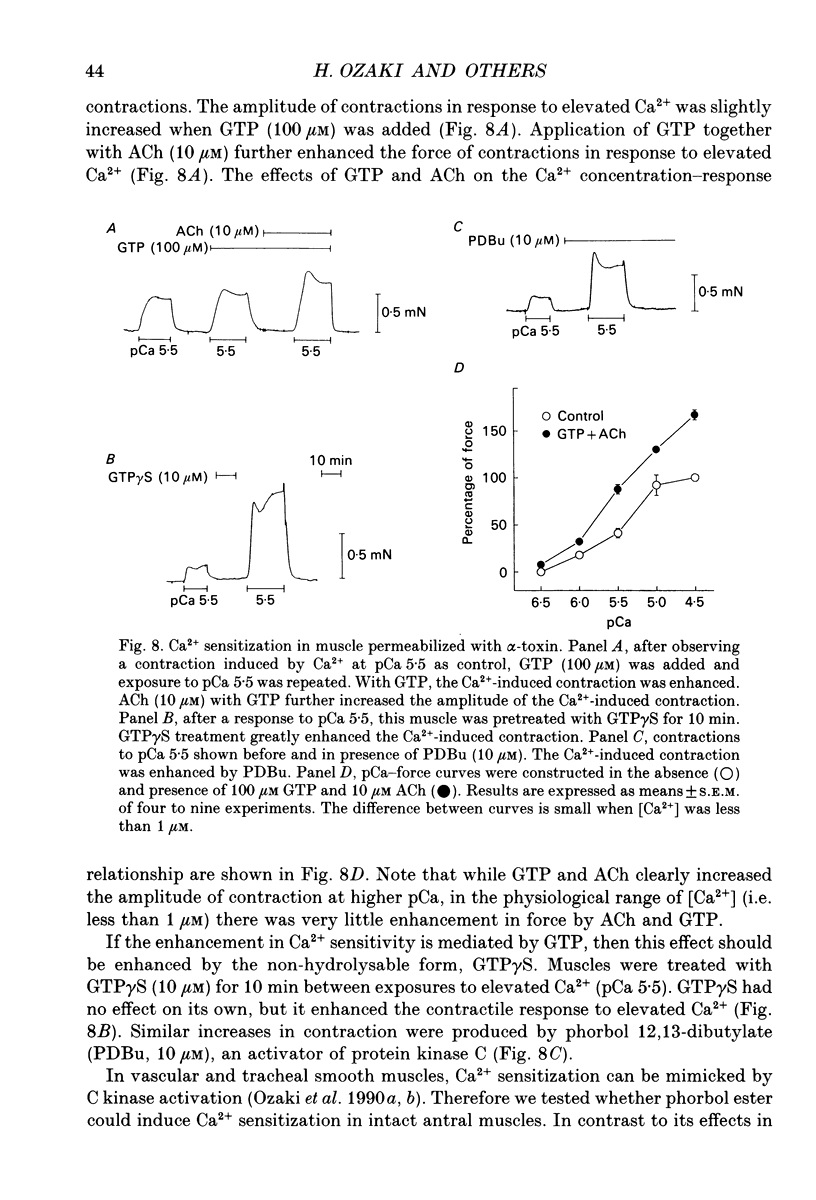
1. The relationships between cytosolic Ca2+ ([Ca2+]cyt; expressed as a fluorescence ratio at 400 nm and 500 nm using Indo-1) and contractile force was examined in strips of circular smooth muscles of canine gastric antrum. Rhythmic increases in [Ca2+]cyt were observed and contractions were biphasic. 2. In most muscles (70%), the amplitude of the second phase of the Ca2+ transient was less than or equal to the first phase of the Ca2+ transient, but the second phase of the contraction was much smaller than the first phase, suggesting a decrease in Ca2+ sensitivity during the second contractile phase. In 30% of muscles, the amplitude of the second phase of the Ca2+ transient was 2- to 3-fold greater than the first phase. In these muscles, the second phase of contraction was 10-fold greater than the first phase of contraction. Thus, a non-linear relationship between [Ca2+]cyt and force greatly amplifies force development when [Ca2+]cyt exceeds a threshold level. 3. Acetylcholine (ACh, 0.3-1 microM) increased the amplitudes of Ca2+ transients and basal [Ca2+]cyt between phasic contractions. The increase in basal [Ca2+]cyt did not cause tone to develop. ACh increased the amplitude of Ca2+ transients 2- to 3-fold and this was associated with a 15 to 20-fold increase in the force of phasic contractions. Pentagastrin (0.5 nM) and cholecystokinin octapeptide (CCK, 40 nM) had similar effects on Ca2+ transients and phasic contractions. 4. Bay K 8644 (0.1 microM) and TEA (5 mM) also increased the amplitudes of Ca2+ transients by 2- to 3-fold and phasic contractions by 15- to 30-fold. There was no significant difference observed between the [Ca2+]cyt-force relationships in the presence of agonists (i.e. ACh, pentagastrin and CCK) or when [Ca2+]cyt was increased by Bay K 8644 or TEA. These data suggest that agonist-dependent increases in Ca2+ sensitivity may not significantly regulate the [Ca2+]cyt-force relationship in antral muscles. 5. D600 (5 microM), added during stimulation with ACh (0.3 M), decreased [Ca2+]cyt and force without affecting the [Ca2+]cyt-force relationship. 6. Mechanisms exist for agonist-mediated enhancement of the Ca(2+)-force relationship. In alpha-toxin-permeabilized antrum, ACh (10 microM) with GTP (100 microM) or GTP gamma S (100 microM) increased the Ca(2+)-induced contraction at clamped levels of Ca2+. Phorbol 12,13-dibutyrate (PDBu, 10 microM) also increased the contractile force at a given level of Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Takahashi K., Hiwada K. Effect of calponin on actin-activated myosin ATPase activity. J Biochem. 1990 Nov;108(5):835–838. doi: 10.1093/oxfordjournals.jbchem.a123289. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Bauer A. J., Publicover N. G., Sanders K. M. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Physiol. 1985 Dec;249(6 Pt 1):G800–G806. doi: 10.1152/ajpgi.1985.249.6.G800. [DOI] [PubMed] [Google Scholar]
