Abstract
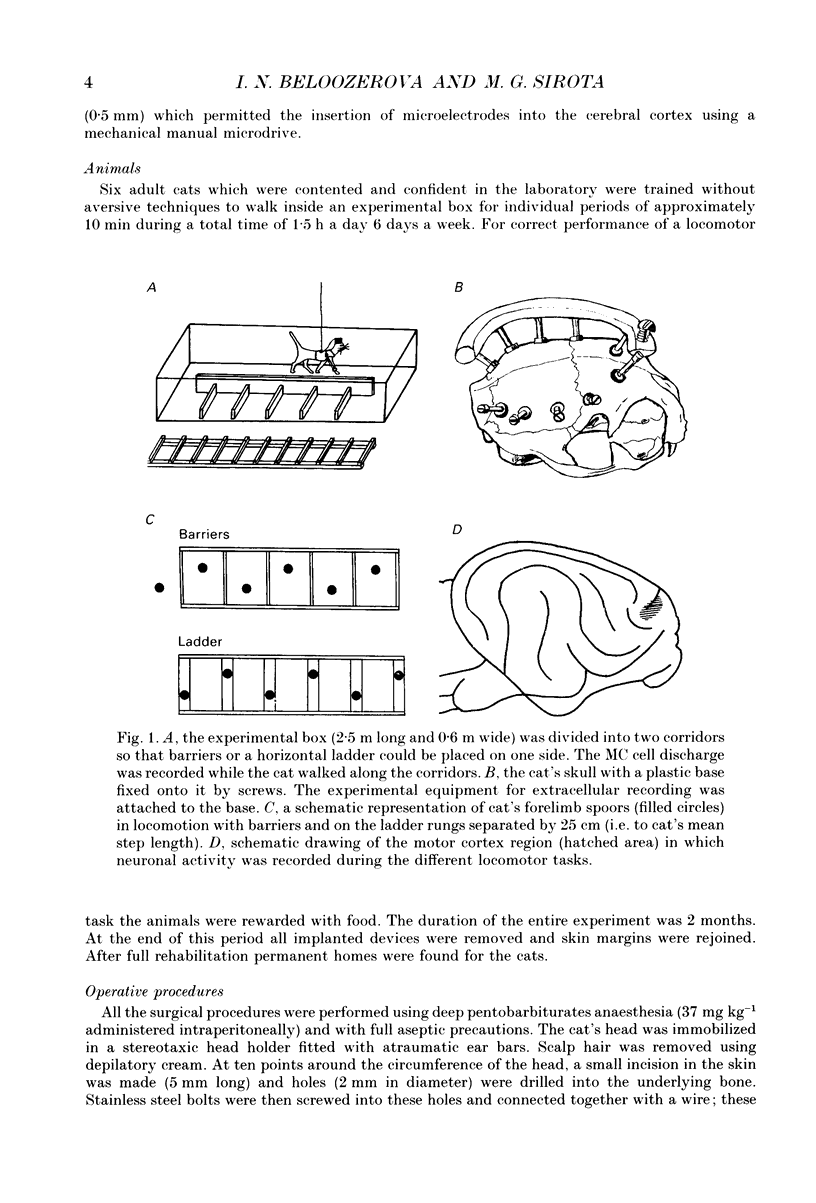
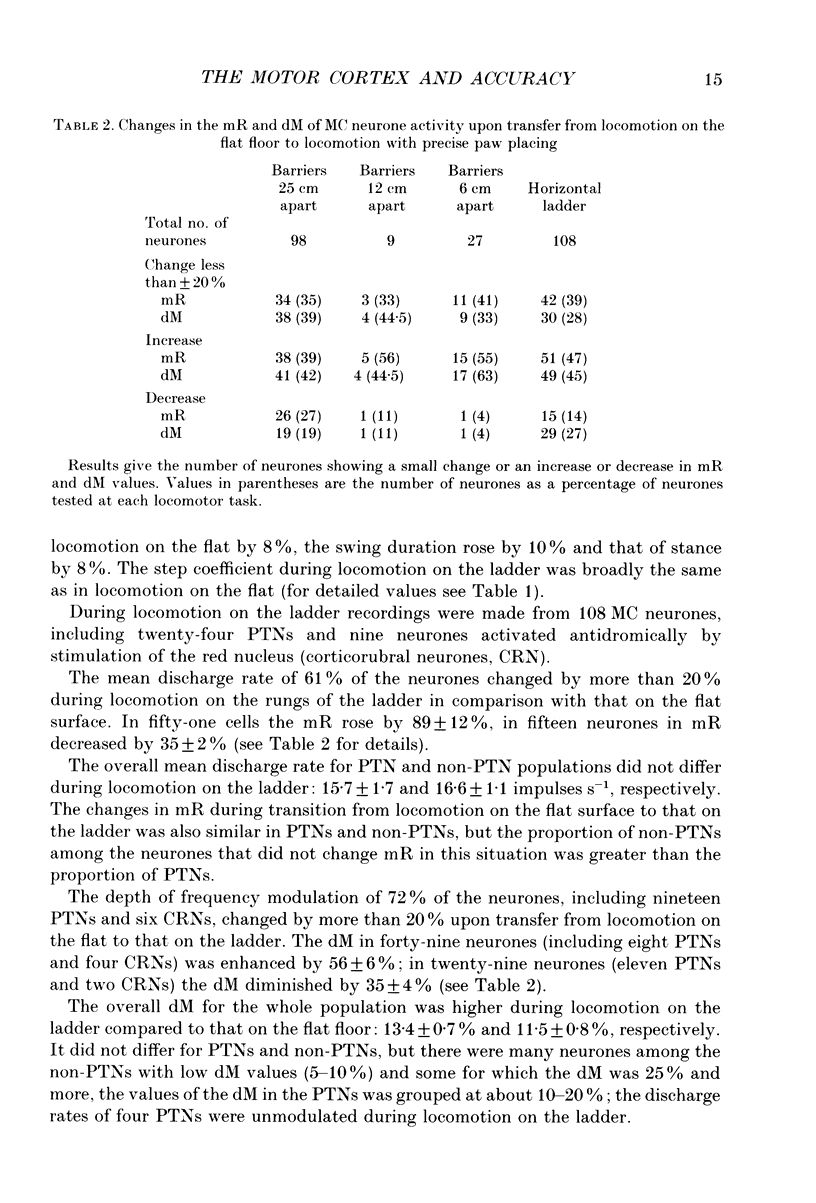
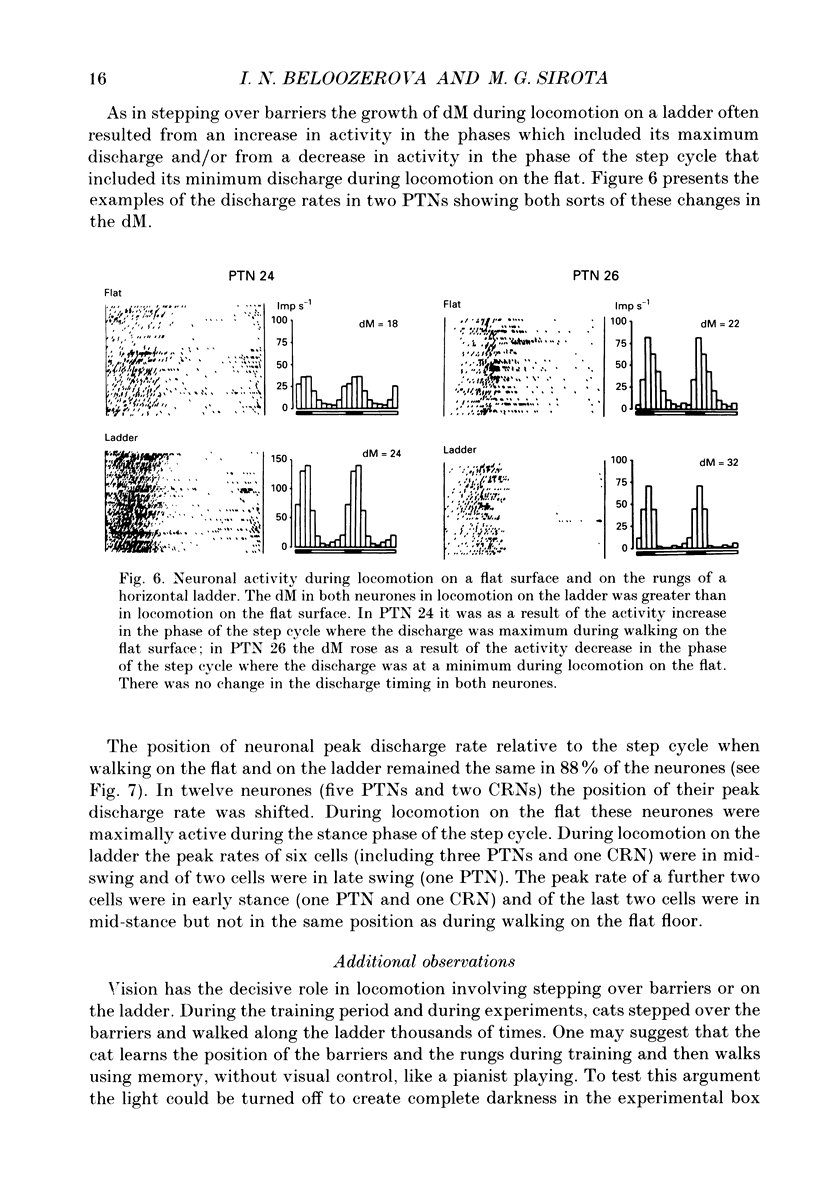
1. The impulse activity of single neurones in the motor cortex (MC) was recorded extracellularly, using movable varnish-insulated tungsten microelectrodes, in six adult, freely moving cats. Neuronal activity was recorded while the cats walked on a flat floor, as they stepped over a series of barriers, and as they walked on the flat rungs of a horizontal ladder. The mean discharge rate (mR) and the depth of frequency modulation (dM) in each cell were estimated over 10-100 steps. 2. The activity of ninety-eight MC cells (Including thirteen pyramidal tract neurones (PTNs)) was recorded during stepping over barriers 25 cm apart. The mR in 66% and the dM in 61% of these cells changed by more than 20% during locomotion with barriers compared to locomotion on the flat (an increase was more often the case). 3. The activity of nine cells was recorded during stepping over barriers 12 cm apart, and the activity of twenty-seven cells (including five PTNs) during walking with barriers only 6 cm apart. The mR in 67% and in 59% of the cells, respectively, and the dM in 56% and in 67% of the cells, respectively, were greater in these locomotor tasks than during locomotion on the flat. 4. The activity of twenty cells was recorded during walking and compared in experiments with different distances between barriers. The mR in 50% and the dM in 75% of the neurones progressively increased when the distance between successive barriers was diminished. 5. The discharge rates of thirteen cells were compared in two different locomotor tasks: (i) when the cat stepped over barriers requiring hyperflexion of the limbs and (ii) when it walked on the flat with loads attached to the distal forelimbs causing a hyperactivity of flexor muscles. The activity of nine cells was different during stepping over the barriers compared to locomotion with loadings on the forelimbs. 