Abstract
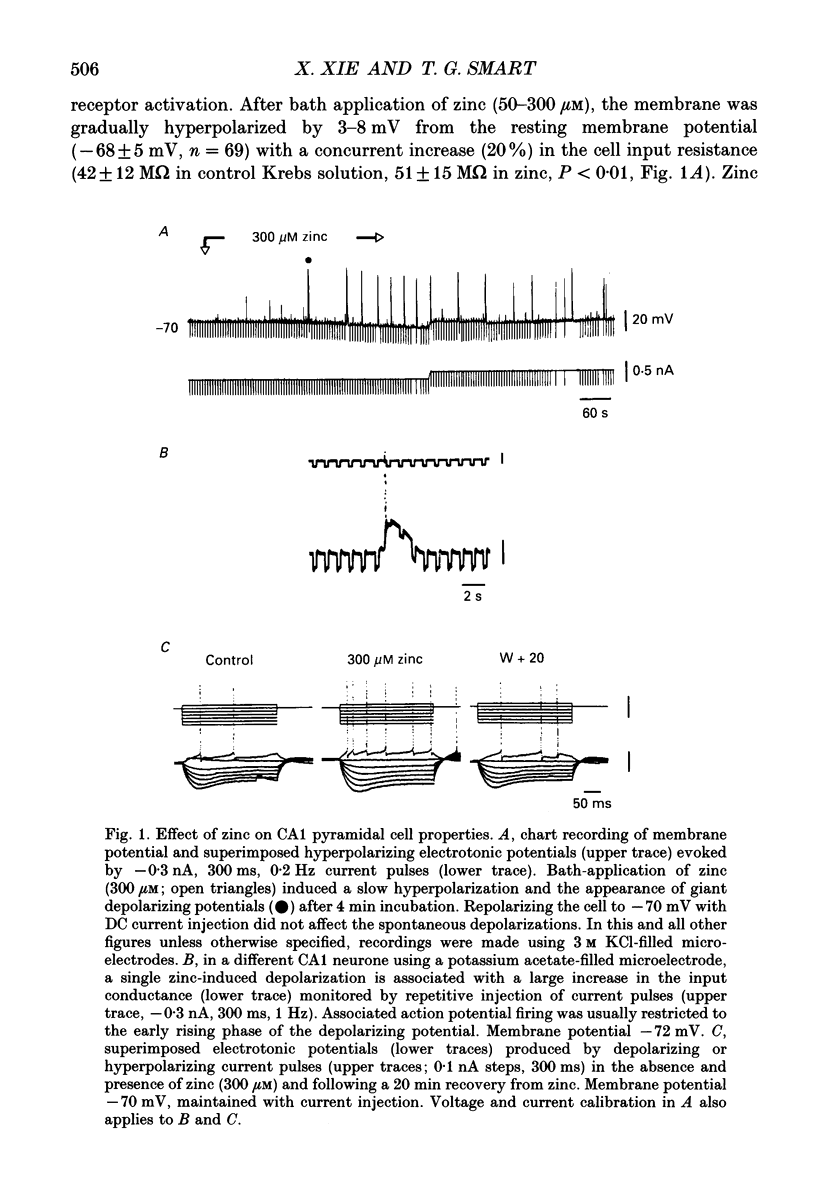
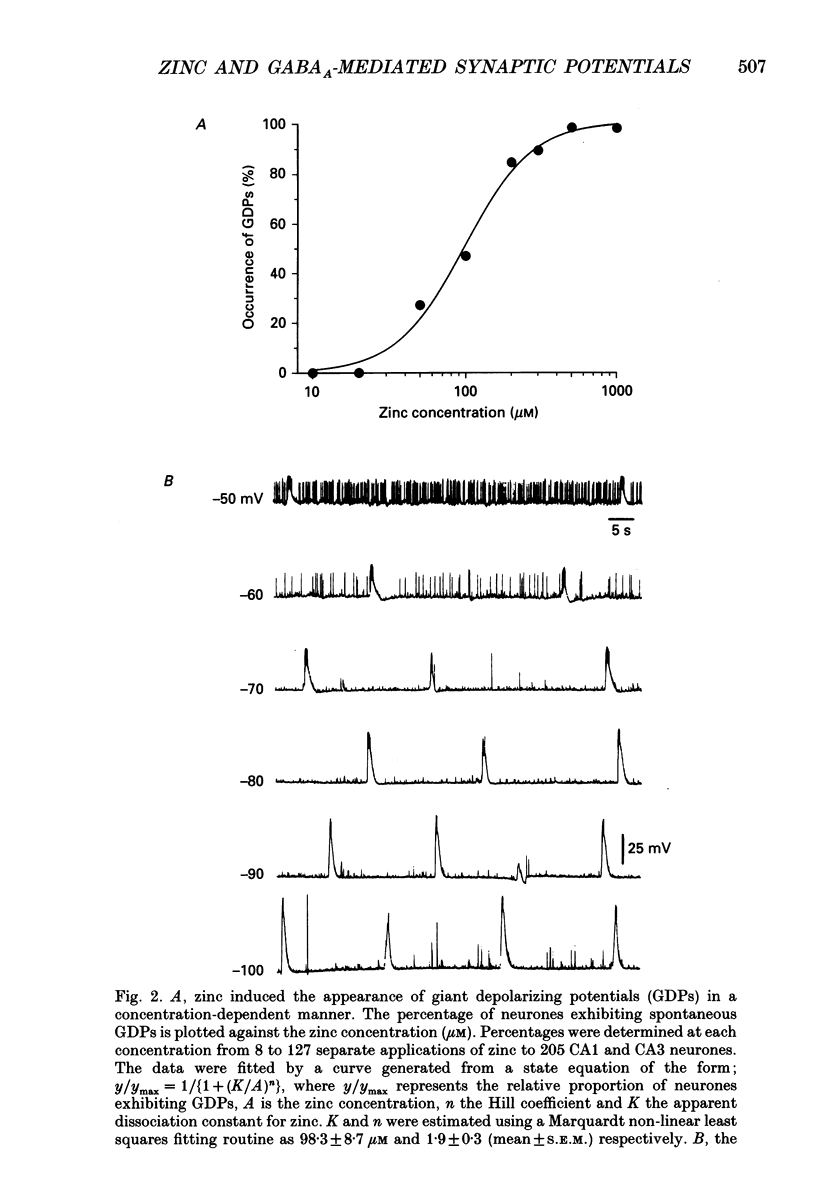
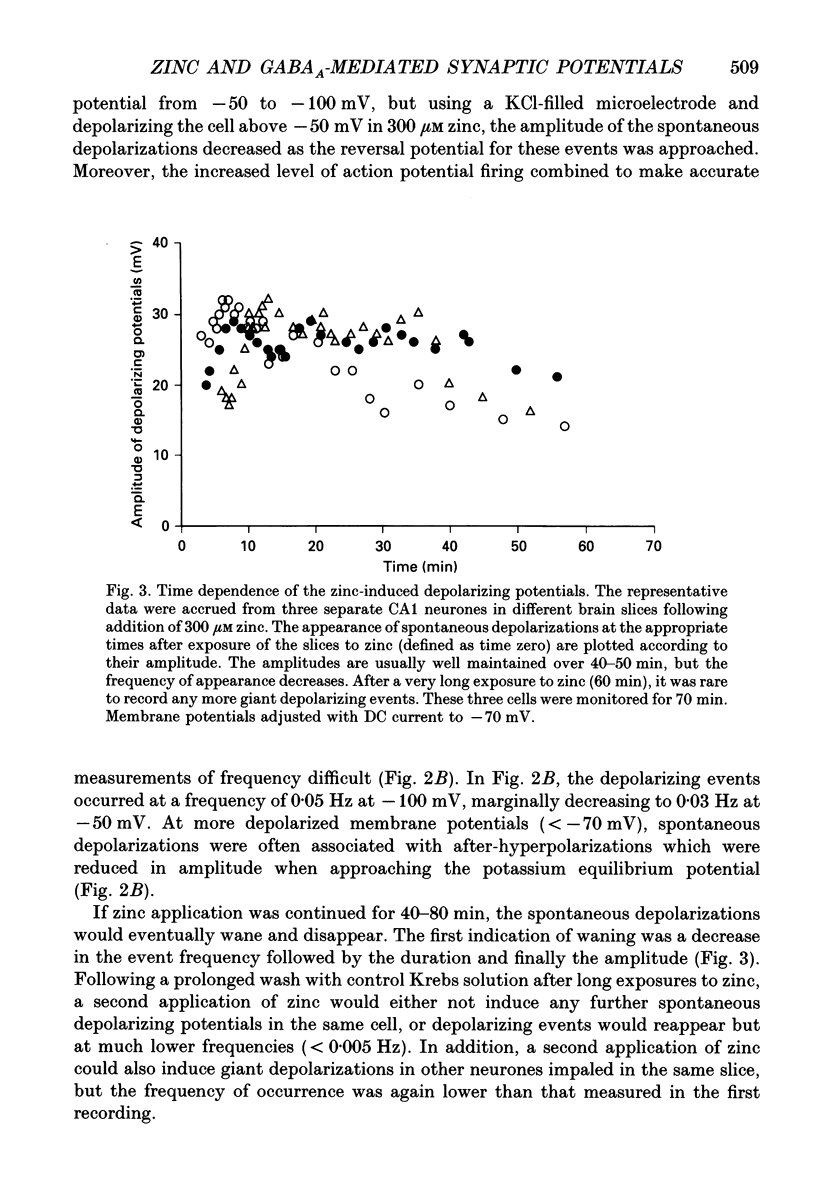
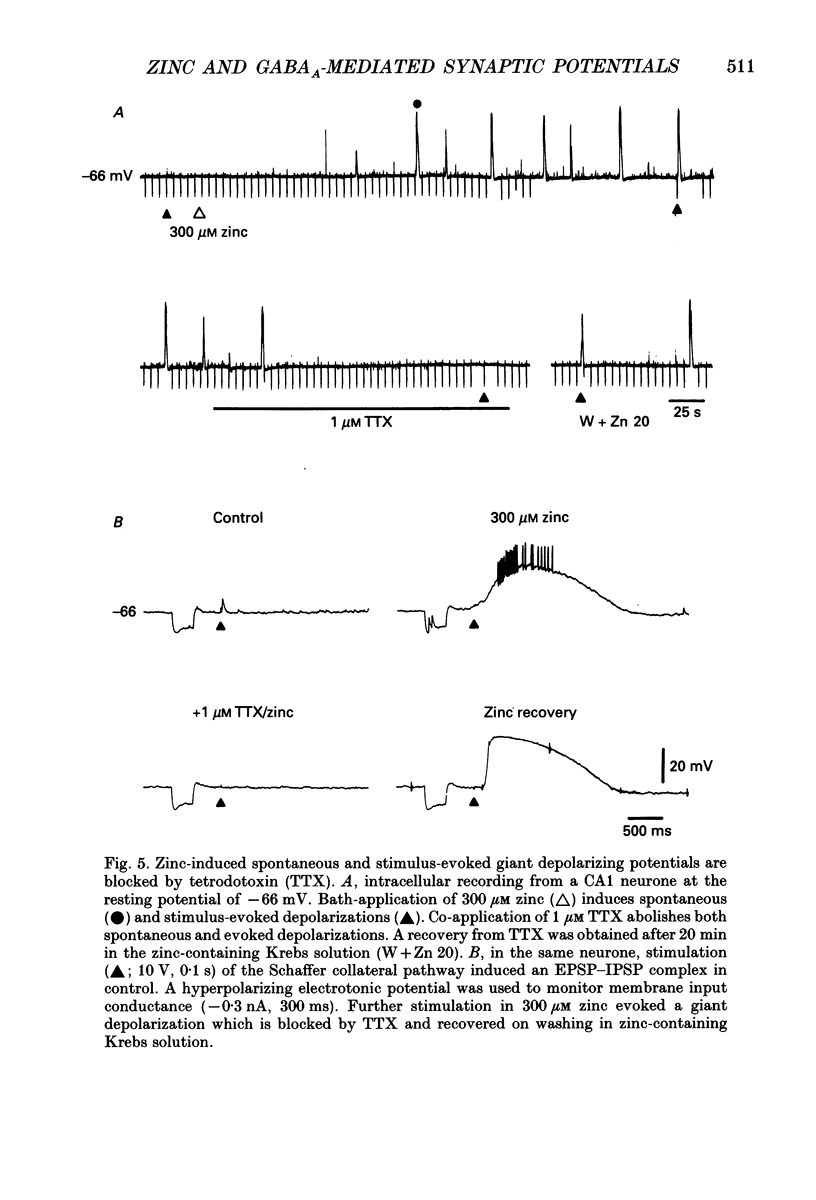
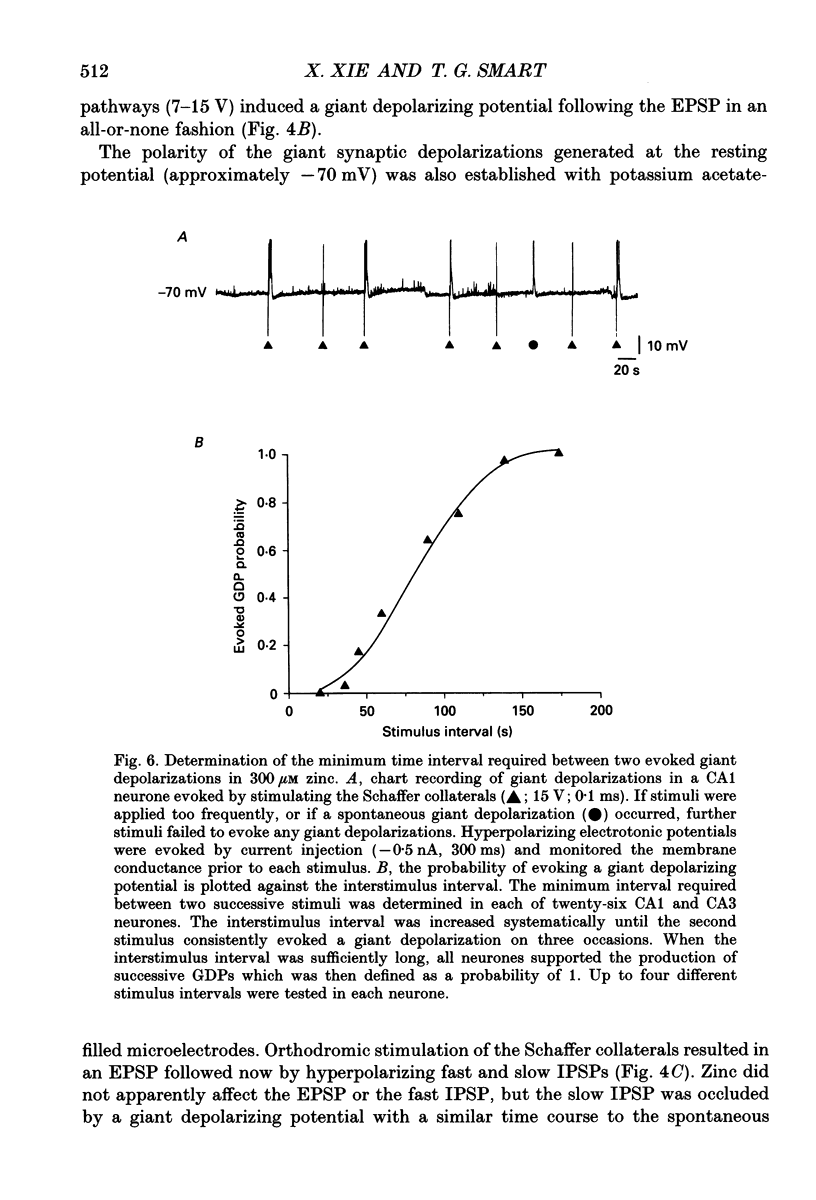
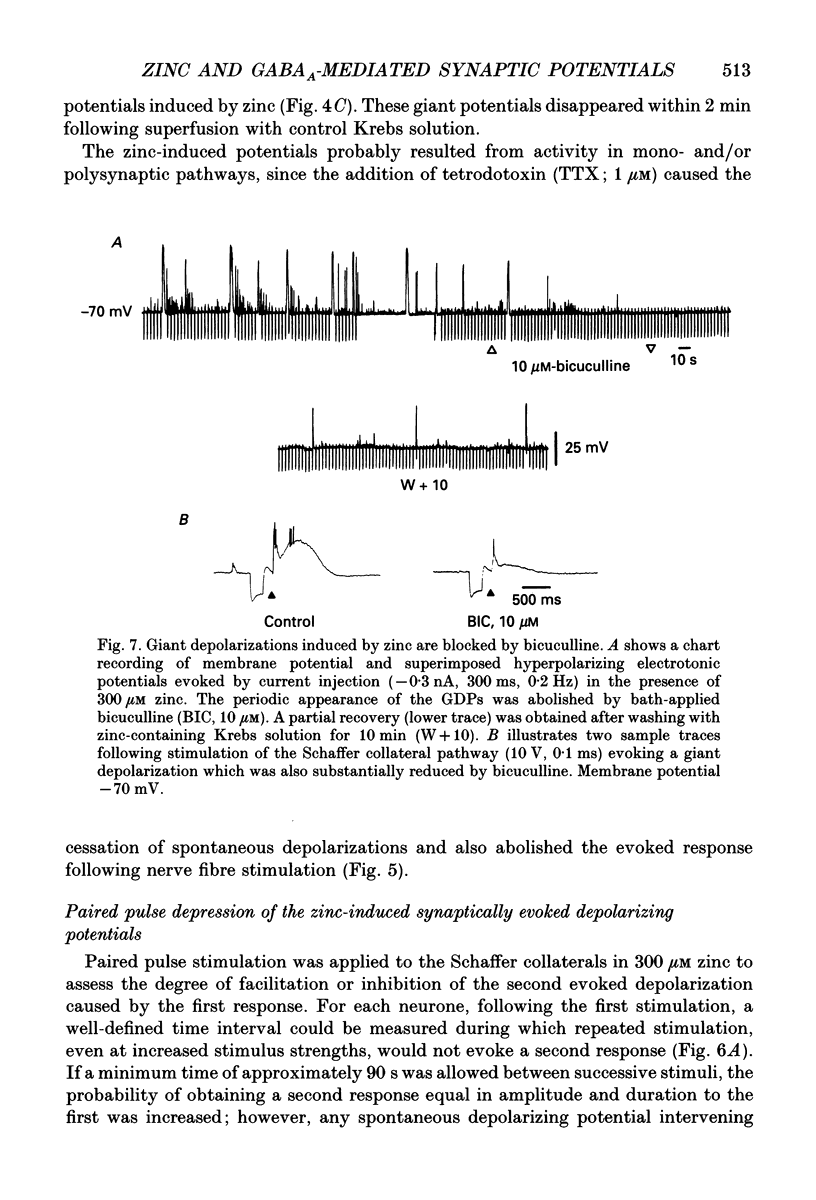
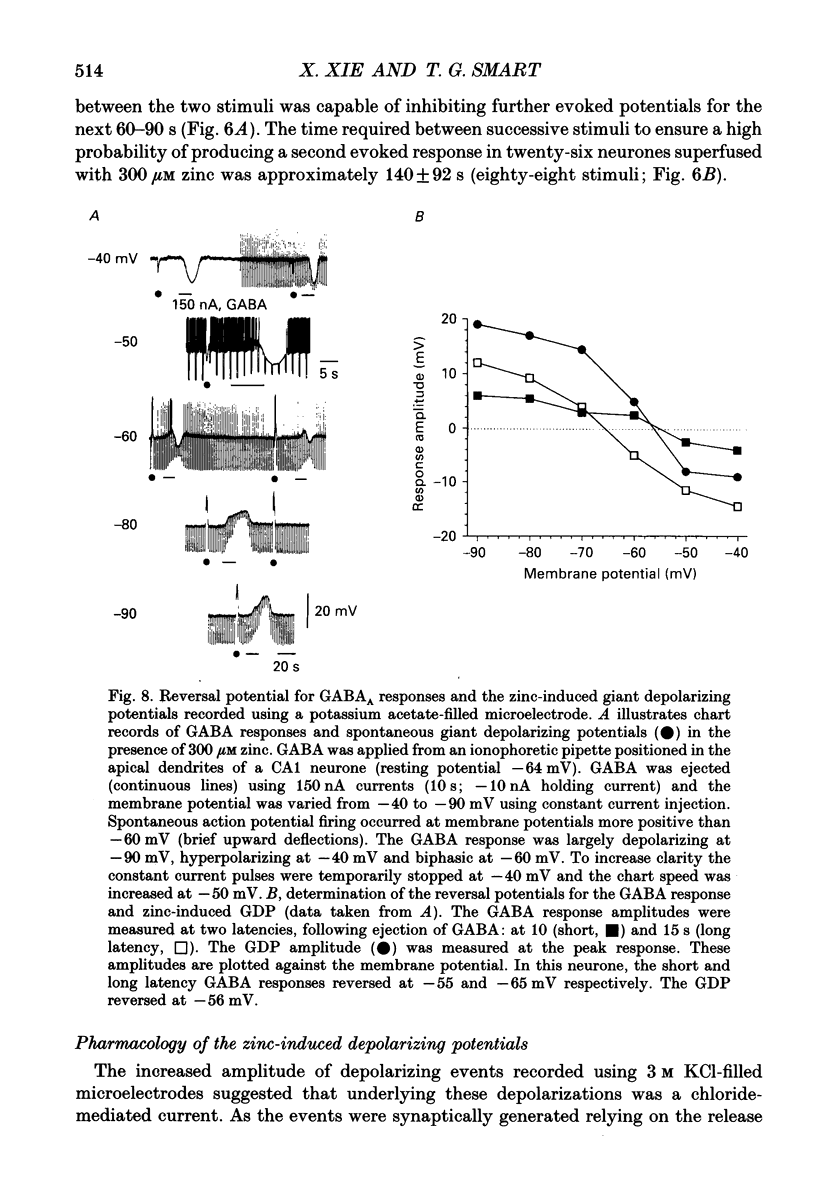
1. Intracellular recording techniques were used to study the actions of the transition ion, zinc, on CA1 and CA3 pyramidal neurones in adult rat hippocampal slices. 2. Zinc (300 microM) hyperpolarized pyramidal neurones, increased the membrane excitability and also