Abstract

Cholangiocarcinoma (CCA) or bile-duct cancer is most prevalent in Southeast Asian counties including Thailand. Patients present at an advanced stage when the cancer is often drug resistant, leading to chemotherapy failure. Curcumin has therapeutic potential with various anticancer properties. However, its effectiveness is limited by its low bioavailability, poor solubility, and instability. This study aimed to synthesize, characterize and evaluate the efficacy of curcumin-loaded maltodextrin-based proniosomes (CMPNs) to overcome the limitations of curcumin for treating gemcitabine-resistant CCA cells (KKU-213BGemR) in vitro and in vivo. Various proniosome formulations were developed and tested for their efficacy against KKU-213BGemR cells using cytotoxicity, clonogenic, migration, and invasion assays. The potential mechanism involving in cell cycle arrest, apoptosis, expression of C/EBP homologous protein (CHOP), a pro-apoptotic transcription factor, and other apoptotic markers were investigated. The results showed that nanoscale CMPNs exhibited a good curcumin loading capacity and an entrapment efficiency of over 97%, as well as good stability and permeability through porcine esophageal mucosa. CMPNs inhibited proliferation, colony formation, migration/invasion and induced apoptosis in KKU-213BGemR cells. Western blot analysis revealed CMPNs significantly increased CHOP, the cleavage products of poly(ADP-ribose) polymerase-1 (PARP-1), apoptosis-inducing factor, and caspase-3 expression in KKU-213BGemR cells. A xenograft model revealed that 62.5 mg/kg BW CMPNs significantly suppressed proliferating cell nuclear antigen and increased CHOP-mediated apoptosis, leading to significantly reduced tumor volume. In conclusion, CMPNs effectively overcome limitations of curcumin and offer an effective strategy against gemcitabine-resistant CCA via CHOP-mediated pathways. These proniosomes are promising as an alternative treatment approach for CCA.
Keywords: bile duct cancer, curcumin, proniosome, drug resistance, nanoparticle, CHOP, apoptosis
1. Introduction
Cholangiocarcinoma (CCA) is a lethal adenocarcinoma of the biliary tract. It is the second most common type of liver cancer and is responsible for approximately 10–15% of all primary liver cancers.1 Globally, CCA has been increasingly common in recent decades with an incidence rate of CCA ranging between 0.3 and 6 cases per 100,000 population per year and a mortality rate of from 1 to 6 cases per 100,000 population per year.1,2 The highest incidence of CCA in the world is in northeastern Thailand, with a reported rate of 85 cases per 100,000 population per year. The liver fluke (Opisthorchis viverrini) is one of the most important risk factors for CCA and is a major public-health problem in Thailand and neighboring countries.1,3 The current treatment strategies for CCA remain insufficient, as surgical resection and medical therapies yield only limited improvements in outcomes.4 Gemcitabine is one of the drugs of choice for chemotherapy of CCA. Patients usually exhibit a good initial response with an improved response rate of 17.5–30%. However, there are no significant gains in survival times, and gemcitabine resistance develops over time.5,6 Therefore, novel alternative treatments and adjuvant therapeutic strategies that focus on drug resistance in CCA are needed.
Curcumin is derived from the rhizomes of turmeric (Curcuma longa) and is known to have antioxidant, anti-inflammatory and anticancer activities. It plays an important role in the prevention and treatment of various diseases such as metabolic syndrome, neurodegenerative diseases, liver diseases, and various cancers.7 Curcumin exerts its therapeutic effects by inhibiting target molecules involved in carcinogenesis by inducing the expression of pro-apoptotic proteins, ultimately leading to cancer cell death in various cancer types,7 including in CCA.8,9 However, the limitations of curcumin are its poor water solubility, poor oral bioavailability, rapid metabolic, rapid systemic excretion, chemical instability, photodegradation and short half-life.10,11 To improve its pharmacological effects, various curcumin formulations have been suggested.12
Nanoparticles, as drug-delivery systems, offer advantages such as increased bioavailability, improved solubility, and sustained delivery.13 Niosomes, formed by self-assembling nonionic surfactants and cholesterol, are promising biodegradable and biocompatible nanocarriers.14 Additionally, proniosomes, a dry powdered form of niosomes, are more stable, minimizing issues such as aggregation, fusion, leaking and provide enhanced convenience in transport and storage.15,16 Niosomes and proniosomes have been studied for delivering various drugs, including curcumin, for treating cancer and infectious diseases. Curcumin-loaded niosomes have shown increased efficacy against breast cancer cells.17 Furthermore, using maltodextrin as a carrier for curcumin enhances oral bioavailability and absorption, leading to higher plasma concentrations compared to native curcumin.18 Previous studies have demonstrated the potential of this delivery system against colorectal cancer.19 Given these promising results, it is worthwhile to evaluate its efficacy against CCA, particularly gemcitabine-resistant CCA.
