Abstract
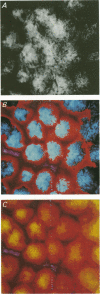
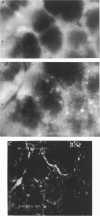
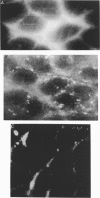
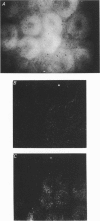
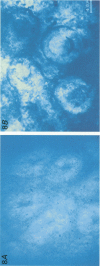
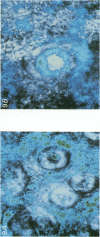
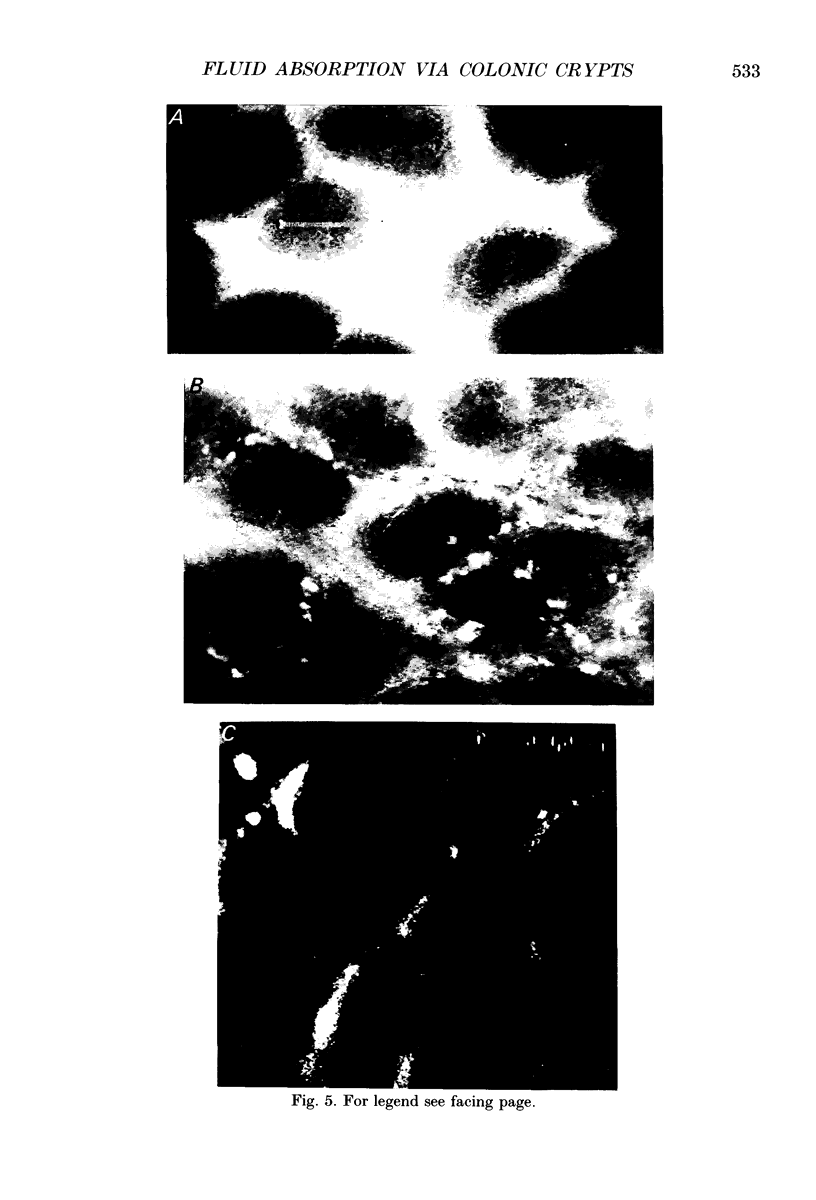
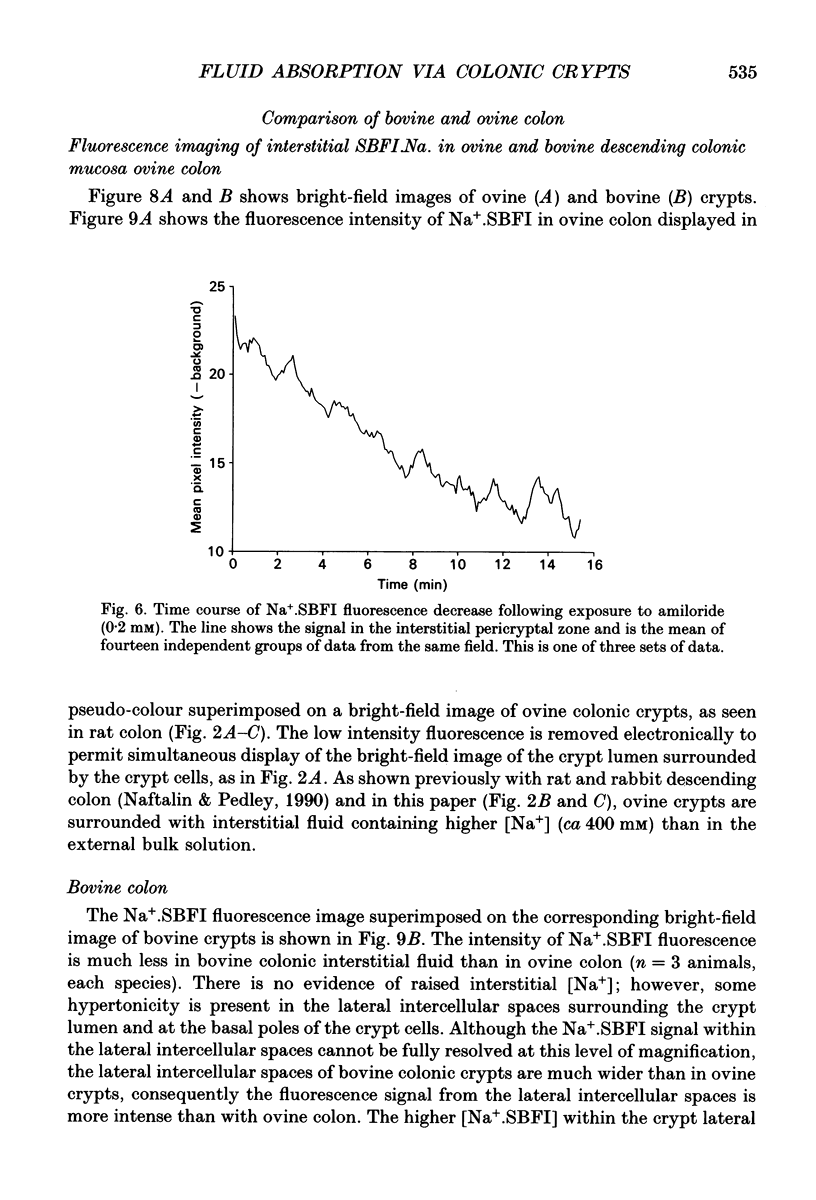
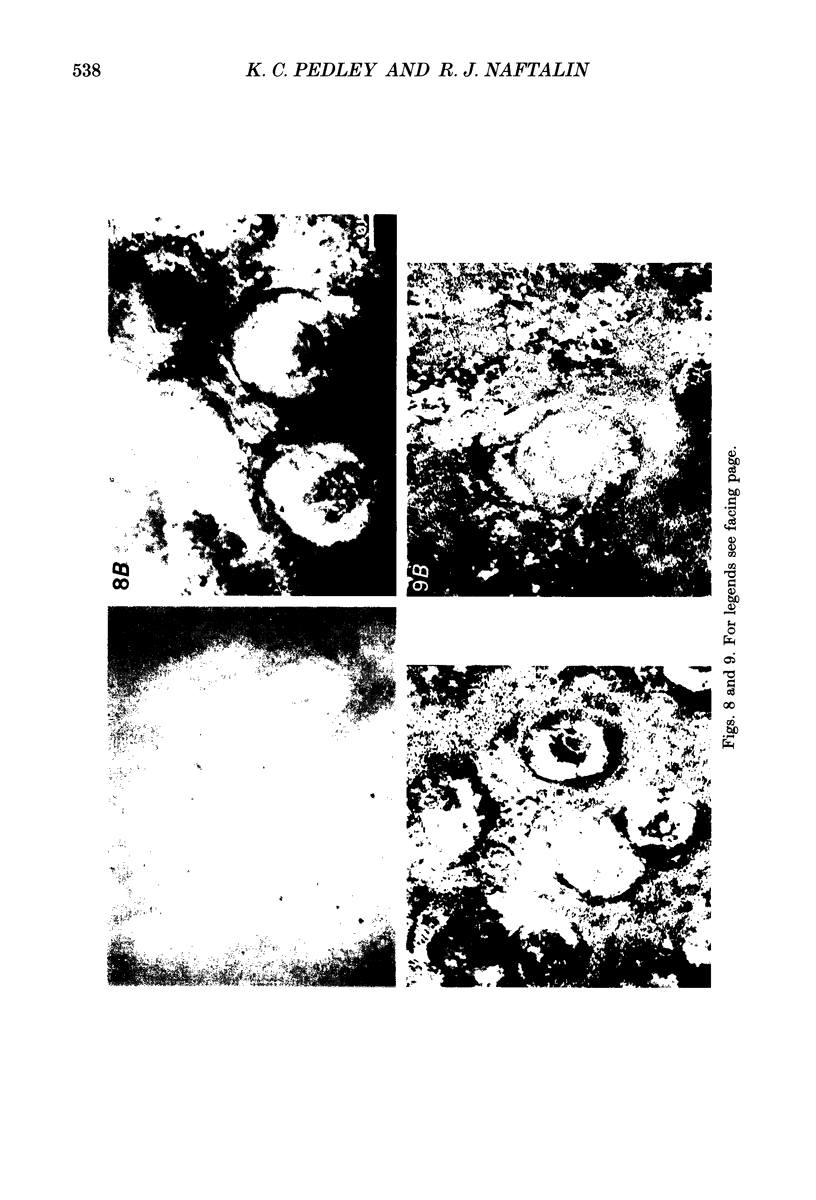
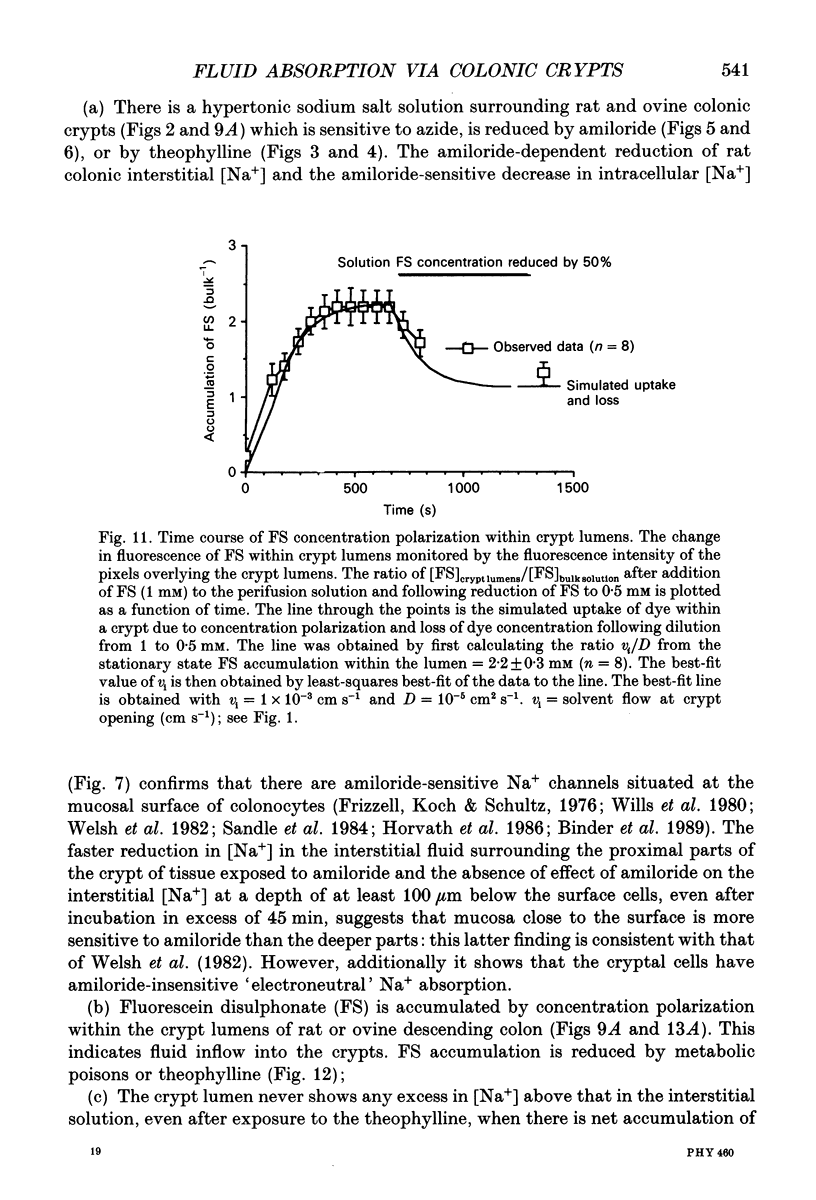
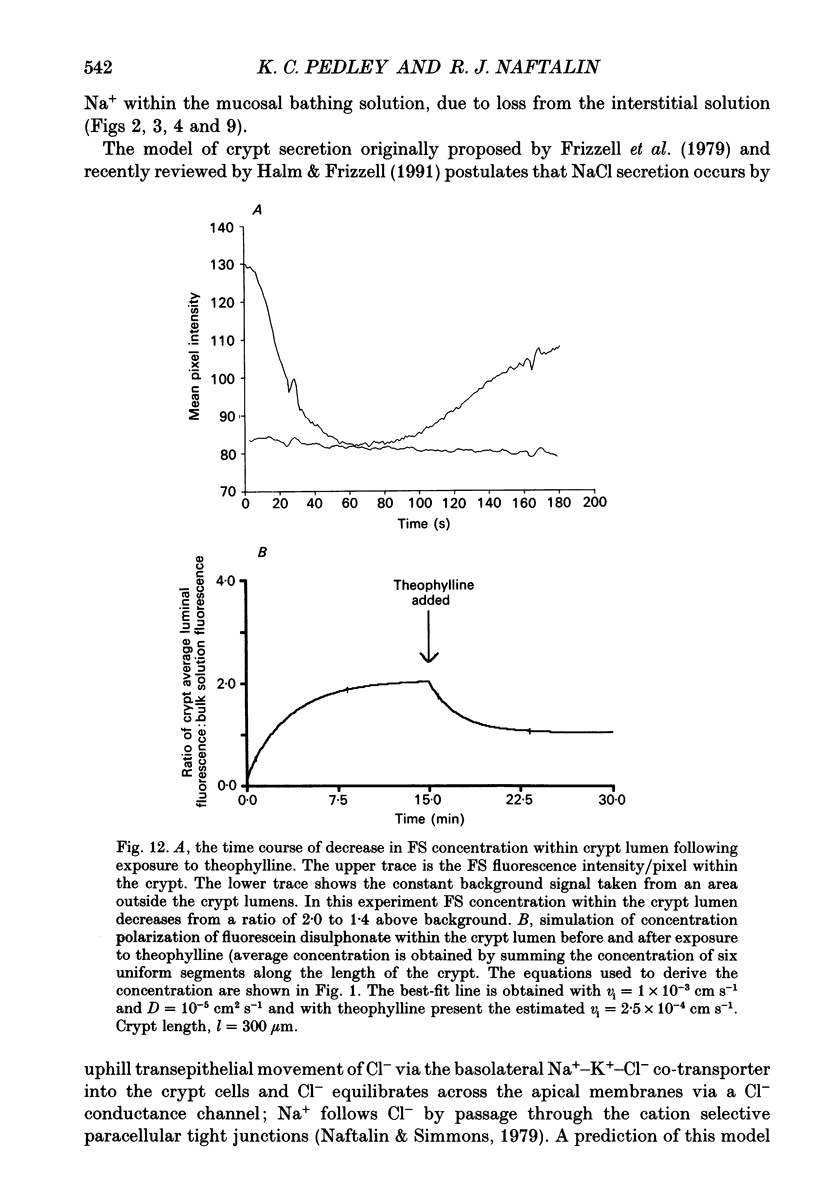
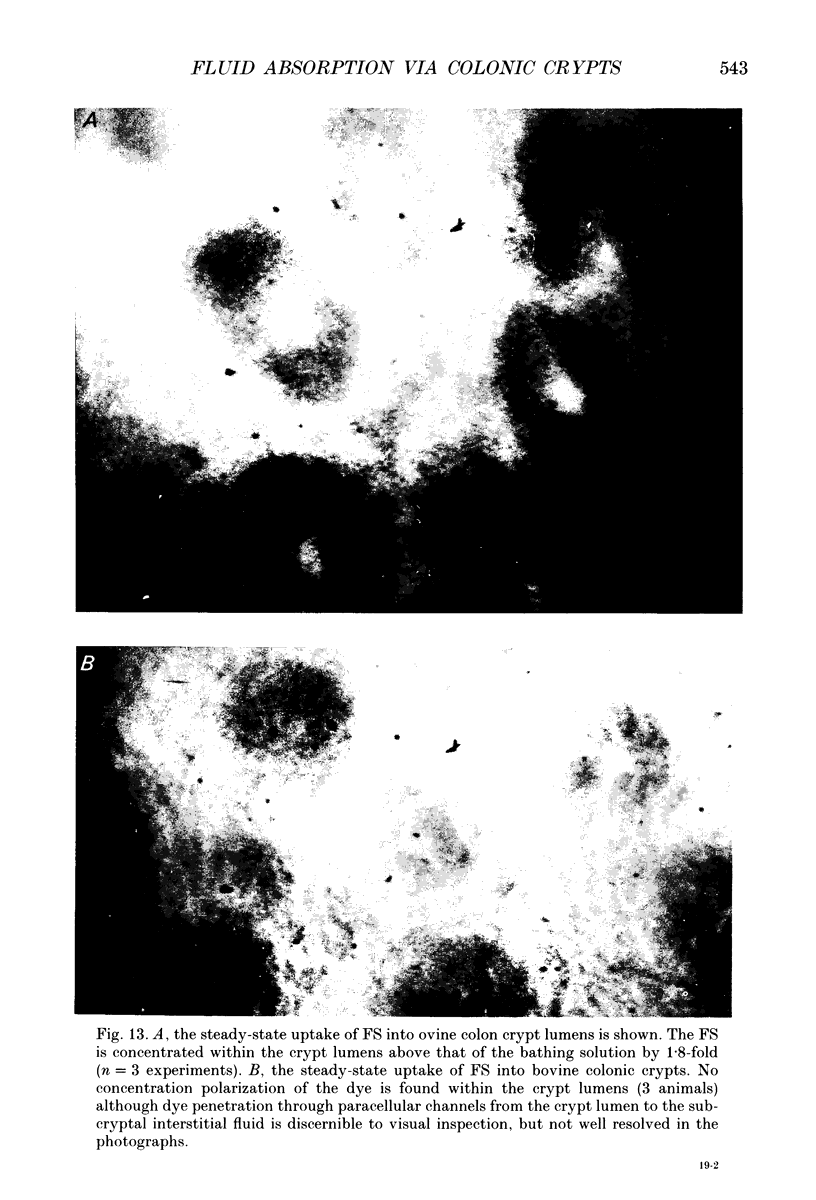
1. To test whether colonic crypts are secretory or absorptive interstitial [Na+] in rat descending colonic mucosa is determined using video-enhanced imaging of the impermeant acid form of the fluorescent Na+ probe