Abstract
1. The present study investigates to what extent increases in resistance to fatigue and aerobic oxidative capacity of energy metabolism are correlated in fast-twitch tibialis anterior muscles of rat and rabbit subjected to chronic low-frequency stimulation. 2. Changes in the aerobic oxidative capacity of the stimulated muscles were judged from increases in citrate synthase activity, representing the constant-proportion enzyme group of the citric acid cycle. 3. Resistance to fatigue reached maximal values in both rat and rabbit tibialis anterior muscles after stimulation periods of 14 days, whereas citrate synthase activity continued to increase with longer stimulation periods. 4. Different time courses of the changes in resistance to fatigue and citrate synthase activity were observed not only with prolonged stimulation periods but also during the first week, when pronounced increases in resistance to fatigue were accompanied by only moderate elevations in citrate synthase activity. 5. The dissociation between the changes of the two parameters studied suggests that factors other than elevated aerobic oxidative capacity contribute to enhanced resistance to fatigue.
Full text
PDF

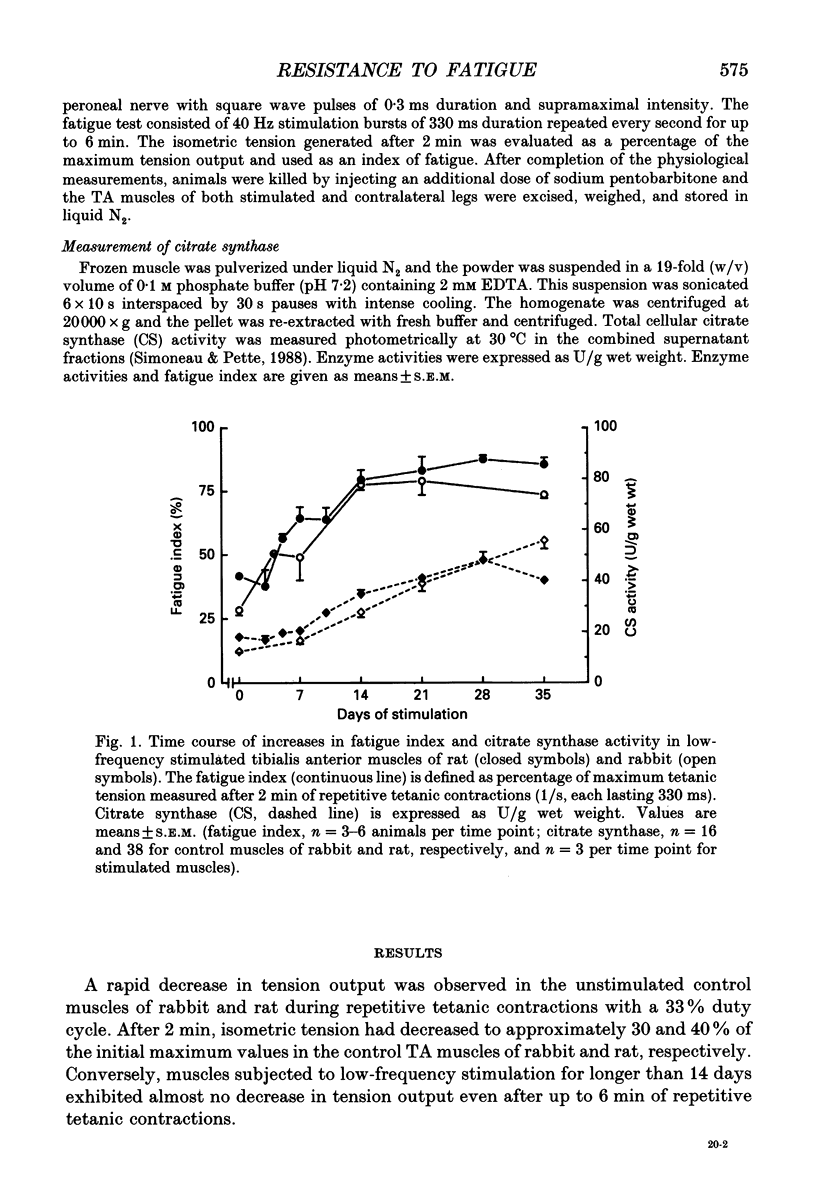
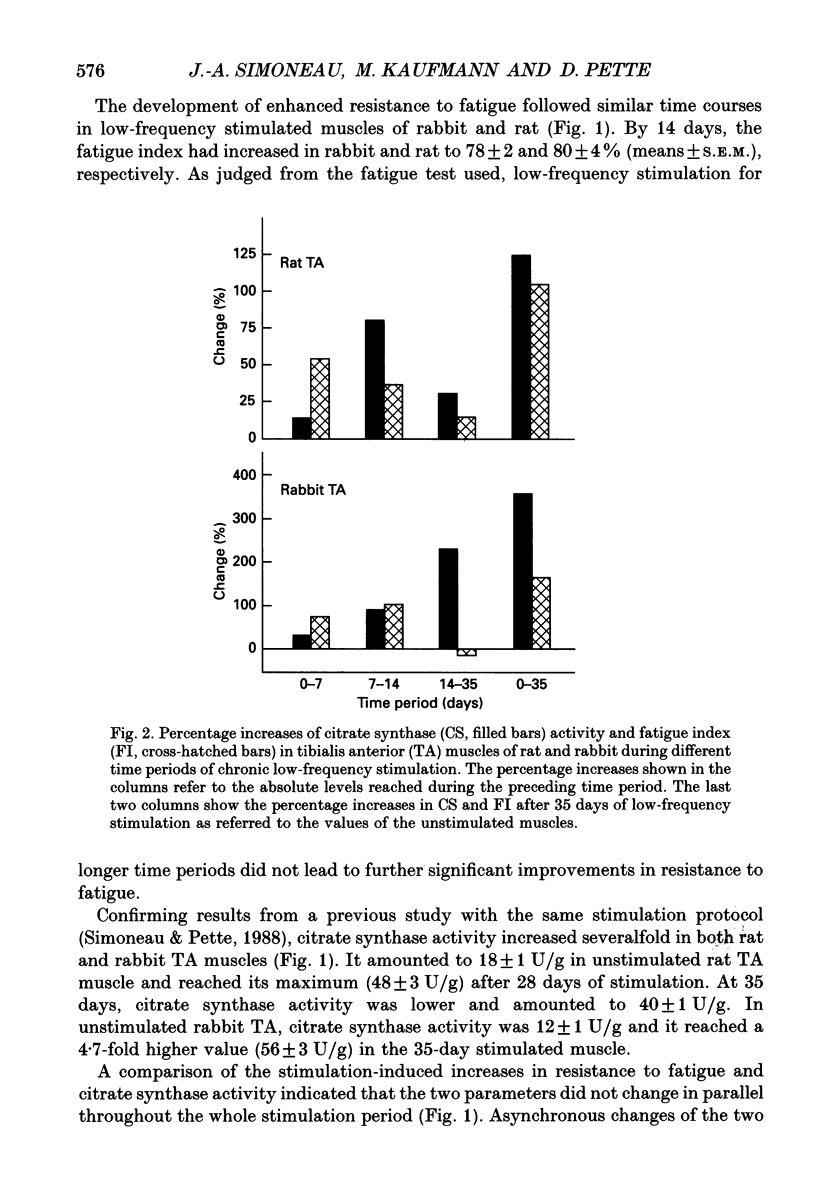
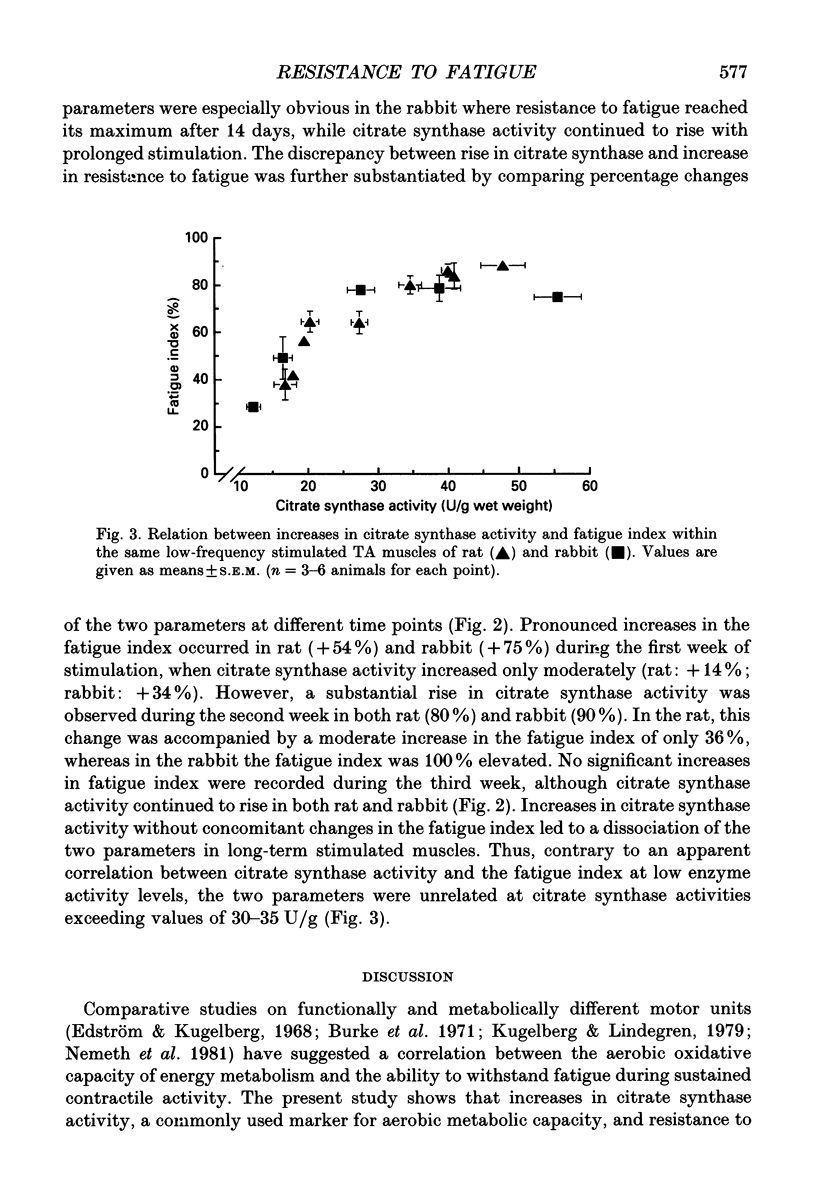



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. D., Cotter M. A., Hudlická O., Vrbová G. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflugers Arch. 1976 Feb 24;361(3):241–250. doi: 10.1007/BF00587288. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. J., Düsterhöft S., Dux L., Pette D. Metabolite patterns related to exhaustion, recovery and transformation of chronically stimulated rabbit fast-twitch muscle. Pflugers Arch. 1992 Mar;420(3-4):359–366. doi: 10.1007/BF00374471. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Brown M., Cotter M., Smith M., Vrbová G. The effect of long-term stimulation of fast muscles on their blood flow, metabolism and ability to withstand fatigue. Pflugers Arch. 1977 Jun 8;369(2):141–149. doi: 10.1007/BF00591570. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Dodd L., Renkin E. M., Gray S. D. Early changes in fiber profile and capillary density in long-term stimulated muscles. Am J Physiol. 1982 Oct;243(4):H528–H535. doi: 10.1152/ajpheart.1982.243.4.H528. [DOI] [PubMed] [Google Scholar]
- Kaufmann M., Simoneau J. A., Veerkamp J. H., Pette D. Electrostimulation-induced increases in fatty acid-binding protein and myoglobin in rat fast-twitch muscle and comparison with tissue levels in heart. FEBS Lett. 1989 Mar 13;245(1-2):181–184. doi: 10.1016/0014-5793(89)80217-0. [DOI] [PubMed] [Google Scholar]
- Kernell D., Donselaar Y., Eerbeek O. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. II. Endurance-related properties. J Neurophysiol. 1987 Sep;58(3):614–627. doi: 10.1152/jn.1987.58.3.614. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Maier A., Gambke B., Pette D. Degeneration-regeneration as a mechanism contributing to the fast to slow conversion of chronically stimulated fast-twitch rabbit muscle. Cell Tissue Res. 1986;244(3):635–643. doi: 10.1007/BF00212544. [DOI] [PubMed] [Google Scholar]
- Maier A., Gorza L., Schiaffino S., Pette D. A combined histochemical and immunohistochemical study on the dynamics of fast-to-slow fiber transformation in chronically stimulated rabbit muscle. Cell Tissue Res. 1988 Oct;254(1):59–68. doi: 10.1007/BF00220017. [DOI] [PubMed] [Google Scholar]
- Maier A., Pette D. The time course of glycogen depletion in single fibers of chronically stimulated rabbit fast-twitch muscle. Pflugers Arch. 1987 Apr;408(4):338–342. doi: 10.1007/BF00581126. [DOI] [PubMed] [Google Scholar]
