Abstract
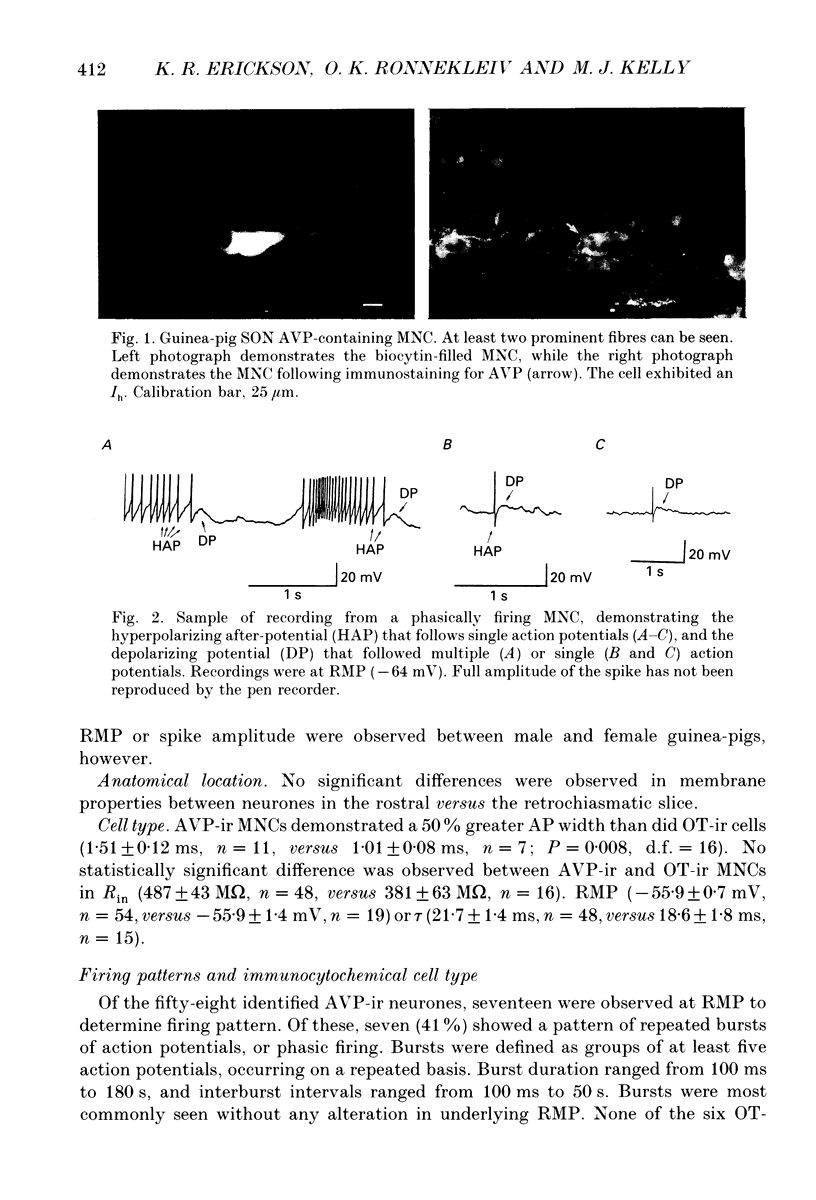
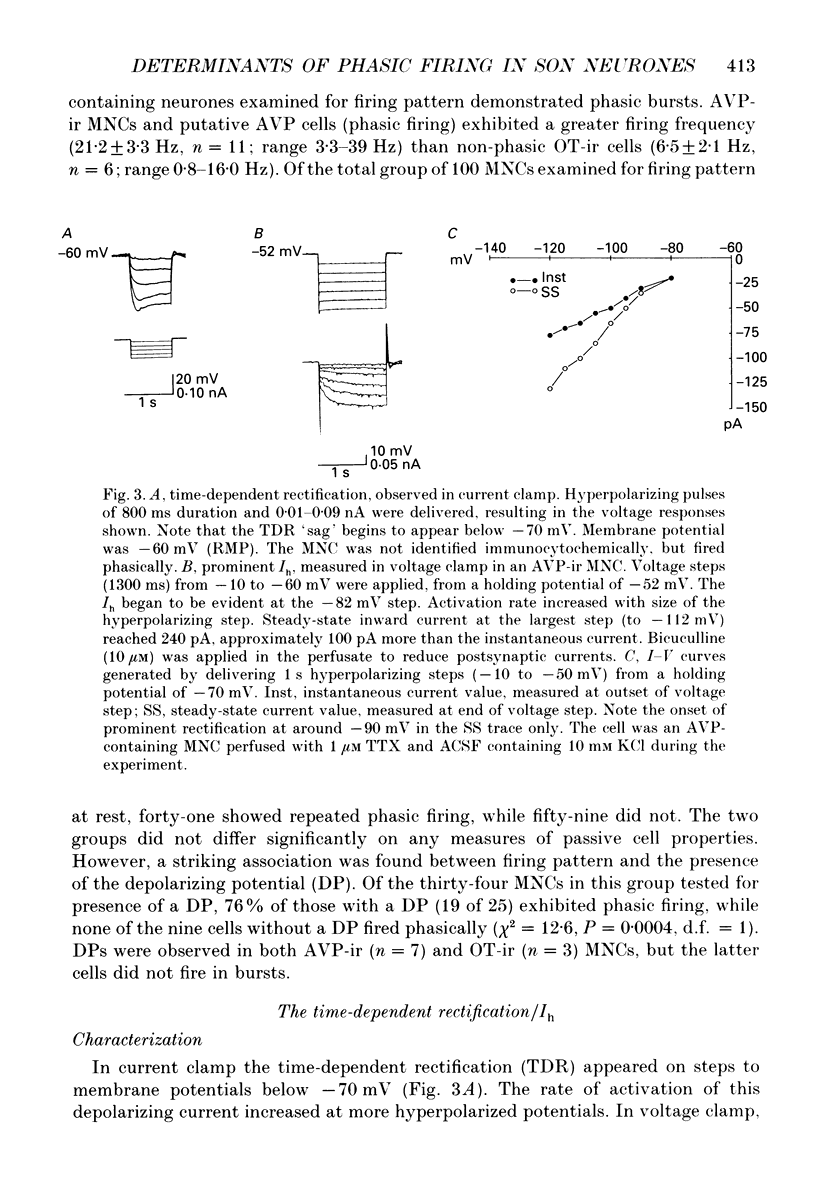
1. Immunocytochemically identified magnocellular neurosecretory cells (MNCs) in the guinea-pig supraoptic nucleus (SON) were studied using the in vitro intracellular recording technique. Cells were identified as containing arginine vasopressin (AVP) or oxytocin (OT) following recordings made with biocytin-filled electrodes. Both AVP and OT MNCs demonstrated a fusiform or pyramidal shape (15-20 microns by 26-39 microns), with two to three processes. There were no significant differences in the proportion of AVP and OT cells in the retrochiasmatic (caudal) versus the rostral slices. 2. No significant differences in passive membrane properties were observed between AVP and OT cells, except that AVP cells exhibited a significantly broader action potential width (1.51 +/- 0.1 ms, n = 11) than did OT cells (1.01 +/- 0.08 ms, n = 7). 3. Firing patterns were recorded for 100 MNCs, 41% of which fired in a phasic manner (repeated clustering of action potentials into bursts). Of the seventy-seven cells which were immunocytochemically identified, only AVP-containing MNCs displayed phasic firing. Phasic firing occurred only in MNCs demonstrating a depolarizing potential which followed hyperpolarizing after-potentials (HAPs). The presence of the depolarizing potential was not always associated with phasic firing, however, as both OT cells and non-phasic AVP cells sometimes exhibited a depolarizing potential. 4. In 160 MNCs examined for the presence of the time-dependent inward rectification (TDR in current clamp, or Ih in voltage clamp), a significant difference in the proportion of cells expressing the Ih was observed in the two cell types. The Ih was expressed in forty-five of fifty-four AVP MNCs (83%) and in six of fifteen OT MNCs (40%). No significant association was found with firing pattern. 5. The Ih exhibited properties similar to those found in other CNS and peripheral tissues. It appeared on steps to potentials more hyperpolarized than -65 mV. It was augmented by raising the extracellular potassium concentration, blocked by 2 mM CsCl, and insensitive to 100-500 microM BaCl2. Activation followed a single exponential, and the time constant of activation was voltage dependent. 6. The adenylate cyclase activator forskolin increased the Ih and shifted its activation curve to more depolarized levels. In cells recorded for several hours, the Ih varied in amplitude, suggesting intrinsic modulation, possibly by intracellular second messenger systems. The Ih in guinea-pig SON MNCs appears to serve an excitatory role, bringing cells closer to firing threshold.