Endoplasmic reticulum (ER) stress-induced apoptosis plays an important role in the progression and treatment-resistance of various cancers20,21 including CCA.22 Prolonged ER stress can trigger apoptosis through the activation of C/EBP homologous protein (CHOP), a key pro-apoptotic transcription factor in ER stress-mediated cell death.23 CHOP upregulation induces pro-apoptotic genes and suppresses antiapoptotic proteins.24 Poly(ADP-ribose) polymerase (PARP), involved in DNA-repair and cell-death pathways, is cleaved during apoptosis, serving as an indicator of apoptotic cell death.25 The interplay between ER stress, CHOP activation, and PARP cleavage is a potential target area for cancer therapeutics, particularly in addressing chemoresistance in CCA, enhancing the efficacy of existing therapies or providing novel therapeutic approaches.
This study aimed to develop curcumin-loaded maltodextrin-based proniosomes (CMPNs) and to evaluate their therapeutic efficacy against gemcitabine-resistant CCA both in vitro and in vivo. We focused on the ability of CMPNs to induce apoptosis through the CHOP-mediated ER stress pathway and PARP cleavage, aiming to overcome drug resistance and establish use of CMPNs as a promising strategy for effective CCA treatment. The findings from this study could serve as a foundation for preclinical research and potentially translate into clinical applications for CCA therapy.
2. Materials and Methods
2.1. Preparation of CMPNs
Nine formulations of curcumin-loaded maltodextrin-based proniosomes (CMPNs) were prepared using the sonication-assisted slurry method, with maltodextrin as a carrier (Table S1). First, curcumin (>97% w/w, Merck-Schuchardt, Hohenbrunn, Germany) was dissolved in ethanol and then mixed with different ratios of Span 60/cholesterol/Tween 20 (formulation A1-A3; 0.06:0.06:1, B1–B3; 0.3:0.3:1, and C1–C3; 0.02:0.02:1). The mixtures were sonicated for 10–30 min at 55 ± 2 °C in pulses using 20 s sonication with 5 s pause at an amplitude of 50% and then mixed with different concentrations of hydroxypropyl methyl cellulose (0.006–0.024% hydroxypropyl methylcellulose, HPMC) followed by 5 min of sonication. The niosomes self-assembled and were homogeneously mixed with maltodextrin to maintain their presence in the proniosome formulations. The mixture was evaporated overnight at 37 °C. Solubility was detected by weighing 3 mg curcumin and 19 mg CMPNs (equivalent to 3 mg curcumin) dissolved in 3 mL distilled water, and the results were observed.
2.2. Size and Morphology of the CMPNs
CMPNs were dispersed in deionized water. For each formulation, their size distribution, polydispersity index (PDI), and zeta potential were determined using Malvern dynamic light scattering (DLS) Zetasizer (ZEN3600, England) and Nano partica SZ-100 series (HORIBA, Kyoto, Japan). CMPNs in dry and dissolved forms were analyzed for particle size and morphology using an environmental scanning electron microscope E SEM (Quattro S, ThermoFisher Scientific, USA) at 5, 30 kV and 28, 39 pA.