SBFI (Molecular Probes) and intracellular [Na+] is monitored with SBFI (AM form). In rat descending colonic mucosa perifused with isotonic Tyrode solution interstitial [Na+] = 500-650 mM. Following exposure to Tyrode solution containing theophylline (10 mM) interstitial [Na+] falls by 300-450 mM within 1 min. Exposure to amiloride (0.2 mM) reduces the intracellular [Na+] from ca 25 to 12 mM within 15 min and concurrently decreases [Na+] in the interstitial fluid surrounding the crypts at the mucosal surface by approximately 200 mM. 2. The route of fluid inflow across the rat colonic mucosa is directly traced by perifusing with Tyrode solution containing the impermeant fluorescent dye, fluorescein disulphonate (FS). FS accumulates rapidly within crypt lumens of control tissues to a 2-fold higher concentration than in the external bathing solution, but FS does not accumulate in crypts of tissues treated with azide (2 mM). The increment in FS accumulation within the crypt lumen above the bulk solution decreases by 80% within 1 min following exposure to theophylline (10 mM), indicating that fluid absorption into crypts is reduced. Estimates of the total fluid influx from the rate and extent of FS concentration polarization within crypts indicate that it is sufficient to account for the entire transcolonic fluid absorption. 3. Comparative studies of isolated bovine and ovine colon were also undertaken to investigate the failure of bovine colon to generate a hypertonic absorbate and hence its incapacity to produce hard faeces. The interstitial fluid surrounding ovine colonic crypts is hypertonic to the bulk solution, whereas the interstitial fluid surrounding bovine colonic crypts is nearly isotonic with the bathing solution. Additionally, fluorescein disulphonate accumulates within ovine colonic crypt lumens by concentration polarization, whereas no concentration of FS occurs within bovine colonic crypt lumens. This corroborates the view that a hypertonic interstitial fluid is absent from bovine colon mainly because of a high rate of transepithelial leakage of low molecular weight solutes via paracellular routes.