induced periodic, spontaneous giant depolarizing potentials associated with a conductance increase mechanism. 3. The occurrence of spontaneous giant depolarizations was dependent on the zinc concentration (10 microM-1 mM) with an apparent dissociation constant of 98 microM. The frequency of zinc-induced depolarizations was unaffected by the membrane potential from -50 to -100 mV. 4. Stimulation of the Schaffer collaterals or mossy fibre pathways evoked an excitatory and inhibitory synaptic potential complex. In the presence of zinc, nerve fibre stimulation evoked, in an all-or-none fashion, a giant depolarizing potential with an increased membrane conductance. Both spontaneous and evoked depolarizations were inhibited by 1 microM tetrodotoxin. 5. Evoked giant depolarizations were labile with too frequent stimulation resulting in a failure of generation. A minimum time of 140 s was required between stimuli to ensure successive giant depolarizations. 6. Spontaneous and evoked zinc-induced depolarizing potentials were inhibited by bicuculline (10 microM) or picrotoxin (40 microM) and enhanced by pentobarbitone (100 microM) or flurazepam (10 microM), suggesting that these potentials are mediated by activation of gamma-aminobutyric acidA (GABAA) receptors. 7. Ionophoretic application of GABA produced biphasic responses at -60 mV membrane potential. The reversal potentials for the depolarizing and hyperpolarizing GABA responses were -56 +/- 5 and -66 +/- 8 mV respectively. The giant depolarizations induced by zinc reversed at -57 +/- 4 mV. This suggests a dendritic location for the generation of these potentials. 8. Excitatory amino acid antagonists, 2-amino-5-phosphonovalerate (APV, 40 microM) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 microM) did not affect the amplitude but slightly reduced the frequency of the giant depolarizations. 9. It is concluded that zinc induces a synchronized release of GABA, quite independent of intact excitatory synaptic transmission, which acts on GABAA receptors producing large depolarizing synaptic potentials. This increased level of GABA release may be of physiological and pathological importance since zinc is a naturally occurring metal ion endogenous to the central nervous system.