2.3. Evaluation of Drug-Loading Efficiency
The total curcumin content in proniosomes was measured by weighing 0.01 g of CMPNs into a 5 mL volumetric flask, adding 1 mL of polyethylene glycol (PEG), and sonicating the mixture for 1 h. After sonication, 30% ethanol was added and mixed thoroughly using a vortex mixer for 5 min. The absorbance of the solution was then measured at 466 nm using a multiwell spectrophotometer (Thermo Scientific Varioskan, USA). To measure unentrapped curcumin, 0.2 g of CMPNs was mixed with 10 g of PBS (pH 7.4) in an ultracentrifuge tube and sonicated for 30 min. The mixture was centrifuged at 25,000 rpm for 30 min at 20 °C to separate unentrapped curcumin.26 The supernatant was collected and its absorbance was measured at 466 nm. The values were compared with the total curcumin content to determine curcumin loading and entrapment efficiency, calculated using standard eqs 1 and 2.
| 1 |
| 2 |
2.4. Stability Testing
The stability of CMPNs were assessed by examining curcumin concentration, particle size and morphology under a range of conditions. CMPNs were stored at two levels of controlled temperature and relative humidity (RH): 25 ± 2 °C, RH 60 ± 5% and 40 ± 2 °C, RH 75 ± 5%. After 0, 3, and 6 months, curcumin content was determined using a spectrophotometer at 466 nm. Additionally, CMPNs stability were investigated in simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 6.8) at 37 °C for 3 and 6 h. After incubation, particle size, PDI, and morphology were analyzed. Particle size and morphology were characterized using DLS with a Nano partica SZ-100 series and E SEM, respectively.
2.5. Permeation Study
We established a Franz cell diffusion system. CMPNs corresponding to a 1% w/v curcumin solution, or pure curcumin at a concentration of 1% w/v, were dispersed in phosphate-buffered saline (PBS) with a pH 6.8 and supplemented with 0.1% Tween 20 as a dispersant. A volume of 1 mL of the dispersion was added to the donor chamber, while the receptor chambers were filled with 5 mL of PBS at pH 7.4. The solutions were maintained at a constant temperature of 37 ± 1 °C and stirred at a speed of 600 rpm. Freshly prepared porcine esophageal mucosa, sourced from a local slaughterhouse,27 was utilized as the barrier membrane. The esophagus was resected to eliminate the muscle and then excised to obtain esophageal specimens measuring 2 × 2 cm with a thickness of 1.5 mm. These were positioned between the chambers with the epithelial side facing the donor chamber and the endothelial side facing the receptor chamber. At specific time intervals (0.5, 1, 2, 3, 4, 6, 12, 24, and 48 h), we collected the receptor medium to analyze the curcumin content using a spectrophotometer at 466 nm. The study protocol was approved by the Animal Ethics Committee [IACUC-KKU (C)-120/67], adhering to the National Research Council of Thailand’s Ethical Guidelines for Animal Experimentation.
2.6. Molecular Dynamics (MD) Simulations
The initial structures of a Span 60-cholesterol-curcumin complex was sketched using Discovery Studio 2017, considering the hydrogen bond (H-bond) between Span 60 and cholesterol according to a previous review.28 The partial atomic charges of each compound were approximated using the AMBER force field version 2 (GAFF2) with the antechamber module in the AMBER 20 software package. The constructed complexes were then simulated under periodic boundary conditions with the isothermal–isobaric scheme (310 K, 1 atm). The AMBER ff14SB force field and the generalized AMBER force field version 2 (GAFF2) were employed to handle bonded and nonbonded interactions, respectively, with solvents modeled using the TIP3P water model. Water molecules were minimized using 500 steps of steepest descent (SD) followed by 1500 steps of conjugate gradient (CG) methods. Subsequently, the entire complex was minimized using the same procedure. The temperature was set at 310 K using a Langevin thermostat with a collision frequency of 2 ps–1, and the pressure was controlled using a Berendsen barostat. Molecular dynamics simulations were run for 500 ns with a time step of 2 fs. Molecular dynamics outputs were obtained from the cpptraj module, including root-mean square deviation (RMSD) and the number of H-bond (#H-bonds). All MD simulations were carried out using the AMBER 20 software package.
