Abstract
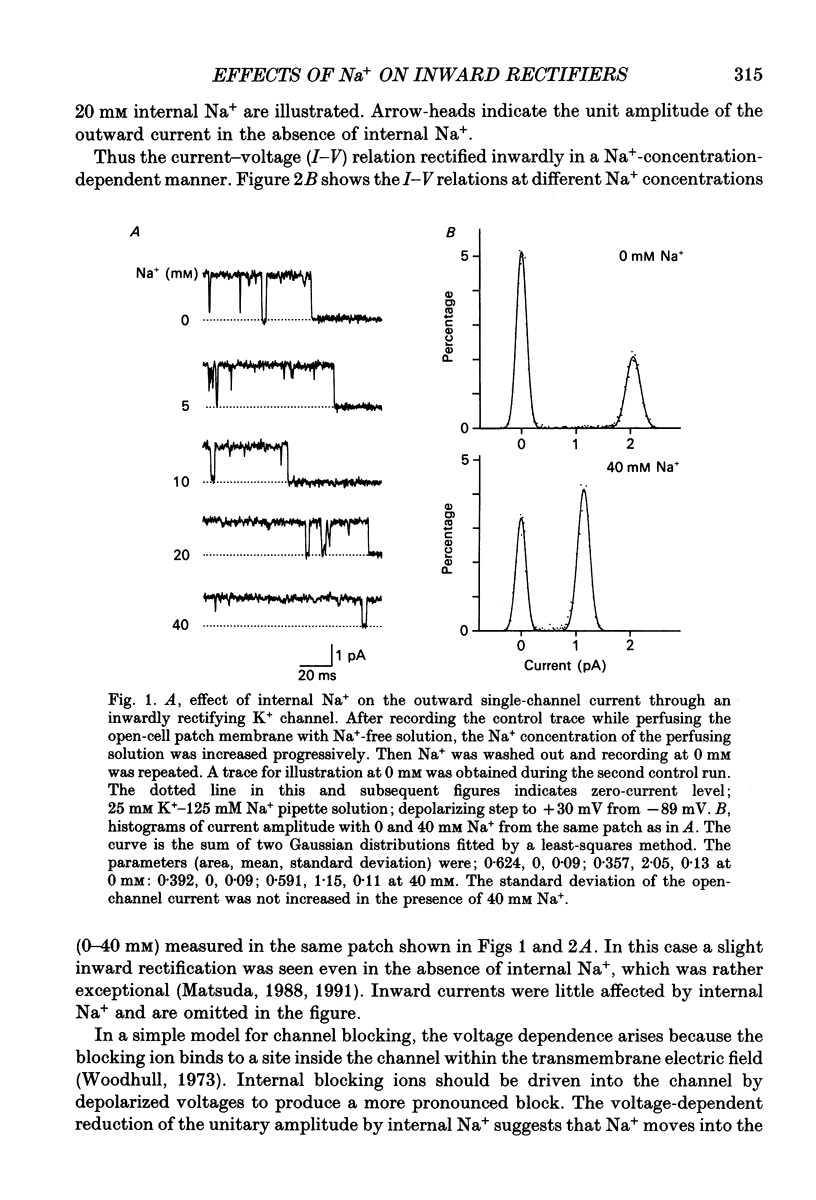
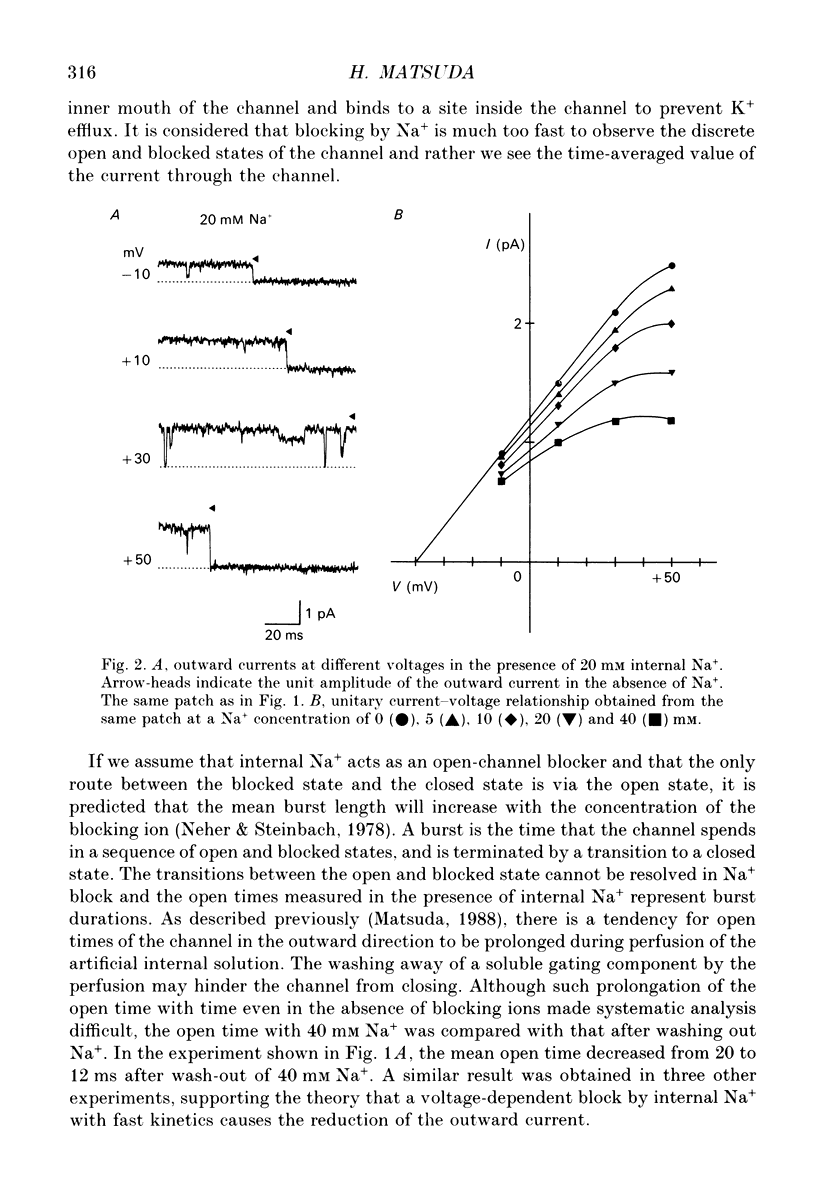
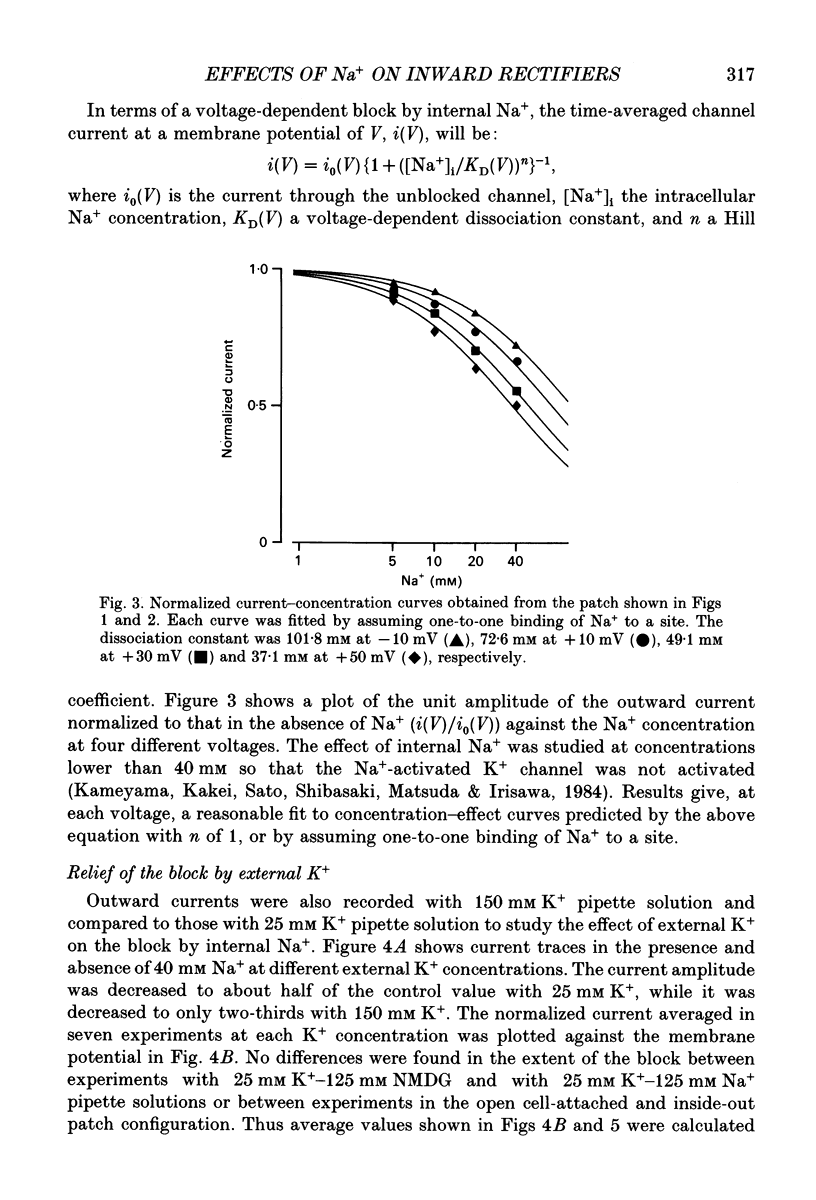
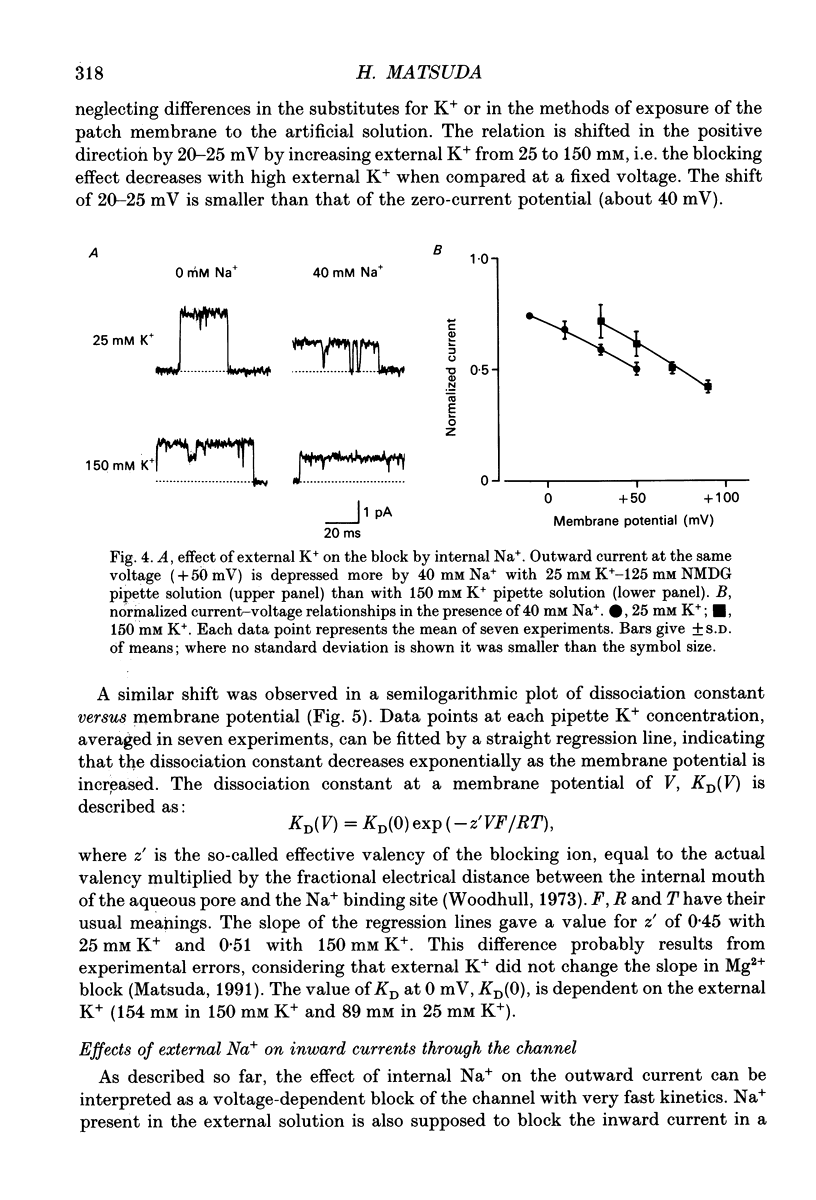
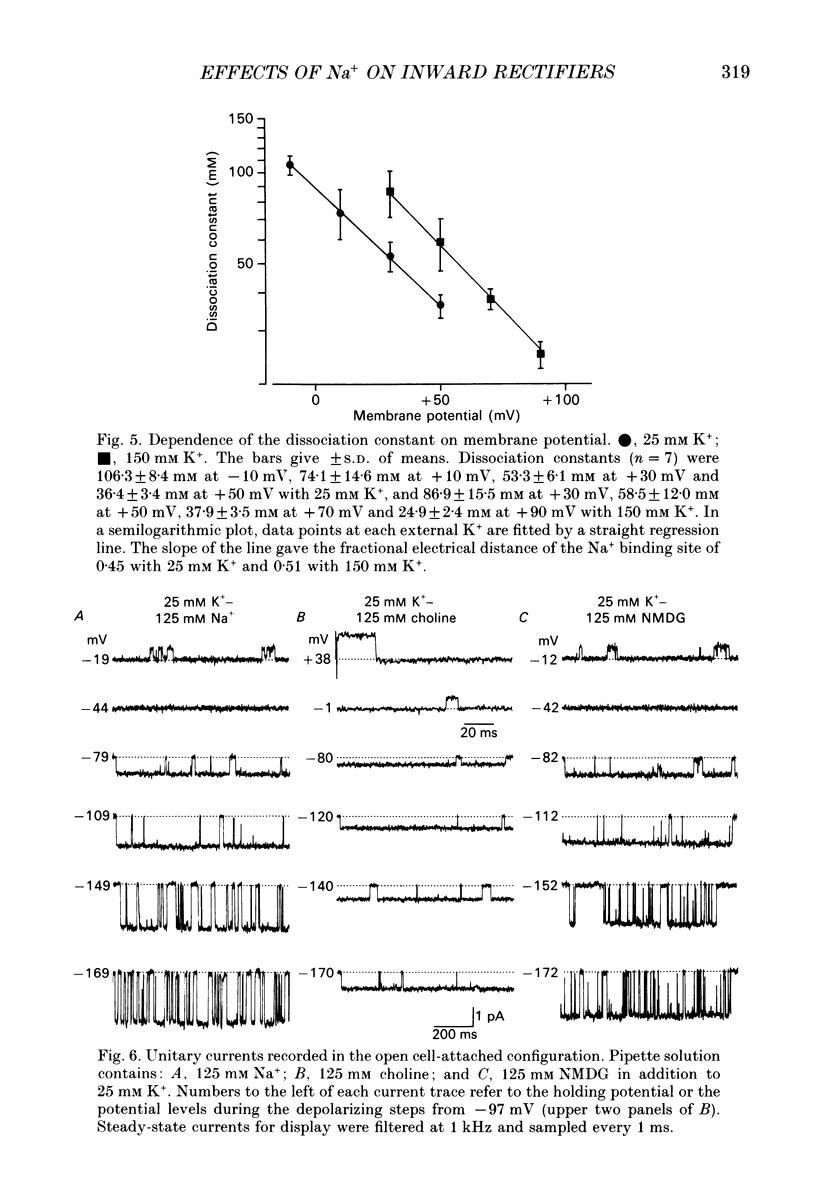
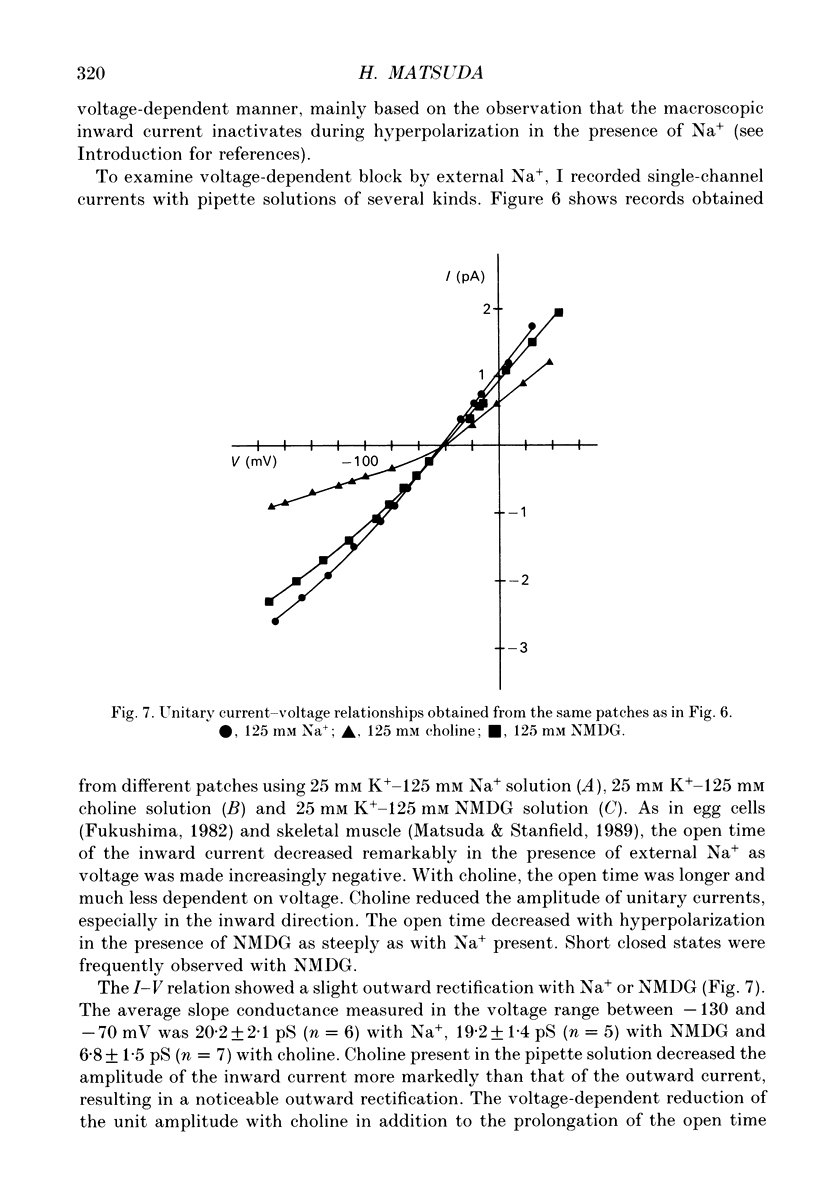
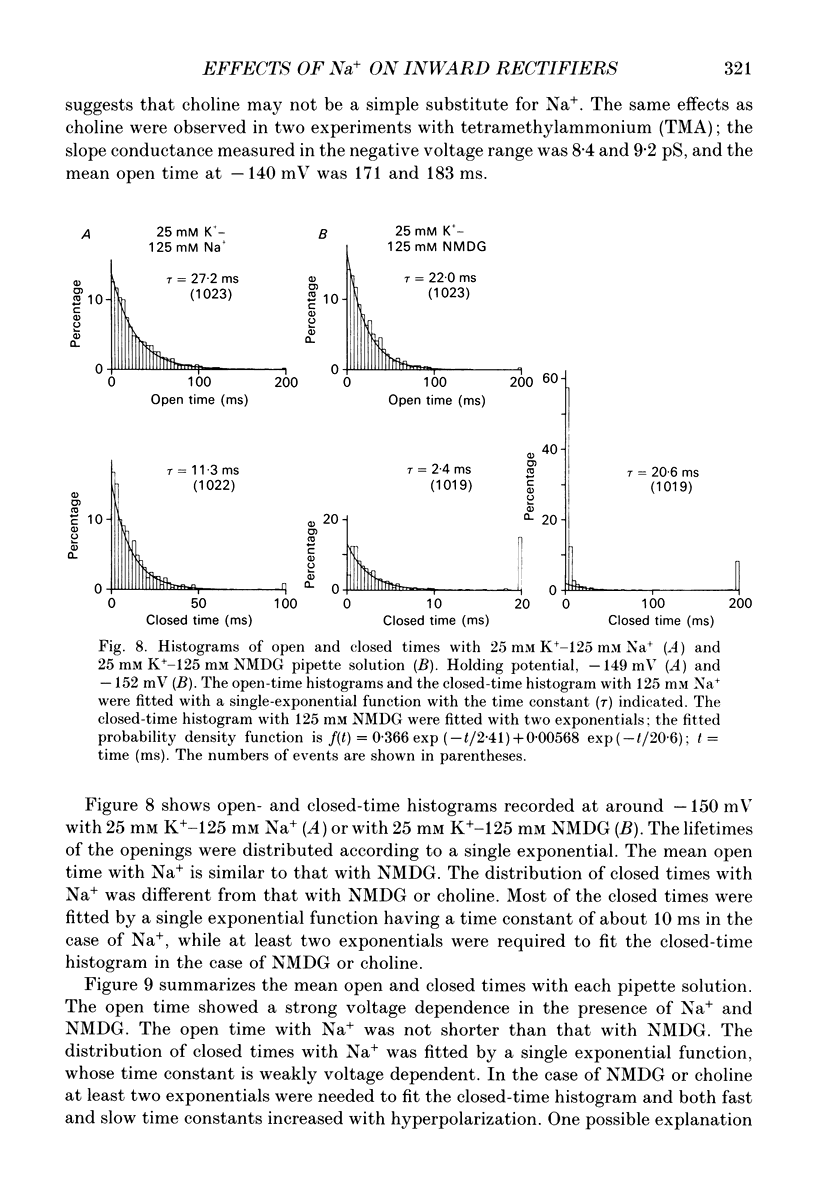
1. The effects of internal and external Na+ ions on the inwardly rectifying K+ channel were studied in guinea-pig ventricular cells. 2. Single-channel currents through the inwardly rectifying K+ channel were recorded in the open cell-attached or inside-out configuration at 150 mM internal K+ and either 150 or 25 mM external K+. Internal Na+, at a concentration of 5-40 mM, reduced the unitary amplitude of the outward current. No increase in open-channel current