Full text
PDF



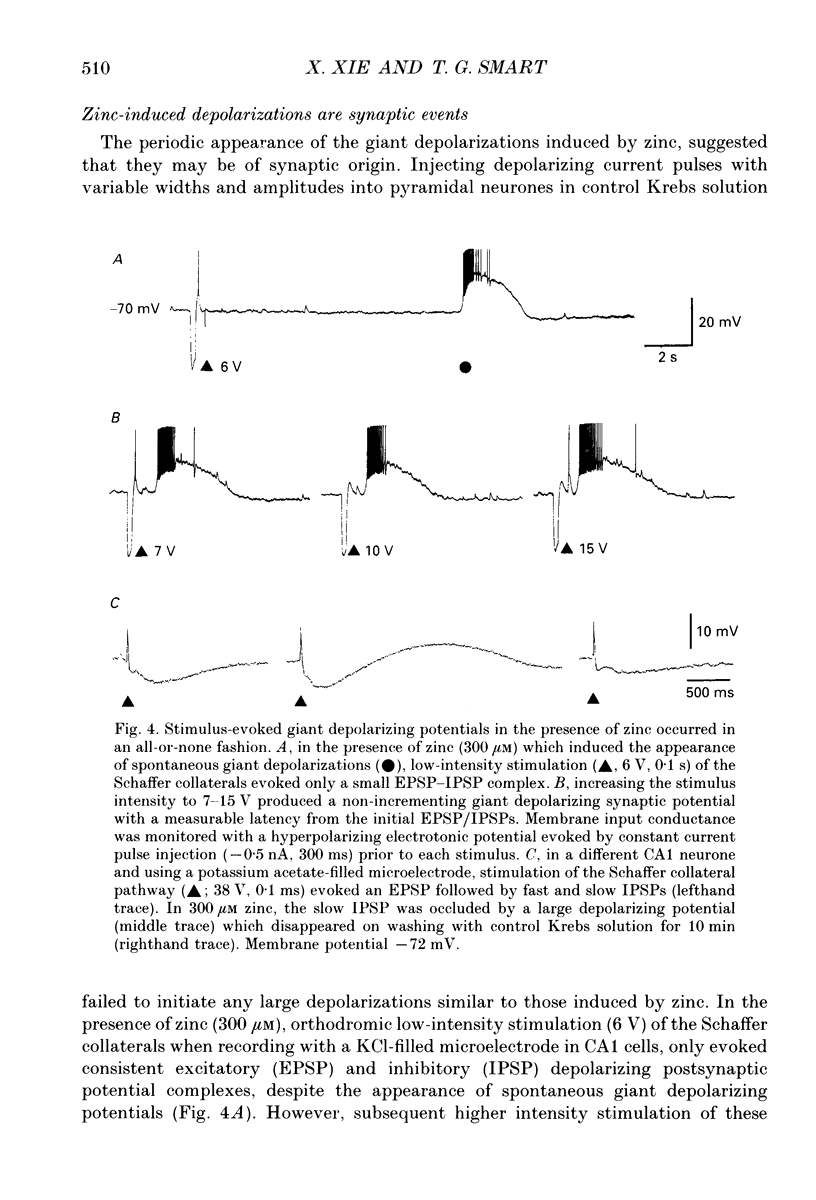







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
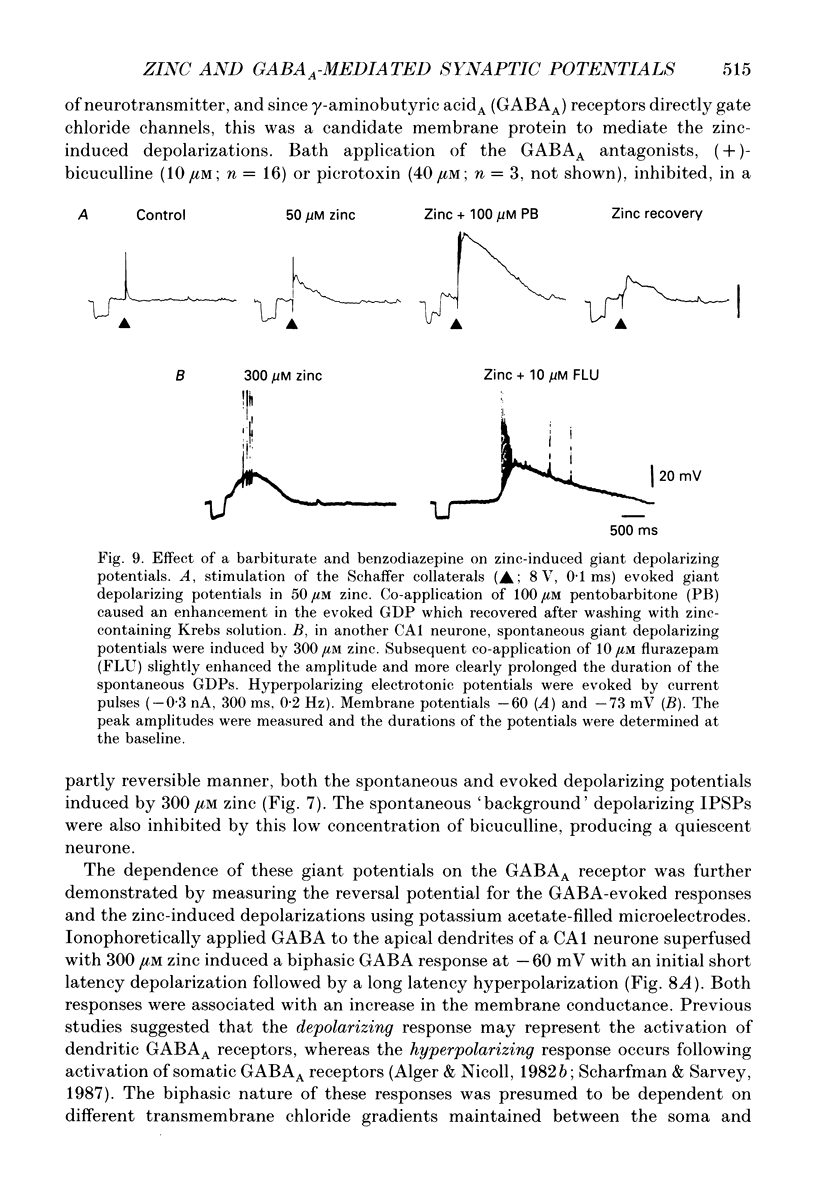
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
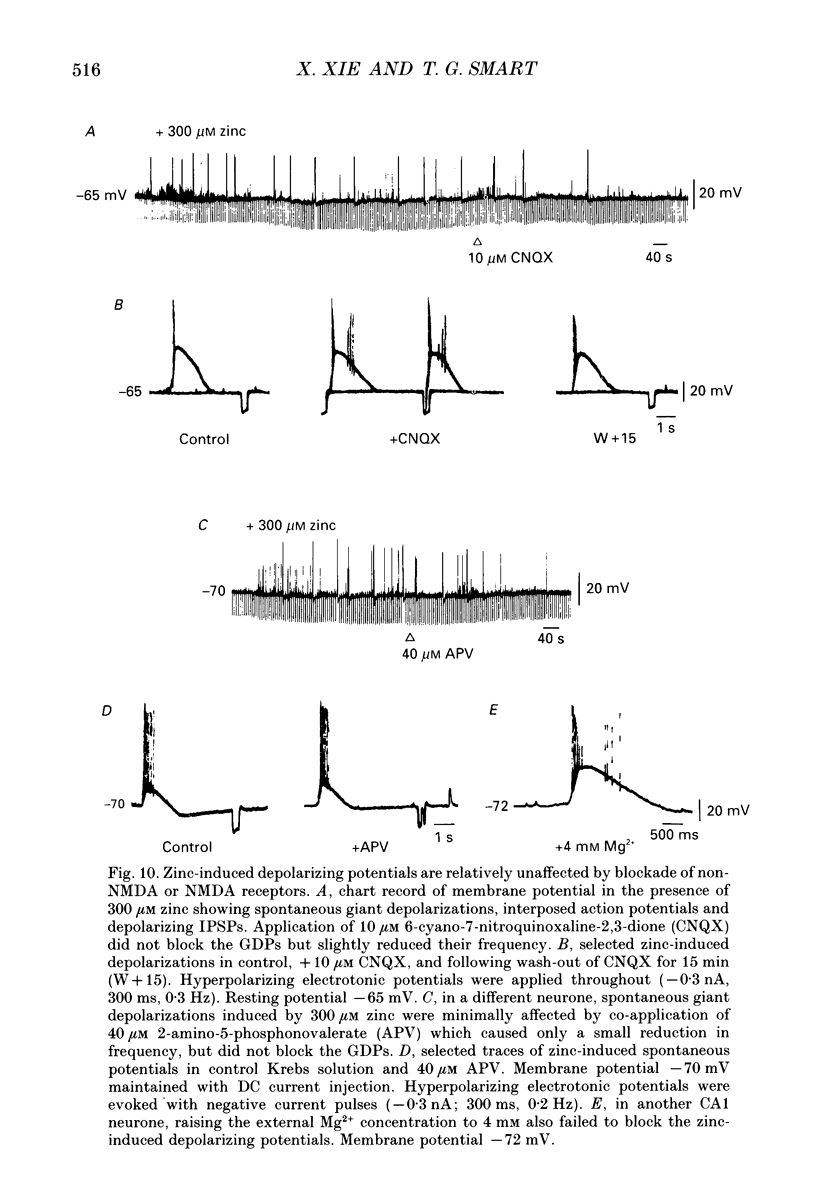
- Aram J. A., Michelson H. B., Wong R. K. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991 May;65(5):1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Avoli M. Epileptiform discharges and a synchronous GABAergic potential induced by 4-aminopyridine in the rat immature hippocampus. Neurosci Lett. 1990 Sep 4;117(1-2):93–98. doi: 10.1016/0304-3940(90)90125-s. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle P. J., Haas H. L. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982 May;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Charton G., Rovira C., Ben-Ari Y., Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58(1):202–205. doi: 10.1007/BF00238969. [DOI] [PubMed] [Google Scholar]
- Claiborne B. J., Amaral D. G., Cowan W. M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986 Apr 22;246(4):435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Crawford I. L., Connor J. D. Zinc in maturing rat brain: hippocampal concentration and localization. J Neurochem. 1972 Jun;19(6):1451–1458. doi: 10.1111/j.1471-4159.1972.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Danscher G., Howell G., Pérez-Clausell J., Hertel N. The dithizone, Timm's sulphide silver and the selenium methods demonstrate a chelatable pool of zinc in CNS. A proton activation (PIXE) analysis of carbon tetrachloride extracts from rat brains and spinal cords intravitally treated with dithizone. Histochemistry. 1985;83(5):419–422. doi: 10.1007/BF00509203. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A., Verdorn T. A., Ewert M., Seeburg P. H., Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990 Dec;5(6):781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Faber H., Braun K., Zuschratter W., Scheich H. System-specific distribution of zinc in the chick brain. A light- and electron-microscopic study using the Timm method. Cell Tissue Res. 1989 Nov;258(2):247–257. doi: 10.1007/BF00239445. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Kasarskis E. J., Ringo D., Frederickson R. E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987 Jun;20(2):91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Friedman B., Price J. L. Fiber systems in the olfactory bulb and cortex: a study in adult and developing rats, using the timm method with the light and electron microscope. J Comp Neurol. 1984 Feb 10;223(1):88–109. doi: 10.1002/cne.902230108. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Mossy fibres form synapses with identified pyramidal basket cells in the CA3 region of the guinea-pig hippocampus: a combined Golgi-electron microscope study. J Neurocytol. 1985 Apr;14(2):245–259. doi: 10.1007/BF01258450. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Holm I. E., Andreasen A., Danscher G., Pérez-Clausell J., Nielsen H. Quantification of vesicular zinc in the rat brain. Histochemistry. 1988;89(3):289–293. doi: 10.1007/BF00493154. [DOI] [PubMed] [Google Scholar]
- Howell G. A., Welch M. G., Frederickson C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984 Apr 19;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Ibata Y., Otsuka N. Electron microscopic demonstration of zinc in the hippocampal formation using Timm's sulfide silver technique. J Histochem Cytochem. 1969 Mar;17(3):171–175. doi: 10.1177/17.3.171. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Giacchino J. L., Chamberlin N. L., Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987 Jan;57(1):325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988 Apr;8(4):1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N. A., Borroni A. M., Grover L. M., Teyler T. J. Hyperpolarizing and depolarizing GABAA receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989 Aug;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarren M., Alger B. E. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985 Feb;53(2):557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Inhibitory role of dentate hilus neurons in guinea pig hippocampal slice. J Neurophysiol. 1990 Jul;64(1):46–56. doi: 10.1152/jn.1990.64.1.46. [DOI] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Picrotoxin- and 4-aminopyridine-induced activity in hilar neurons in the guinea pig hippocampal slice. J Neurophysiol. 1991 Jan;65(1):141–147. doi: 10.1152/jn.1991.65.1.141. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. A depolarizing inhibitory postsynaptic potential activated by synaptically released gamma-aminobutyric acid under physiological conditions in rat hippocampal pyramidal cells. Can J Physiol Pharmacol. 1988 Aug;66(8):1100–1102. doi: 10.1139/y88-180. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989 May;61(5):953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Rutecki P. A., Lebeda F. J., Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987 Jun;57(6):1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Sandler R., Smith A. D. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991 Jan 8;303(2):177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. Responses to GABA recorded from identified rat visual cortical neurons. Neuroscience. 1987 Nov;23(2):407–422. doi: 10.1016/0306-4522(87)90065-0. [DOI] [PubMed] [Google Scholar]