2.7. Fourier Transform Infrared Spectrometer (FT-IR)
Proniosome powders were assayed using FT-IR spectra with a Bruker Tensor 27 benchtop spectrometer (Bruker Optik GmbH, Ettlingen, Germany). Spectra were recorded over a wavenumber range of 400–4000 cm–1.
2.8. Cell Lines
This study was approved by Khon Kaen University Human Research Ethics Committee ICH (HE651479) based on the Declaration of Helsinki and Good Clinical Practice Guidelines. A CCA cell line (KKU-213B), derived from a CCA patient was established by Sripa et al.29 The gemcitabine-resistant CCA cell line (KKU-213BGemR) was developed by stepwise dose-escalation with gemcitabine as previous described.9 KKU-213BGemR was grown in Dulbecco’s modified Eagle’s medium, DMEM (Gibco, NY, USA) with the addition of 10% fetal bovine serum (Corning, NY, USA), and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco). KKU-213BGemR was cultured in a humidified incubator at 37 °C and 5% CO2.
2.9. Drug Uptake
Cellular uptake by KKU-213BGemR cells of green fluorescent protein-loaded niosomes (GFP-NI) was evaluated. Cells were seeded on sterile coverslips in 24-well plates at a density of 1 × 104 cells per well. After removing the culture medium and washing twice with PBS, cells were incubated with 0.1 μM of either free GFP (Abcam plc, Cambridge, UK) or GFP-NI for 12 h. For the endocytosis inhibition study, cells were pretreated with 10 μg/mL cytochalasin D, CyD (Sigma-Aldrich, Saint Louis, USA) for 30 min before GFP-NI treatment for 12 h. Following incubation, cells were washed twice with PBS, stained with Hoechst 33,342 (Invitrogen, CA, USA) for 15 min, and fixed with 4% paraformaldehyde. Coverslips were mounted using mounting medium and images captured using a fluorescence microscope (ECLIPSE Ni-U, Nikon Instruments Inc., Japan) at 100× magnification. Fluorescence intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.10. Measurement of Cell Proliferation Using the MTT Assay
The effect of CMPNs on the proliferation of gemcitabine-resistant CCA cells was assessed using the MTT assay (Invitrogen). KKU-213BGemR cells were seeded at a density of 3000 cells per well in flat-bottomed 96-well plates (Corning). The following day, cells were incubated with various concentrations of curcumin, 11.50, 23.00, 34.48, 46.00, 57.50, 75.01, and 80.50 μg/mL blank maltodextrin-based proniosomes (BMPNs) or CMPNs (equivalent to 5, 10, 15, 20, 25, 30, and 35 μM curcumin, respectively) for 24–72 h in a humidified incubator. Then, 10 μL of MTT reagent was added to achieve a final concentration of 0.5 mg/mL. The plates were then placed in a humidified incubator for a 2 h incubation period. The insoluble formazan crystals were dissolved using DMSO and the resulting colored solution was quantified by measuring the absorbance at 540 nm using a spectrophotometer.
2.11. Clonogenic Assay
The clonogenic assay assesses cell survival by measuring colony formation. In this study, KKU-213BGemR cells were initially seeded at a density of 1000 cells per well in 6-well plates (Corning). The next day, cells were treated with 10 or 15 μM curcumin, 23.00 or 34.48 μg/mL BMPNs or CMPNs and incubated for 24 h in a humidified incubator. Subsequently, the cells were transferred to normal growth medium, allowing them to form colonies over a span of 12 days. Then, the colonies were fixed with a 4% paraformaldehyde solution and stained with crystal violet. Finally, the colonies were dissolved with 33% acetic acid, and their absorbance was measured at 620 nm using a spectrophotometer.