noise was detected with the filter cut-off frequency of 3 kHz. Substate behaviour seen with internal Mg2+ at a micromolar level was not observed. The inward currents were little affected by internal Na+. 3. The unitary current-voltage relation rectified inwardly in the presence of internal Na+ in a concentration-dependent manner. 4. Outward unitary currents were normalized to those measured in the absence of Na+. The normalized current-voltage relation was shifted in the negative direction by 20-25 mV by decreasing external K+ from 150 to 25 mM, indicating that the blocking effect increases with low external K+ when compared at a fixed voltage. 5. The normalized current-Na+ concentration curve was fitted by a one-to-one binding curve at each voltage. In a semi-logarithmic plot of dissociation constant versus membrane potential, data points for 150 and 25 mM external K+ were fitted by straight lines with nearly the same slope. The dissociation constant at 0 mV is 154 mM in 150 mM external K+ and 89 mM in 25 mM external K+. The voltage dependence of dissociation constants gives a value for the effective valency of the Na+ ion of around 0.5. 6. To study effects of external Na+, single-channel currents were recorded with pipette solutions containing 125 mM Na+, 125 mM choline or 125 mM N-methyl-D-glucamine (NMDG) in addition to 25 mM K+. Current amplitude was smaller with choline than with Na+ or NMDG. The reduction in current amplitude with choline was more evident in the inward current, resulting