- Nemeth P. M., Pette D., Vrbová G. Comparison of enzyme activities among single muscle fibres within defined motor units. J Physiol. 1981 Feb;311:489–495. doi: 10.1113/jphysiol.1981.sp013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham P. H., Mortimer J. T., Van Der Meulen J. P. Physiologic and metabolic changes in white muscle of cat following induced exercise. Brain Res. 1973 Feb 28;50(2):424–429. doi: 10.1016/0006-8993(73)90745-2. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Reichmann H., Hoppeler H., Mathieu-Costello O., von Bergen F., Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch. 1985 May;404(1):1–9. doi: 10.1007/BF00581484. [DOI] [PubMed] [Google Scholar]
- Reichmann H., Wasl R., Simoneau J. A., Pette D. Enzyme activities of fatty acid oxidation and the respiratory chain in chronically stimulated fast-twitch muscle of the rabbit. Pflugers Arch. 1991 Jul;418(6):572–574. doi: 10.1007/BF00370573. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Simoneau J. A., Kaufmann M., Härtner K. T., Pette D. Relations between chronic stimulation-induced changes in contractile properties and the Ca2+-sequestering system of rat and rabbit fast-twitch muscles. Pflugers Arch. 1989 Sep;414(6):629–633. doi: 10.1007/BF00582127. [DOI] [PubMed] [Google Scholar]
- Simoneau J. A., Pette D. Species-specific effects of chronic nerve stimulation upon tibialis anterior muscle in mouse, rat, guinea pig, and rabbit. Pflugers Arch. 1988 Jul;412(1-2):86–92. doi: 10.1007/BF00583735. [DOI] [PubMed] [Google Scholar]
- Weber F. E., Pette D. Changes in free and bound forms and total amount of hexokinase isozyme II of rat muscle in response to contractile activity. Eur J Biochem. 1990 Jul 20;191(1):85–90. doi: 10.1111/j.1432-1033.1990.tb19096.x. [DOI] [PubMed] [Google Scholar]