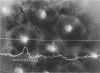
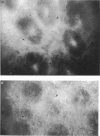
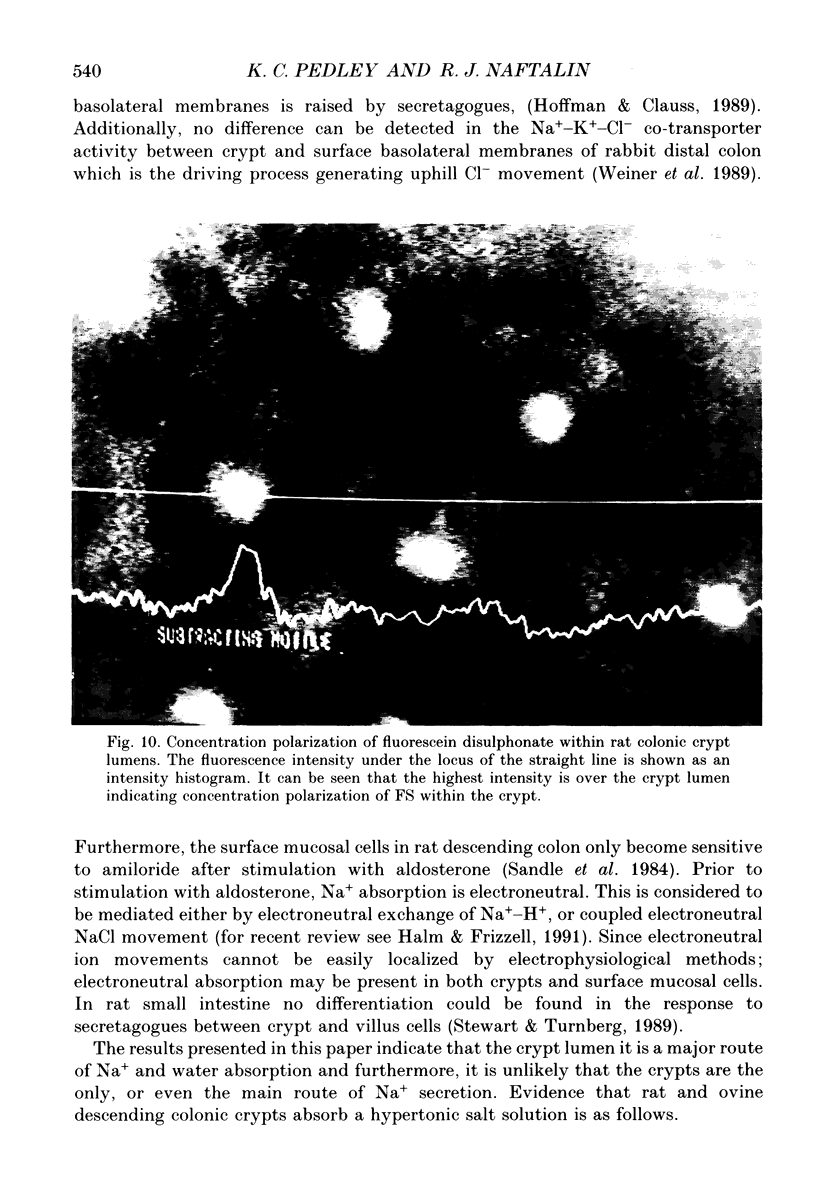
Full text
PDF

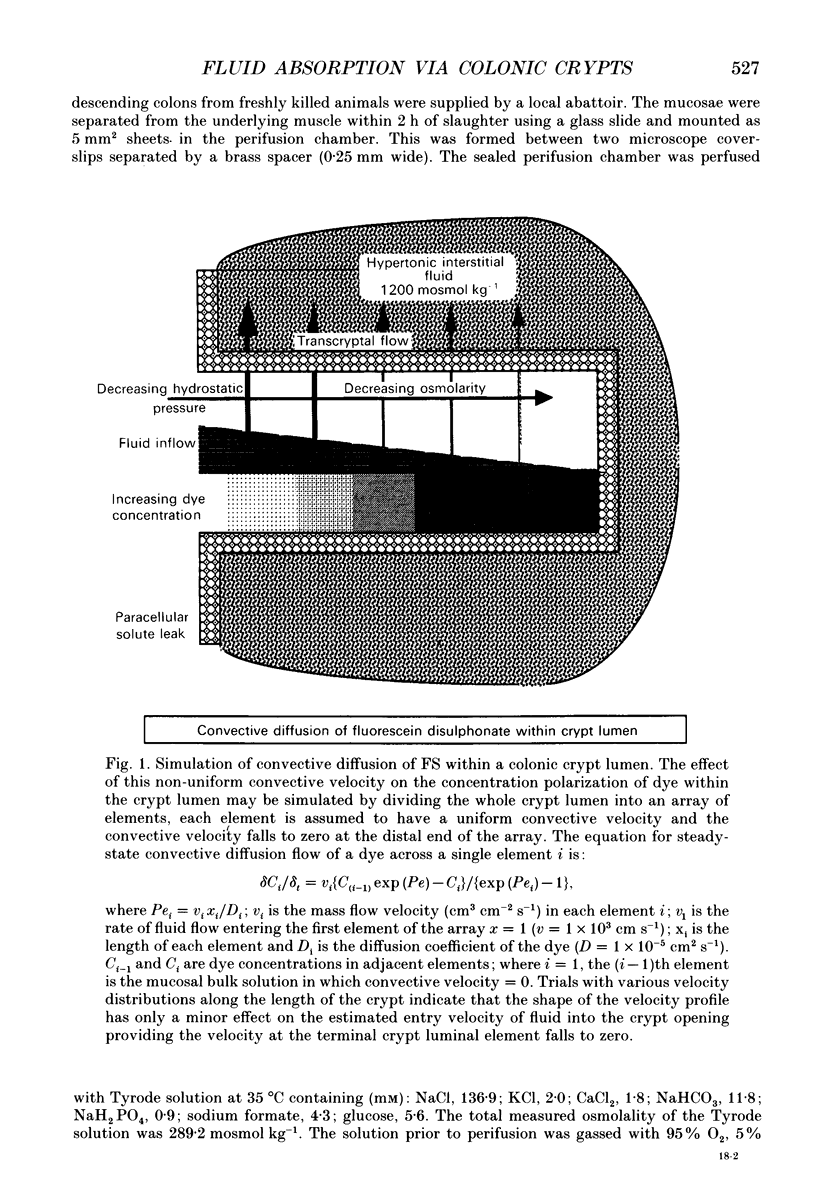
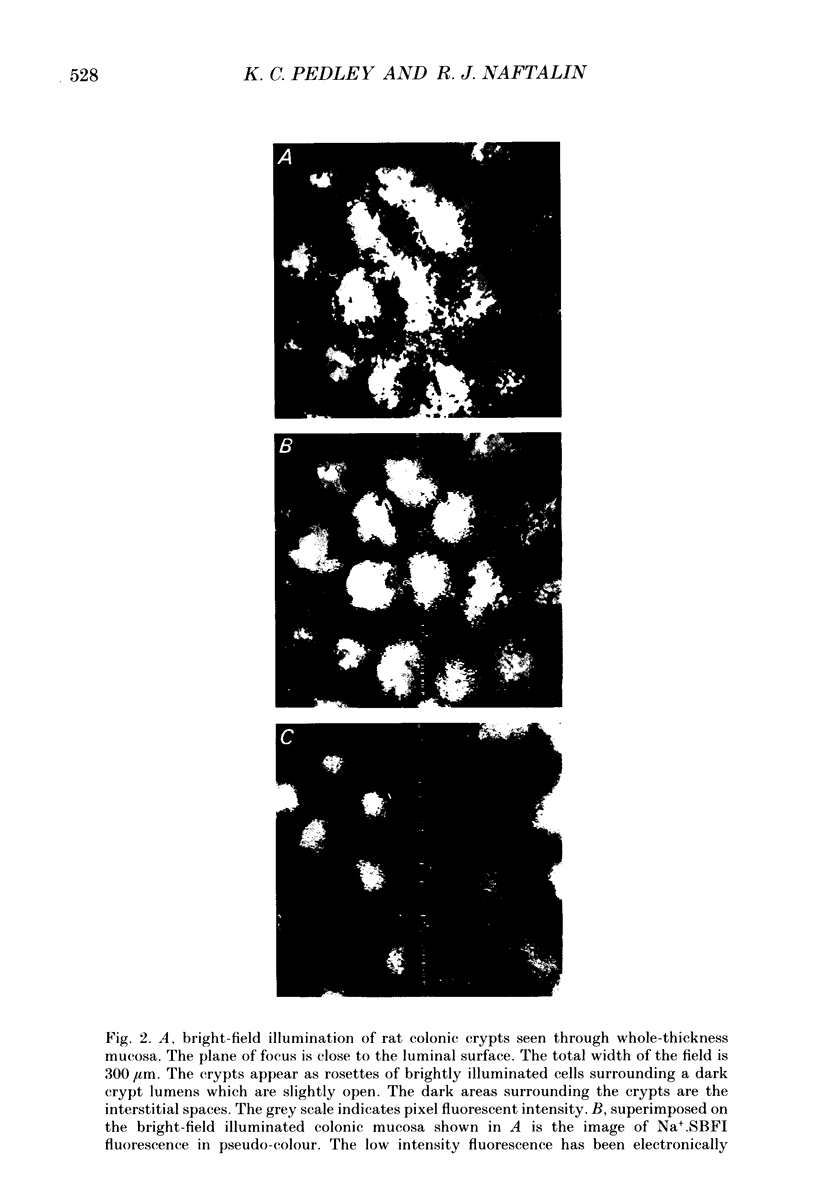

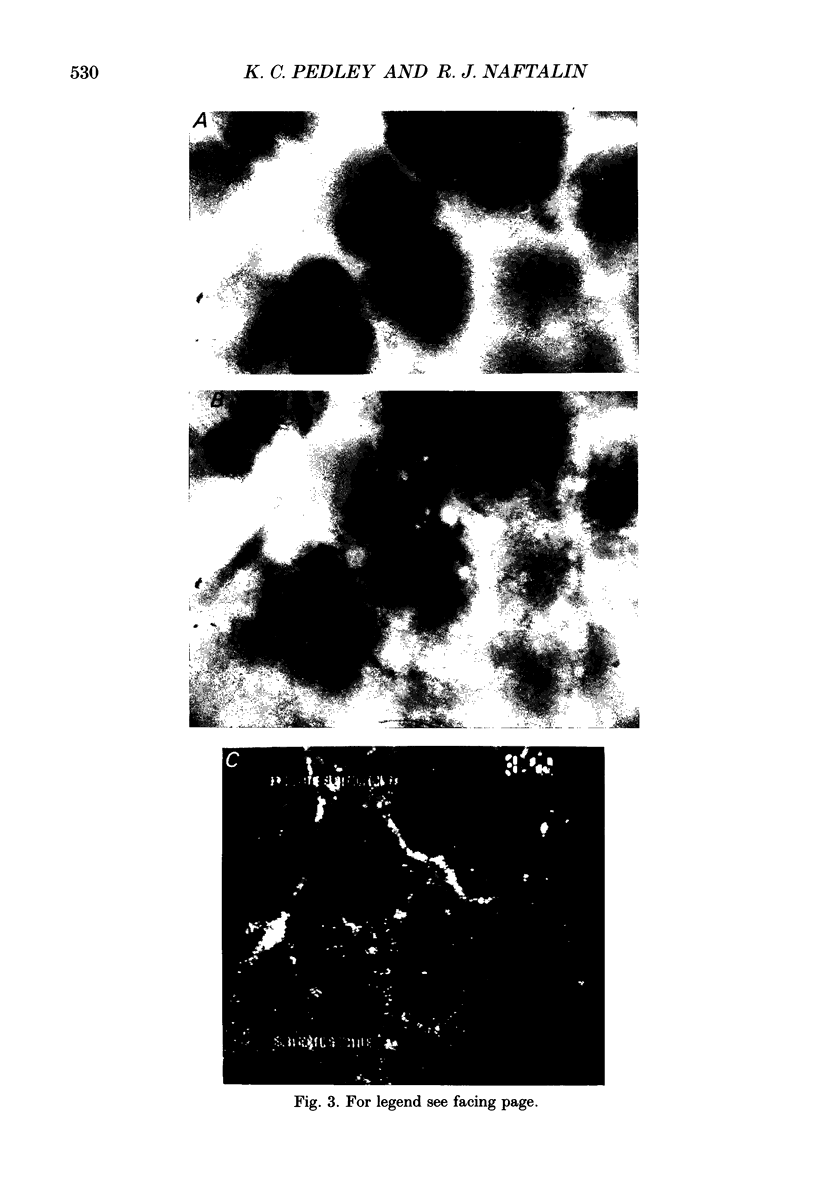

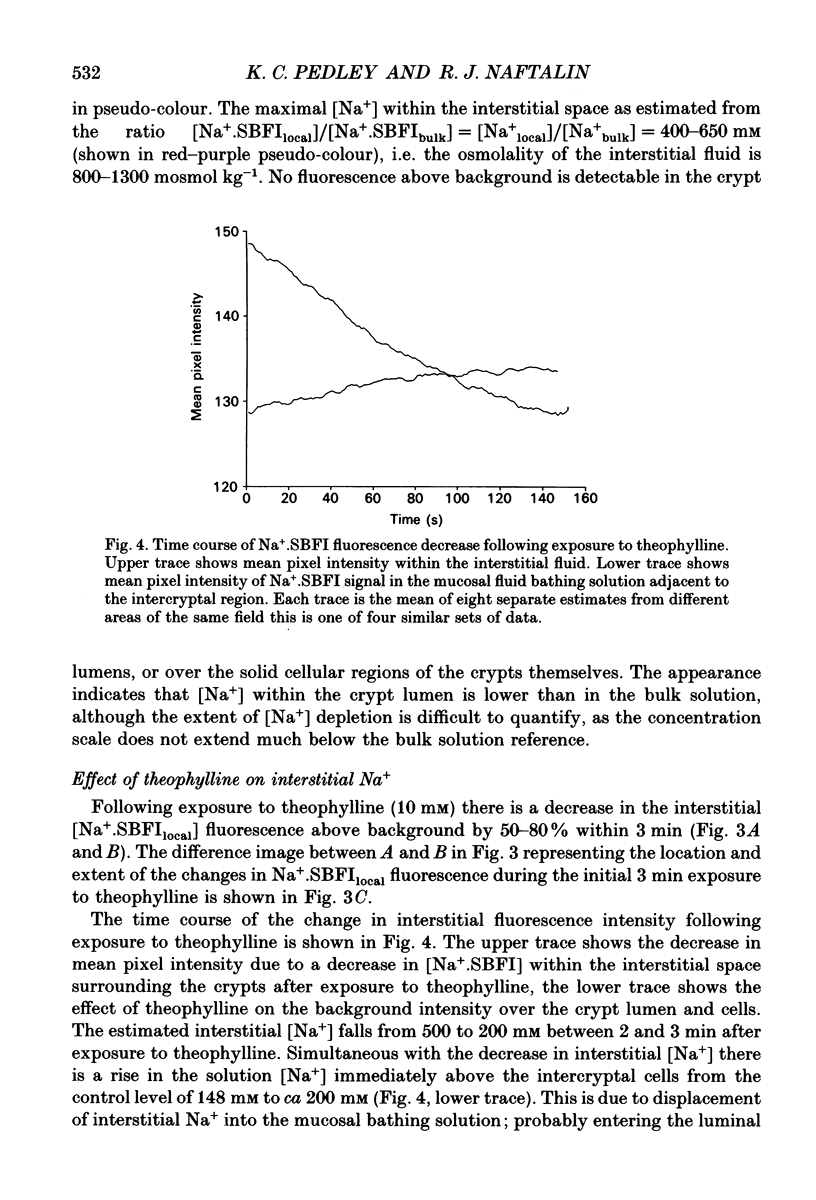








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahsan M. A., Ilundain A., Naftalin R. J., Sandhu B. K., Smith P. M. Effects of theophylline, choleragen and loperamide on rabbit ileal fluid and electrolyte transport in vitro. Br J Pharmacol. 1987 Dec;92(4):743–754. doi: 10.1111/j.1476-5381.1987.tb11378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan M. A., Naftalin R. J., Smith P. M. A submucosal mechanism for catecholamine-induced increases in fluid absorption in rabbit ileum in vitro. J Physiol. 1988 Oct;404:385–405. doi: 10.1113/jphysiol.1988.sp017295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H., Diamond J. M. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984 Jul;64(3):763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- Binder H. J., McGlone F., Sandle G. I. Effects of corticosteroid hormones on the electrophysiology of rat distal colon: implications for Na+ and K+ transport. J Physiol. 1989 Mar;410:425–441. doi: 10.1113/jphysiol.1989.sp017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D., Naftalin R. J. Hypertonic fluid absorption from rabbit descending colon in vitro. Am J Physiol. 