- Sim J. A., Cherubini E. Submicromolar concentrations of zinc irreversibly reduce a calcium-dependent potassium current in rat hippocampal neurons in vitro. Neuroscience. 1990;36(3):623–629. doi: 10.1016/0306-4522(90)90005-o. [DOI] [PubMed] [Google Scholar]
- Smart T. G. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992 Feb;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. Differential effect of zinc on the vertebrate GABAA-receptor complex. Br J Pharmacol. 1990 Apr;99(4):643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. Pre- and postsynaptic effects of zinc on in vitro prepyriform neurones. Neurosci Lett. 1983 Sep 30;40(2):205–211. doi: 10.1016/0304-3940(83)90303-8. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Moss S. J., Xie X., Huganir R. L. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991 Aug;103(4):1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G. Uncultured lobster muscle, cultured neurons and brain slices: the neurophysiology of zinc. J Pharm Pharmacol. 1990 Jun;42(6):377–387. doi: 10.1111/j.2042-7158.1990.tb06576.x. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H. Blockade of a late inhibitory postsynaptic potential in hippocampal CA3 neurons in vitro reveals a late depolarizing potential that is augmented by pentobarbital. Neurosci Lett. 1988 Dec 19;95(1-3):155–160. doi: 10.1016/0304-3940(88)90649-0. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Deisz R. A., Prince D. A. Relative contributions of passive equilibrium and active transport to the distribution of chloride in mammalian cortical neurons. J Neurophysiol. 1988 Jul;60(1):105–124. doi: 10.1152/jn.1988.60.1.105. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl- in hippocampal CA3 neurons. J Neurophysiol. 1989 Mar;61(3):512–523. doi: 10.1152/jn.1989.61.3.512. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):524–533. doi: 10.1152/jn.1989.61.3.524. [DOI] [PubMed] [Google Scholar]
- Wensink J., Molenaar A. J., Woroniecka U. D., Van den Hamer C. J. Zinc uptake into synaptosomes. J Neurochem. 1988 Mar;50(3):782–789. doi: 10.1111/j.1471-4159.1988.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Winegar B. D., Lansman J. B. Voltage-dependent block by zinc of single calcium channels in mouse myotubes. J Physiol. 1990 Jun;425:563–578. doi: 10.1113/jphysiol.1990.sp018118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G., Schütte M., Römhild W. Uptake and subcellular distribution of 65zinc in brain structures during the postnatal development of the rat. Neurosci Lett. 1984 Oct 12;51(2):277–280. doi: 10.1016/0304-3940(84)90564-0. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]