6. The activity of 108 cells (twenty-four PTNs) was recorded during walking along a horizontal ladder with flat rungs. The mR of 61% and the dM of 72% of cells changed by more than 20% during locomotion on the ladder compared with that on the flat (most often they increased). 7. The position of the peak rate relative to the step cycle did not differ in the majority of cells (in 78-91% depending on the task) during locomotion on the flat, with the barriers or on the ladder.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins R. J., Cegnar M. R., Rafuse D. D. Differential effects of lesions of the anterior and posterior sigmoid gyri in cats. Brain Res. 1971 Jul 23;30(2):411–414. doi: 10.1016/0006-8993(71)90092-8. [DOI] [PubMed] [Google Scholar]
- Amos A., Armstrong D. M., Marple-Horvat D. E. Changes in the discharge patterns of motor cortical neurones associated with volitional changes in stepping in the cat. Neurosci Lett. 1990 Feb 5;109(1-2):107–112. doi: 10.1016/0304-3940(90)90546-l. [DOI] [PubMed] [Google Scholar]
- Amos A., Armstrong D. M., Marple-Horvat D. E. Responses of motor cortical neurones in the cat to unexpected perturbations of locomotion. Neurosci Lett. 1989 Sep 25;104(1-2):147–151. doi: 10.1016/0304-3940(89)90345-5. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Electromyographic responses evoked in muscles of the forelimb by intracortical stimulation in the cat. J Physiol. 1985 Oct;367:309–326. doi: 10.1113/jphysiol.1985.sp015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Topographical localization in the motor cortex of the cat for somatic afferent responses and evoked movements. J Physiol. 