1990 Mar;258(3 Pt 1):G377–G390. doi: 10.1152/ajpgi.1990.258.3.G377. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Rummel W., Wollenberg P. Effects of vasopressin on electrolyte transport across isolated colon from normal and dexamethasone-treated rats. J Physiol. 1984 Oct;355:11–23. doi: 10.1113/jphysiol.1984.sp015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Hecker J. F., Grovum W. L. Rates of passage of digesta and water absorption along the larg intestines of sheep, cows and pigs. Aust J Biol Sci. 1975 Apr;28(2):161–167. doi: 10.1071/bi9750161. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Clauss W. Time-dependent effects of aldosterone on sodium transport and cell membrane resistances in rabbit distal colon. Pflugers Arch. 1989 Nov;415(2):156–164. doi: 10.1007/BF00370587. [DOI] [PubMed] [Google Scholar]
- Horvath P. J., Ferriola P. C., Weiser M. M., Duffey M. E. Localization of chloride secretion in rabbit colon: inhibition by anthracene-9-carboxylic acid. Am J Physiol. 1986 Feb;250(2 Pt 1):G185–G190. doi: 10.1152/ajpgi.1986.250.2.G185. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987 Jan;92(1):68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Berry C. A., Verkman A. S. Rapid development of vasopressin-induced hydroosmosis in kidney collecting tubules measured by a new fluorescence technique. Biophys J. 1988 Oct;54(4):595–602. doi: 10.1016/S0006-3495(88)82994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie A. T., Goecke I. A., Naftalin R. J. Comparison of fluid absorption by bovine and ovine descending colon in vitro. Am J Physiol. 