2.12. Apoptosis
KKU-213BGemR cells were plated in 6-well Corning plates at a density of 2 × 105 cells per well and left to grow in culture medium for 24 h. The cells were then exposed to either 30 μM curcumin, 75.01 μg/mL BMPNs or CMPNs for a 24 h period. Following treatment, both the supernatant and cells were harvested. After washing with cold 1× PBS, the samples underwent a dual staining procedure using Annexin V-FITC and propidium iodide (PI) from BioLegend (San Diego, CA, USA), following the protocol provided by the manufacturer. A BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA) was used to quantify apoptotic cells. The data were analyzed using BD FACS Diva software (BD Biosciences).
2.13. Cell Cycle
KKU-213BGemR cells were cultured in 6-well plates at 2 × 105 cells per well for 24 h. The cells were subsequently exposed to 30 μM curcumin, 75.01 μg/mL BMPNs or CMPNs for an additional 24 h. After treatment, cells were harvested, washed with cold 1X PBS, and fixed overnight in 70% ethanol at −80 °C. Following fixation, the cells were centrifuged and rinsed again with cold 1× PBS. The cell samples were then stained using FxCycle PI/Rnase solution (Molecular Probes, Life Technologies, CA, USA) as per the manufacturer’s protocol. The stained cell samples were analyzed using a BD FACSCantoII flow cytometer, and the resulting data were processed with BD FACS Diva software (BD Biosciences).
2.14. Cell Wound-Healing Assay
The gemcitabine-resistant CCA cell line (KKU-213BGemR) was seeded (8 × 104 cells per well) in 42-well plates (Corning). When the cells reached approximately 80% confluence, the plates were scratched using a sterile pipet tip and washed with PBS to remove cell debris. The wound-healing area was immediately photographed using an inverted fluorescence microscope at 4× magnification. Then, cells were incubated with 34.48 μg/mL BMPNs or different concentrations of CMPNs (11.50, 23.00, and 34.48 μg/mL). The wound-healing area was observed at 18 and 24 h after treatment and the closure rate evaluated.
2.15. Transwell Invasion Assay
The Transwell insert membrane (24 wells, Corning) was coated overnight with 400 μg/mL Matrigel-coating solution. The following day, 2 × 104 KKU-213BGemR cells per well were seeded in 200 μL of serum-free medium in the upper chamber, and 700 μL growth medium was added to the lower chamber and cultured for 24 h. Then, the cells were treated with 34.48 μg/mL BMPNs or 11.50, 23.00, and 34.48 μg/mL in serum-free medium for 48 h. Cells were then fixed in methanol solution and stained with crystal violet. The invasion results were photographed using an imaging microscope at 4× magnification and quantified using ImageJ (National Institutes of Health).
2.16. Western Blot
CCA cells and tumor tissue were homogenized using 1× RIPA reagent (Cell Signaling Technology, Danvers, MA, USA). Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc.). SDS-PAGE was used to separate 25 μg of total protein lysates, which were then transferred to PVDF membranes (Cytiva, Dreieich, Germany). The membranes were blocked for 1 min with BlockPRO protein-free blocking buffer (Energenesis Biomedical Co., Ltd., Taipei, Taiwan). Primary antibodies (1:1000 dilution) were applied overnight at 4 °C. These included anti-CHOP (A0221), sourced from Abclonal (Wuhan, China). Anti-PARP/Cleaved-PARP (9542) and anti-AIF (5318), sourced from Cell Signaling Technology (Danvers, MA, USA), anti-Bcl-2 (ab7973), sourced from Abcam plc and anticytochrome c (sc-7159) and anti-GAPDH (sc-25778) from Santa Cruz Biotechnology in Dallas, TX, USA. HRP-conjugated goat antirabbit IgG (1:2000) (Jackson Immuno Research Inc., West Grove, PA, USA) was used as the secondary antibody. Protein bands were visualized using an enhanced chemiluminescence system (EMD Millipore Corporation, MA, USA). Band intensity was quantified using NIH ImageJ software and normalized to GAPDH levels.