Full text
PDF





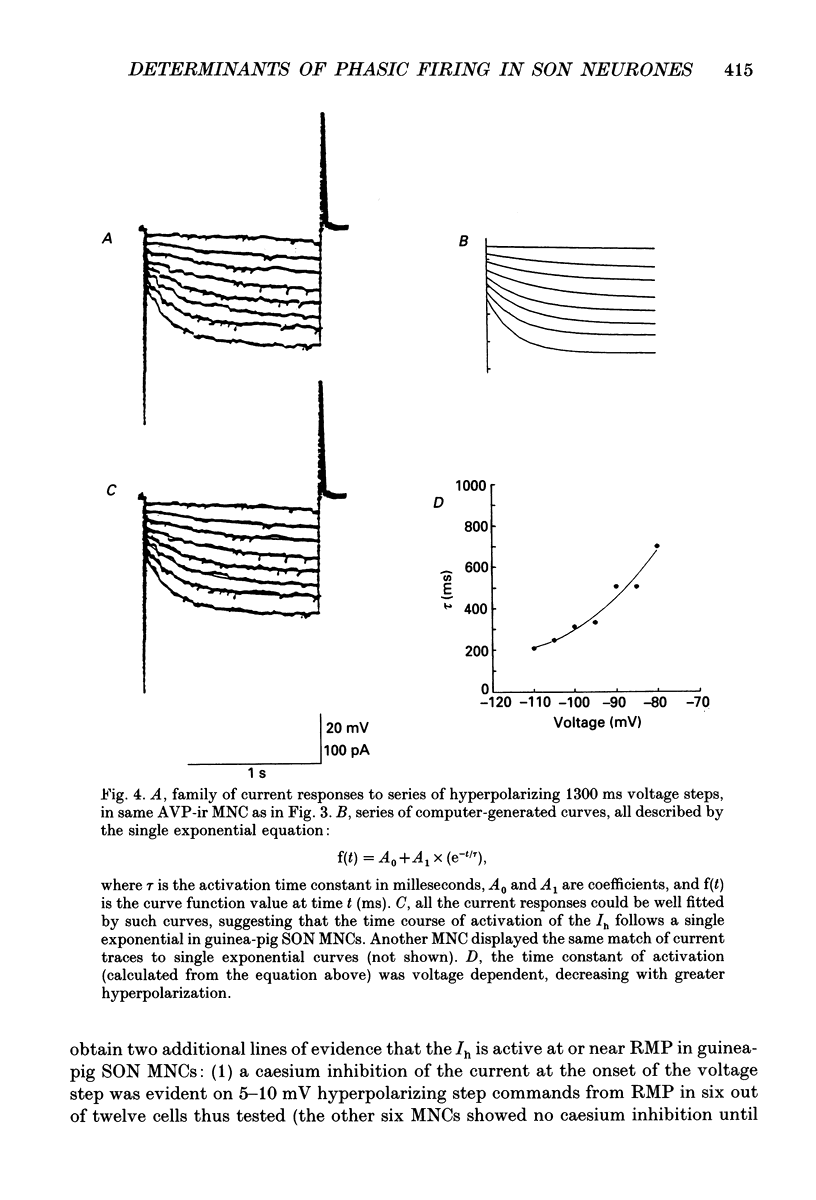
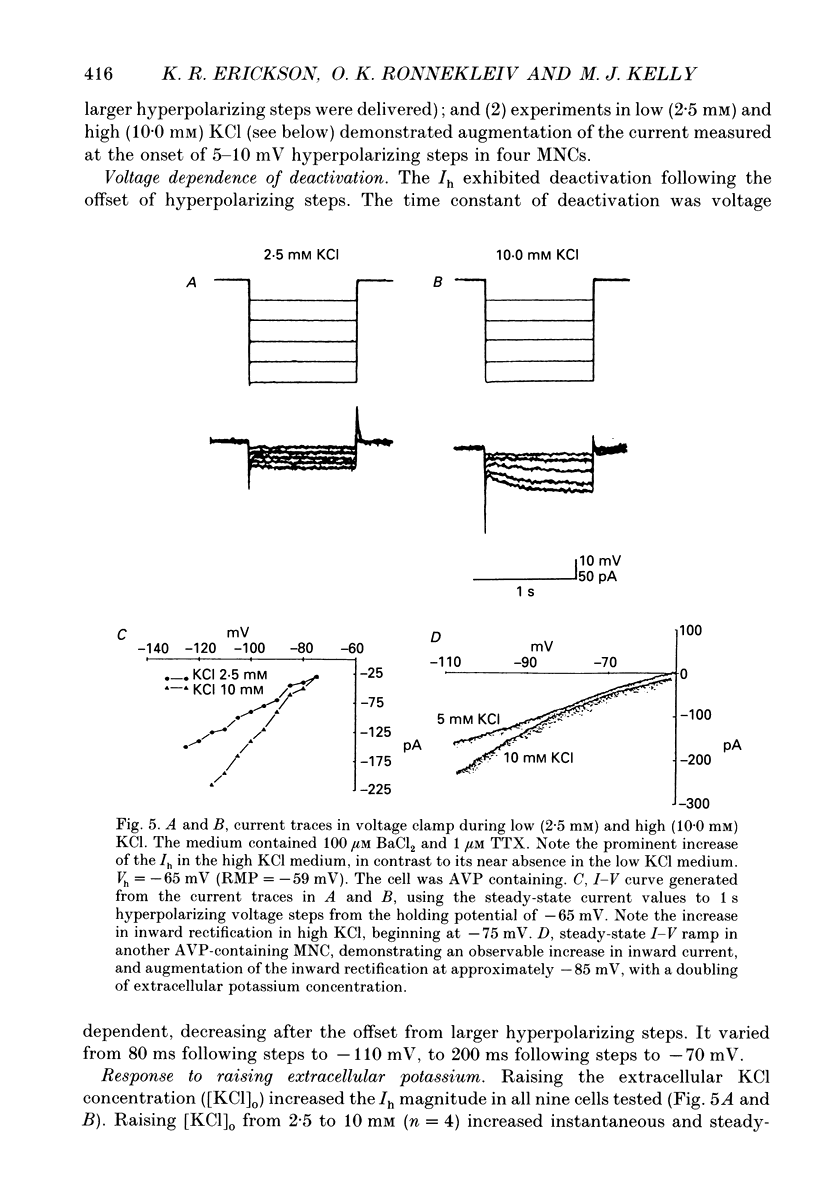
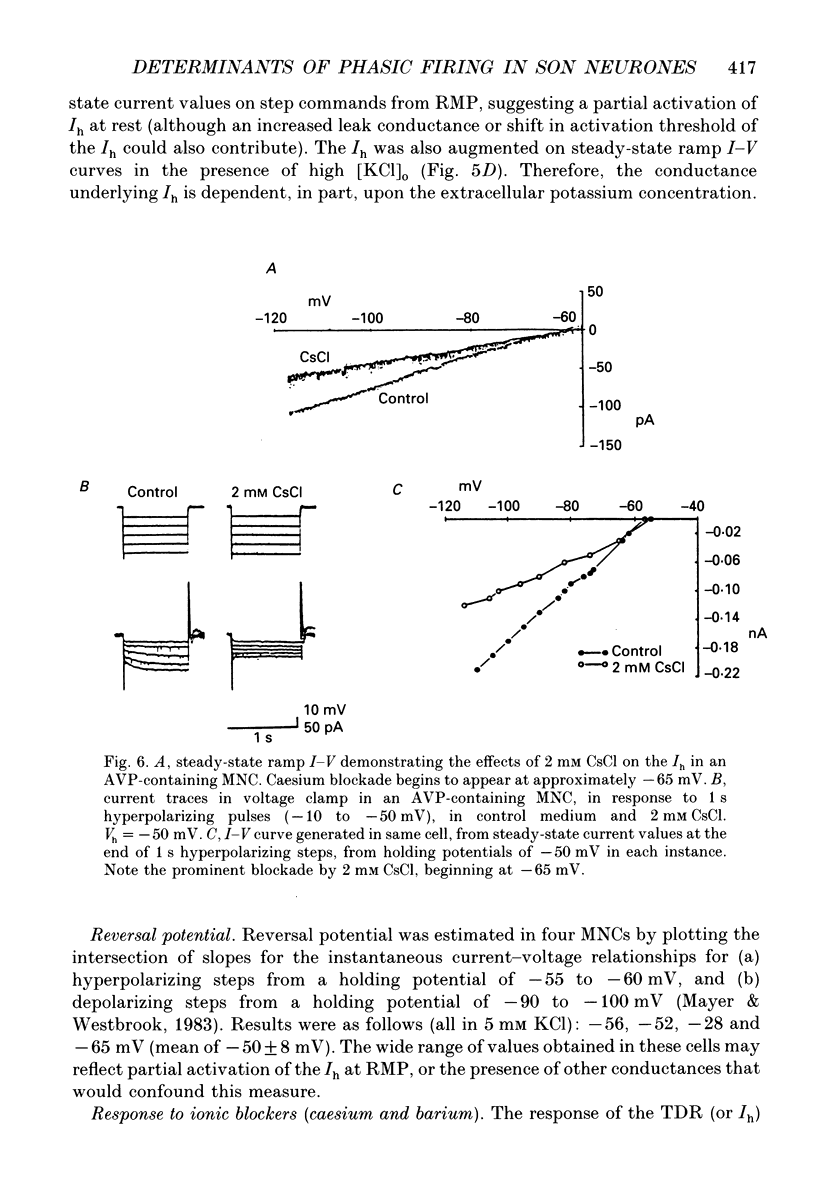
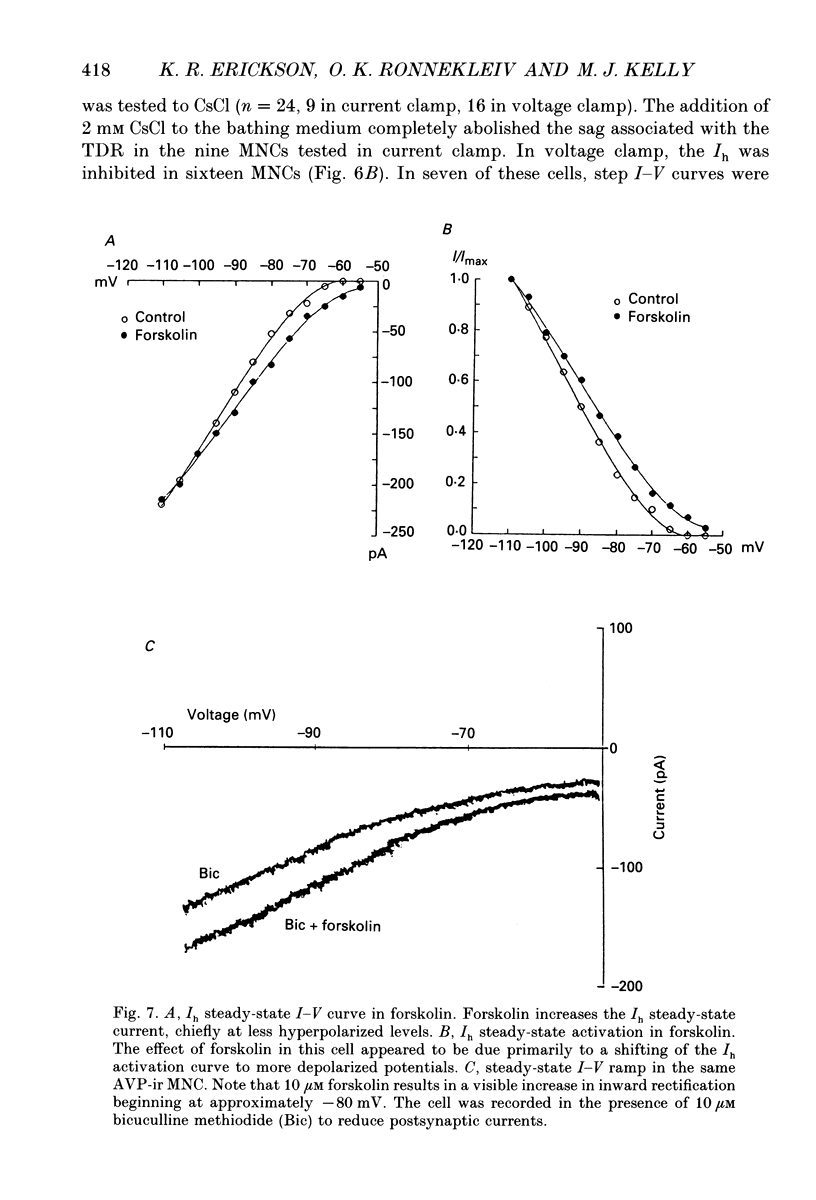







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982 Jun;327:157–171. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983 Sep 9;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Andrew R. D. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. J Physiol. 1987 Mar;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. E., Sladek C. D. Spontaneous "phasic-firing' in supraoptic neurons recorded from hypothalamo-neurohypophysial explants in vitro. Neuroendocrinology. 1982 Jun;34(6):405–409. doi: 10.1159/000123336. [DOI] [PubMed] [Google Scholar]
- Belin V., Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986 Aug;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn R. E., Leng G., Russell J. A. Control of magnocellular oxytocin neurones by the region anterior and ventral to the third ventricle (AV3V region) in rats. J Endocrinol. 1987 Aug;114(2):253–261. doi: 10.1677/joe.0.1140253. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. J Physiol. 