1984 May;350:33–54. doi: 10.1113/jphysiol.1984.sp015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of interpositus and Purkinje cells of the cat cerebellum during locomotion under different conditions. J Physiol. 1988 Jun;400:425–445. doi: 10.1113/jphysiol.1988.sp017130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A., Lidierth M. Complex spikes in Purkinje cells of the paravermal part of the anterior lobe of the cat cerebellum during locomotion. J Physiol. 1988 Jun;400:405–414. doi: 10.1113/jphysiol.1988.sp017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H., Babb R. S., Mori A., Waters R. S. Input-output relationships in cat's motor cortex after pyramidal section. J Neurophysiol. 1981 Sep;46(3):694–703. doi: 10.1152/jn.1981.46.3.694. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Fernandez J., Scheibel M. E., Scheibel A. B. Characteristics of projections from the nucleus ventralis lateralis to the motor cortex in the cats: an anatomical and physiological study. Exp Brain Res. 1974;20(4):315–330. doi: 10.1007/BF00237378. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Tyner C. F., Towe A. L. Observations on single neurons recorded in the sigmoid gyri of awake, nonparalyzed cats. Exp Neurol. 1971 Sep;32(3):388–403. doi: 10.1016/0014-4886(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Beloozerova I. N., Sirota M. G. Aktivnost' neironov motosensornoi kory koshki vo vremia estestvennoi khod'by s pereshagivaniem cherez prepiatstviia. Neirofiziologiia. 1986;18(4):546–549. [PubMed] [Google Scholar]
- Beloozerova I. N., Sirota M. G. Aktivnost' neironov motosensornoi kory vo vremia estestvennoi lokomotsii koshki. Neirofiziologiia. 1985;17(3):406–408. [PubMed] [Google Scholar]