in a stronger outward rectification of the current-voltage relation. This finding and prolonged mean open time (see Summary point 7) was interpreted by assuming that choline is an open-channel blocker. 7. The lifetimes of the openings in the inward currents were distributed according to a single exponential. The mean open time with Na+ was similar to that with NMDG, which decreased with hyperpolarization. The mean open time with choline was much longer and less voltage dependent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., McGuigan J. A. Intracellular pH regulation in ferret ventricular muscle. The role of Na-H exchange and the influence of metabolic substrates. Circ Res. 1991 Jan;68(1):150–161. doi: 10.1161/01.res.68.1.150. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Coray A., McGuigan J. A. Sodium/calcium exchange in mammalian ventricular muscle: a study with sodium-sensitive micro-electrodes. J Physiol. 1983 Oct;343:253–276. doi: 10.1113/jphysiol.1983.sp014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Ribalet B. Ion permeation and rectification in ATP-sensitive channels from insulin-secreting cells (RINm5F): effects of K+, Na+ and Mg2+. J Membr Biol. 1988 Jul;103(2):171–180. doi: 10.1007/BF01870947. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol. 1988 Apr;91(4):593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. On the role of sodium ions in the regulation of the inward-rectifying potassium conductance in cat ventricular myocytes. J Gen Physiol. 1989 Aug;94(2):329–348. doi: 10.1085/jgp.94.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Voltage-dependent block of cardiac inward-rectifying potassium current by monovalent cations. J Gen Physiol. 1989 Aug;94(2):349–361. doi: 10.1085/jgp.94.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Dual effects of intracellular magnesium on muscarinic potassium channel current in single guinea-pig atrial cells. J Physiol. 1989 Jan;408:313–332. doi: 10.1113/jphysiol.1989.sp017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Marty A. Blocking of large unitary calcium-dependent potassium currents by internal sodium ions. Pflugers Arch. 1983 Feb;396(2):179–181. doi: 10.1007/BF00615524. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Stanfield P. R. Single inwardly rectifying potassium channels in cultured muscle cells from rat and mouse. J Physiol. 1989 Jul;414:111–124. doi: 10.1113/jphysiol.1989.sp017679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet M. D., Rousseau E., Sauvé R. Single-channel analysis of a potassium inward rectifier in myocytes of newborn rat heart. J Membr Biol. 1985;86(2):79–88. doi: 10.1007/BF01870774. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. J Physiol. 1979 Sep;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M., Qin D. Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am J Physiol. 1990 Jan;258(1 Pt 2):H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J Gen Physiol. 1984 Aug;84(2):187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