1984 Apr;349:631–642. doi: 10.1113/jphysiol.1984.sp015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. In vitro neurophysiology of identified rat hypothalamic 'neuroendocrine' neurons. Neuroendocrinology. 1983 Feb;36(2):161–164. doi: 10.1159/000123453. [DOI] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino M., Renaud L. P. Influence of lateral septum and amygdala stimulation on the excitability of hypothalamic supraoptic neurons. An electrophysiological study in the rat. Brain Res. 1985 Feb 11;326(2):357–361. doi: 10.1016/0006-8993(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Cobbett P., Legendre P., Mason W. T. Characterization of three types of potassium current in cultured neurones of rat supraoptic nucleus area. J Physiol. 1989 Mar;410:443–462. doi: 10.1113/jphysiol.1989.sp017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Smithson K. G., Hatton G. I. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986 Jan 1;362(1):7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave J. R., Rubinstein N., Eskay R. L. Evidence that beta-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3',5'-monophosphate system. Endocrinology. 1985 Oct;117(4):1389–1396. doi: 10.1210/endo-117-4-1389. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The contribution of the 'pacemaker' current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991 Mar;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F. E., Gribkoff V. K. Synaptic activation of slow depolarization in rat supraoptic nucleus neurones in vitro. J Physiol. 1987 Jun;387:273–296. doi: 10.1113/jphysiol.1987.sp016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan M., Andrew R. D. Intracellular study of calcium-related events in cat magnocellular neuroendocrine cells. J Physiol. 1991 Mar;434:337–349. doi: 10.1113/jphysiol.1991.sp018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Legendre P., Poulain D. A., Vincent J. D. A study of ionic conductances involved in plateau potential activity in putative vasopressinergic neurons in primary cell culture. Brain Res. 1988 Aug 9;457(2):386–391. doi: 10.1016/0006-8993(88)90713-5. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos F., Richard P. Serotonergic control of oxytocin release during suckling in the rat: opposite effects in conscious and anesthetized rats. Neuroendocrinology. 1983;36(4):300–306. doi: 10.1159/000123471. [DOI] [PubMed] [Google Scholar]
- Morris M., Stevens S. W., Adams M. R. Plasma oxytocin during pregnancy and lactation in the cynomolgus monkey. Biol Reprod. 1980 Nov;23(4):782–787. doi: 10.1095/biolreprod23.4.782. [DOI] [PubMed] [Google Scholar]