2.17. Immunofluorescence Assay (IFA)
KKU-213BGemR cells were plated on sterile cover slides in 24-well plates at a density of 2.5 × 104 cells per well. The cells were then exposed to 30 μM curcumin, 75.01 μg/mL BMPNs or CMPNs for 24 h. Following treatment, cells were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100 (Amresco, Solon, OH, USA). The samples were then blocked with PBS containing 5% BSA and 0.1% Triton X-100. Primary antibodies against CHOP (1:200; A0221, Abclonal) and Cleaved-PARP (1:200; 5625S, Cell Signaling Technology) were applied overnight. The cells were then incubated with ABflo 594-conjugated Goat anti-Rabbit IgG (H + L) (1:150; AS039, Abclonal) or FITC-conjugated Goat anti-Mouse IgG (H + L) (1:150; AS001, Abclonal). To visualize plasma membranes, CellMask Deep Red (1:200; C10046, Invitrogen) was used, while Hoechst 33,342 (Invitrogen) was employed for nuclear staining. Using a fluorescence imaging microscope (ECLIPSE Ni-U, Nikon Instruments Inc.), 10 fields per experimental group were imaged at 100× magnification. Fluorescence intensity was quantified using ImageJ software 30 (National Institutes of Health, Bethesda).
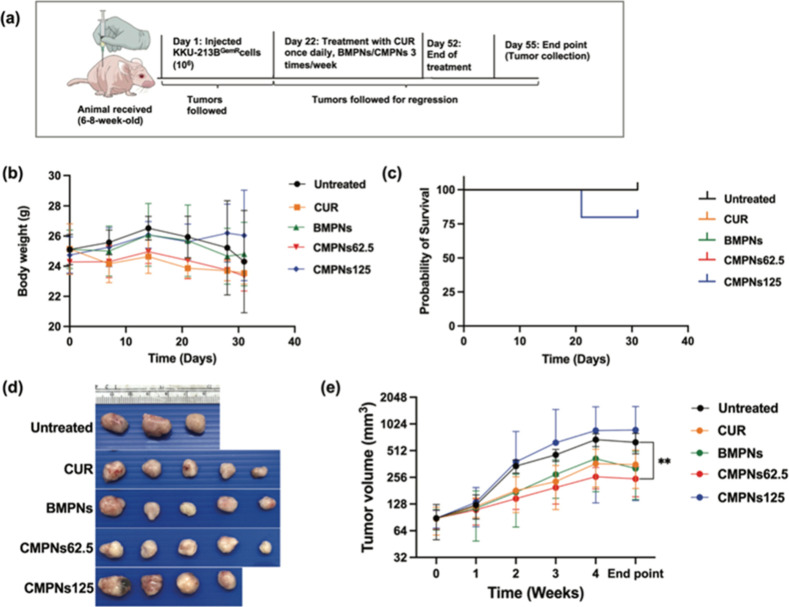
2.18. Animal Study
The study protocol was approved by the Animal Ethics Committee (IACUC-KKU-65/65), adhering to the National Research Council of Thailand’s ethical guidelines for Animal Experimentation. Female BALB/cAJcl-nu mice, aged 6–8 weeks, were injected subcutaneously with KKU-213BGemR cells (1 × 106 cells each) mixed with Matrigel in a 1:1 ratio. Tumors were allowed to grow for 21 days until reaching a minimum size of 100 mm3. Treatment began on day 22 postinjection, with dosages based on a previous study.30 The treatment groups were: daily oral gavage of 20 mg/kg BW curcumin, or thrice-weekly oral gavage of 125 mg/kg BW BMPNs, 62.5 mg/kg BW CMPNs (equivalent to 10 mg curcumin), and 125 mg/kg BW CMPNs (equivalent to 20 mg/kg of curcumin). Tumor dimensions were measured weekly using calipers. After 4 weeks of treatment, mice were euthanized and tumors were harvested for immunohistochemical analysis.