1991 Sep;261(3 Pt 1):G433–G442. doi: 10.1152/ajpgi.1991.261.3.G433. [DOI] [PubMed] [Google Scholar]
- McKie A. T., Powrie W., Naftalin R. J. Mechanical aspects of rabbit fecal dehydration. Am J Physiol. 1990 Mar;258(3 Pt 1):G391–G394. doi: 10.1152/ajpgi.1990.258.3.G391. [DOI] [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Naftalin R. J., Pedley K. C. Video enhanced imaging of the fluorescent Na+ probe SBFI indicates that colonic crypts absorb fluid by generating a hypertonic interstitial fluid. FEBS Lett. 1990 Jan 29;260(2):187–194. doi: 10.1016/0014-5793(90)80100-w. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Simmons N. L. The effects of theophylline and choleragen on sodium and chloride ion movements within isolated rabbit ileum. J Physiol. 1979 May;290(2):331–350. doi: 10.1113/jphysiol.1979.sp012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J Physiol. 1985 Mar;360:27–50. doi: 10.1113/jphysiol.1985.sp015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. The roles of paracellular and transcellular pathways and submucosal space in isotonic water absorption by rabbit ileum. J Physiol. 1986 Jan;370:409–432. doi: 10.1113/jphysiol.1986.sp015942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Malawer S. J. Relationship between water and solute transport from isosmotic solutions by rat intestine in vivo. Am J Physiol. 