- Ogata N., Matsuo T. The effects of catecholamines on electrical activity of neurons in the guinea pig supraoptic nucleus in vitro. Brain Res. 1986 Oct 15;385(1):122–135. doi: 10.1016/0006-8993(86)91553-2. [DOI] [PubMed] [Google Scholar]
- Ogata N. gamma-Aminobutyric acid (GABA) causes consistent depolarization of neurons in the guinea pig supraoptic nucleus due to an absence of GABAB recognition sites. Brain Res. 1987 Feb 17;403(2):225–233. doi: 10.1016/0006-8993(87)90059-x. [DOI] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Resko J. A., Ellinwood W. E., Pasztor L. M., Huhl A. E. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab. 1980 May;50(5):900–905. doi: 10.1210/jcem-50-5-900. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv O. K., Loose M. D., Erickson K. R., Kelly M. J. A method for immunocytochemical identification of biocytin-labeled neurons following intracellular recording. Biotechniques. 1990 Oct;9(4):432–438. [PubMed] [Google Scholar]
- Silverman A. J., Zimmerman E. A. Magnocellular neurosecretory system. Annu Rev Neurosci. 1983;6:357–380. doi: 10.1146/annurev.ne.06.030183.002041. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Weindl A., Schinko I., Wetzstein R. The distribution of vasopressin-, oxytocin-, and neurophysin-producing neurons in the guinea pig brain. I. The classical hypothalamo-neurophypophyseal system. Cell Tissue Res. 1979 Feb 28;196(3):367–384. doi: 10.1007/BF00234734. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990 Jan;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N., Cherubini E., North R. A. Cation current activated by hyperpolarization in a subset of rat nucleus accumbens neurons. J Neurophysiol. 1990 Dec;64(6):1847–1850. doi: 10.1152/jn.1990.64.6.1847. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Colmers W. F., Pan Z. Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988 Sep;8(9):3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yatani A., Okabe K., Codina J., Birnbaumer L., Brown A. M. Heart rate regulation by G proteins acting on the cardiac pacemaker channel. Science. 1990 Sep 7;249(4973):1163–1166. doi: 10.1126/science.1697697. [DOI] [PubMed] [Google Scholar]
- van Ginneken A. C., Giles W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J Physiol. 1991 Mar;434:57–83. doi: 10.1113/jphysiol.1991.sp018459. [DOI] [PMC free article] [PubMed] [Google Scholar]