2.19. Immunohistochemistry
Tumor tissues in paraffin wax were cut into 4 μm thick sections, which were then placed on specially coated slides. The paraffin-embedded sections were deparaffinized and then rehydrated. Antigen retrieval was achieved by autoclaving the samples at 110 °C for 10 min in 1× (10 mM) citrate buffer. To block endogenous peroxidase activity, a 3% H2O2 solution in methanol was applied and incubated at 25 °C for 15 min. Slides were treated with 5% fetal bovine serum in PBS for 30 min to prevent nonspecific binding. Rabbit anti-PCNA antibodies (diluted 1:500, AB2426, Abcam, Cambridge UK) or rabbit anti-CHOP antibodies (1:100; A0221, Abclonal) were then applied to the slides as primary antibodies and incubated overnight. The slides were then incubated with a 1:1000 dilution of horseradish peroxidase (HRP)-labeled goat antirabbit IgG for 1 h at 25 °C. The presence of immunoreactivity was visualized with 3,3-diaminobenzidine, and counterstaining was performed with Mayer’s hematoxylin. Sections were scanned at 10× and 20× magnification using the NanoZoomer S360 Digital Slide Scanner (Hamamatsu, Japan). Proliferating (PCNA/CHOP-positive) cells were counted from three different individuals using imageJ in 10 random areas/each tumor (N = 3, magnified 20×).
2.20. Statistical Analysis
Data were expressed as mean ± SD and analyzed using GraphPad Prism 9.0. The Kruskal–Wallis test with Dunn’s posthoc test was used for nonparametric comparisons, while one-way ANOVA with Tukey’s HSD test was employed for parametric comparisons. Student’s t test was used to compare two groups. Significance was set at 0.05 for all comparisons.
3. Results and Discussion
3.1. Characterization of CMPNs
The main components of the proniosome structure are nonionic surfactants and cholesterol, which are similar to cell membranes. Proniosomes transform into a niosomal suspension upon addition of water and can be prepared as dry powders or gels.16,28 Proniosomes loaded with curcumin were developed using sonication-assisted slurry method. We prepared nine formulations and evaluated the properties of CMPNs to determine the optimal formulation for the treatment of CCA (Table S1). The different ratios of Span 60/cholesterol/Tween 20, including A: 0.06:0.06:1, B: 0.3:0.3:1, and C: 0.02:0.02:1 were selected based on previous study.31 All formulations resulted in a yellow powder with good water solubility, especially formulation B, which had the best solubility. In addition, formulation B had a softer texture than A and C. Despite these differences, all formulations contained only 5% curcumin. Particles of formulation B were slightly larger than A and C, with sizes ranging from 367 to 617, 170 to 235, and 142 to 752 nm, respectively. The polydispersity index (PDI) for formulations A, B, and C was <0.454, <0.740, and <0.538, respectively. Consistent with a previous study using the probe sonication method, the particle sizes ranged from 165 to 893 nm, and the PDI values ranged from 0.333 to 0.725.32 However, the zeta potential values of formulation B were more negative. The zeta potential of vesicles varied based on the amount of curcumin loaded. As the quantity of curcumin increased, a trend toward more negative zeta potential values was observed.33 Additionally, drug-encapsulating niosomes were slightly larger in size compared to empty niosomes.34 We aimed to maximize the curcumin content. Thus, formulation B seemed to have the most potential to encapsulate curcumin. Prolonging the probe sonication time was effective in decreasing and stabilizing the dimensions of niosomes.35 Subsequently, we successfully developed CMPNs, using formulation B3, in the form of a soft yellow powder (Figure S1a). This was achieved by increasing the curcumin content and reducing the particle size through a 30 min sonication process. This formulation was used in all subsequent experiments. We found that CMPNs exhibited better water solubility than native curcumin, which tended to precipitate upon mixing (Figure S1b,c). Formulation B3 achieved an impressive entrapment efficiency (EE) of 97.68% ± 0.11%, particle sizes 170.7 ± 14.5 nm, a PDI of 0.170 ± 0.032 and a curcumin loading of 15.66 ± 0.95%. It has been well documented that EE can vary depending on several factors, such as the type of carrier system, preparation methods, and physicochemical properties of the encapsulated drug.36 In this study, the optimized CMPNs formulation achieved a high entrapment efficiency (97.68%) through the synergistic combination of carefully selected components. The mixture of Span 60 and cholesterol forms rigid, stable bilayers that effectively prevent drug leakage, while Tween 20 provides additional stability.37 The high phase transition temperature of Span 60 contributes to forming less permeable vesicles, and cholesterol enhances membrane rigidity by filling void spaces in the bilayer structure.38,39 The sonication method employed ensures uniform size distribution, improving drug delivery efficiency. Advantages include increased entrapment efficiency, enhanced zeta potential, and better stability of the formulation.40 These findings are consistent with a previous study reporting niosomes with high EE (96–99%).32 Notably, these combined factors contribute to a significantly higher EE % in CMPNs formulations compared to conventional carriers like liposomes, some liposomal formulations report EE% between 73–84%,41 highlighting that entrapment efficiency depends on multiple formulation parameters rather than carrier type alone.