1968 Jul;215(1):49–55. doi: 10.1152/ajplegacy.1968.215.1.49. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Hayslett J. P., Binder H. J. Effect of chronic hyperaldosteronism on the electrophysiology of rat distal colon. Pflugers Arch. 1984 May;401(1):22–26. doi: 10.1007/BF00581528. [DOI] [PubMed] [Google Scholar]
- Smaje L. H., Fraser P. A., Clough G. The distensibility of single capillaries and venules in the cat mesentery. Microvasc Res. 1980 Nov;20(3):358–370. doi: 10.1016/0026-2862(80)90064-3. [DOI] [PubMed] [Google Scholar]
- Stewart C. P., Turnberg L. A. A microelectrode study of responses to secretagogues by epithelial cells on villus and crypt of rat small intestine. Am J Physiol. 1989 Sep;257(3 Pt 1):G334–G343. doi: 10.1152/ajpgi.1989.257.3.G334. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Boulpaep E. L. Mechanisms of water transport by epithelial cells. Q J Exp Physiol. 1989 Jul;74(4):385–417. doi: 10.1113/expphysiol.1989.sp003288. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Fromm M., Frizzell R. A. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982 Dec 17;218(4578):1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]
- Wiener H., Turnheim K., van Os C. H. Rabbit distal colon epithelium: I. Isolation and characterization of basolateral plasma membrane vesicles from surface and crypt cells. J Membr Biol. 1989 Sep;110(2):147–162. doi: 10.1007/BF01869470. [DOI] [PubMed] [Google Scholar]