- Beloozerova I. N., Sirota M. G. The role of the motor cortex in the control of vigour of locomotor movements in the cat. J Physiol. 1993 Feb;461:27–46. doi: 10.1113/jphysiol.1993.sp019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS W. W., LIU C. N. Corticospinal tract of the cat: an attempt to correlate the pattern of degeneration with deficits in reflex activity following neocortical lesions. J Comp Neurol. 1957 Aug;108(1):23–55. doi: 10.1002/cne.901080103. [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical cell discharge during voluntary gait modification. Brain Res. 1988 Aug 2;457(1):181–187. doi: 10.1016/0006-8993(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Halbertsma J., Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand. 1980 Mar;108(3):283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand. 1980 Mar;108(3):269–281. doi: 10.1111/j.1748-1716.1980.tb06533.x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A. P., Kettner R. E., Schwartz A. B. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988 Aug;8(8):2928–2937. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979 Jan 15;34(2):241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Palmer C. I., Marks W. B., Bak M. J. The responses of cat motor cortical units to electrical cutaneous stimulation during locomotion and during lifting, falling and landing. Exp Brain Res. 1985;58(1):102–116. doi: 10.1007/BF00238958. [DOI] [PubMed] [Google Scholar]
- Rispal-Padel L., Grangetto A. The cerebello-thalamo-cortical pathway. Topographical investigation at the unitary level in the cat. Exp Brain Res. 1977 May 23;28(1-2):101–123. doi: 10.1007/BF00237089. [DOI] [PubMed] [Google Scholar]
- Tanji J., Evarts E. V. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976 Sep;39(5):1062–1068. doi: 10.1152/jn.1976.39.5.1062. [DOI] [PubMed] [Google Scholar]