- Bauer A. J., Reed J. B., Sanders K. M. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985 Sep;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T., Ngai P. K., Sutherland C., Gröschel-Stewart U., Walsh M. P. Vascular smooth muscle caldesmon. J Biol Chem. 1986 Jun 15;261(17):8028–8035. [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol. 1985 Dec;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Inoue R., Yamanaka K., Yoshitomi T. Effects of calcium antagonists on smooth muscle membranes of the canine stomach. Gen Pharmacol. 1985;16(3):217–221. doi: 10.1016/0306-3623(85)90072-2. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphey K. A., Gunst S. J. Aequorin luminescence, myosin phosphorylation, and active stress in tracheal smooth muscle. Am J Physiol. 1989 Dec;257(6 Pt 1):C1062–C1068. doi: 10.1152/ajpcell.1989.257.6.C1062. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphey K. A., Mangini J., Boman S., Lattanzio F. A., Jr Myosin phosphorylation and calcium in tonic and phasic contractions of colonic smooth muscle. Am J Physiol. 1991 Jun;260(6 Pt 1):G958–G964. doi: 10.1152/ajpgi.1991.260.6.G958. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haeberle J. R., Hathaway D. R., Smith C. L. Caldesmon content of mammalian smooth muscles. J Muscle Res Cell Motil. 1992 Feb;13(1):81–89. doi: 10.1007/BF01738431. [DOI] [PubMed] [Google Scholar]
- Himpens B., Casteels R. Different effects of depolarization and muscarinic stimulation on the Ca2+/force relationship during the contraction-relaxation cycle in the guinea pig ileum. Pflugers Arch. 1990 Apr;416(1-2):28–35. doi: 10.1007/BF00370218. [DOI] [PubMed] [Google Scholar]
- Himpens B., Kitazawa T., Somlyo A. P. Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Arch. 1990 Sep;417(1):21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989 Jun;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnsbein J., Golenhofen K. Differentiation of the action potential in the smooth muscle of canine gastric antrum using calcium-inhibitory drugs. J Auton Pharmacol. 1985 Mar;5(1):1–12. doi: 10.1111/j.1474-8673.1985.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Activation of smooth muscle contraction: relation between myosin phosphorylation and stiffness. Science. 1986 Apr 4;232(4746):80–82. doi: 10.1126/science.3754063. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H. Different effects of verapamil on cytosolic Ca2+ and contraction in norepinephrine-stimulated vascular smooth muscle. Jpn J Pharmacol. 1991 Jan;55(1):35–42. doi: 10.1254/jjp.55.35. [DOI] [PubMed] [Google Scholar]
- Kelly K. A., Code C. F. Canine gastric pacemaker. Am J Physiol. 1971 Jan;220(1):112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobayashi S., Gong M. C., Somlyo A. V., Somlyo A. P. Ca2+ channel blockers distinguish between G protein-coupled pharmacomechanical Ca2+ release and Ca2+ sensitization. Am J Physiol. 1991 Feb;260(2 Pt 1):C364–C370. doi: 10.1152/ajpcell.1991.260.2.C364. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Gerthoffer W. T., Publicover N. G., Fusetani N., Sanders K. M. Time-dependent changes in Ca2+ sensitivity during phasic contraction of canine antral smooth muscle. J Physiol. 1991;440:207–224. doi: 10.1113/jphysiol.1991.sp018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Gerthoffer W. T., Publicover N. G., Sanders K. M. Time-dependent decrease in Ca(2+)-sensitivity in "phasic smooth muscle". Adv Exp Med Biol. 1991;304:481–489. [PubMed] [Google Scholar]
- Ozaki H., Kwon S. C., Tajimi M., Karaki H. Changes in cytosolic CA2+ and contraction induced by various stimulants and relaxants in canine tracheal smooth muscle. Pflugers Arch. 1990 Jun;416(4):351–359. doi: 10.1007/BF00370740. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Ohyama T., Sato K., Karaki H. Ca2(+)-dependent and independent mechanisms of sustained contraction in vascular smooth muscle of rat aorta. Jpn J Pharmacol. 1990 Mar;52(3):509–512. doi: 10.1254/jjp.52.509. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Satoh T., Karaki H., Ishida Y. Regulation of metabolism and contraction by cytoplasmic calcium in the intestinal smooth muscle. J Biol Chem. 1988 Oct 5;263(28):14074–14079. [PubMed] [Google Scholar]
- Ozaki H., Stevens R. J., Blondfield D. P., Publicover N. G., Sanders K. M. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991 May;260(5 Pt 1):C917–C925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Sakata K., Ozaki H., Kwon S. C., Karaki H. Effects of endothelin on the mechanical activity and cytosolic calcium level of various types of smooth muscle. Br J Pharmacol. 1989 Oct;98(2):483–492. doi: 10.1111/j.1476-5381.1989.tb12621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Somlyo A. P., Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989 Sep;3(11):2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Goldman Y. E., Fujimori T., Bond M., Trentham D. R., Somlyo A. P. Cross-bridge kinetics, cooperativity, and negatively strained cross-bridges in vertebrate smooth muscle. A laser-flash photolysis study. J Gen Physiol. 1988 Feb;91(2):165–192. doi: 10.1085/jgp.91.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Szurszewski J. H. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978 Apr;277:91–102. doi: 10.1113/jphysiol.1978.sp012262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Hiwada K., Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986 Nov 26;141(1):20–26. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nadal-Ginard B. Molecular cloning and sequence analysis of smooth muscle calponin. J Biol Chem. 1991 Jul 15;266(20):13284–13288. [PubMed] [Google Scholar]
- Vogalis F., Publicover N. G., Hume J. R., Sanders K. M. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C1012–C1018. doi: 10.1152/ajpcell.1991.260.5.C1012. [DOI] [PubMed] [Google Scholar]
- Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 Jun 15;265(17):10148–10155. [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]