3.2. Structural Dynamics and FTIR Spectrum Properties of CMPNs
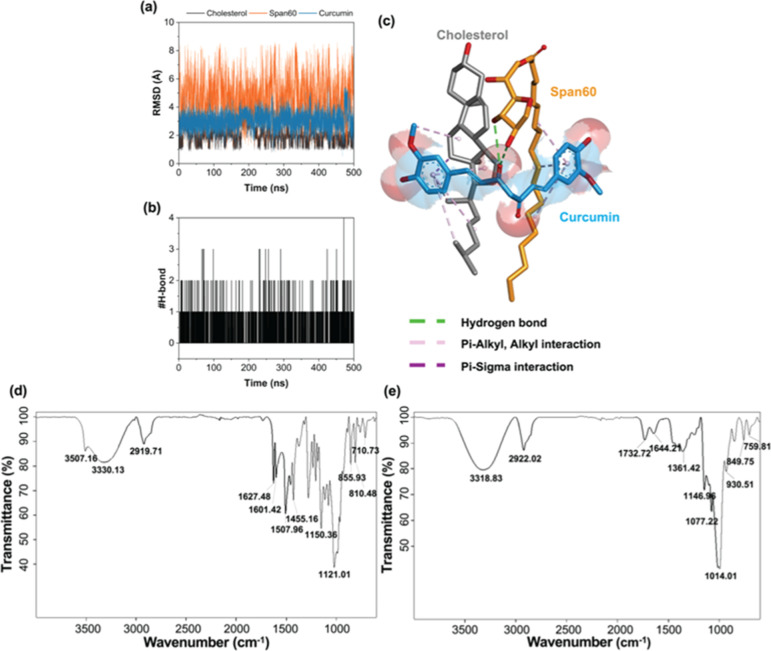
To elucidate the binding mode of curcumin in complex with Span 60, and cholesterol, MD simulation was performed. The results revealed that the RMSD values of Span 60 (∼2–8 Å) were higher than those of curcumin (∼2–4 Å) and cholesterol (∼1–2 Å) due to its long hydrophobic tail. However, the stability of these three ligands was high in an aqueous environment throughout the simulation period (RMSD of ∼1–8 Å) (Figure 1a). Consistent with this, the #H-bonds in the complex were stable at approximately 1–2 bonds (Figure 1b). The final MD snapshot of the simulated Span 60-cholesterol–curcumin complex revealed that two aromatic rings of curcumin formed several pi-alkyl, alkyl (pink), and pi-sigma (purple) interactions with the hydrophobic moieties of the Span 60 and the cholesterol, whereas hydrogen bond (green) was found between the carbonyl group of curcumin and the hydroxyl groups of Span 60 (Figure 1c). These findings suggested that the main interactions between curcumin and Span 60/cholesterol were hydrophobic forces.
Figure 1.
Structural dynamics and FT-IR spectra of CMPNs and BMPNs. (a) Time evolution of (a) RMSD and (b) #H-bonds of curcumin in complex with Span 60 and cholesterol. (c) Final MD snapshot of Span 60-cholesterol-curcumin complex. FT-IR spectrum of (d) CMPNs and (e) BMPNs.
The FTIR spectra of CMPNs and BMPNs were analyzed to identify the presence of specific functional groups and to confirm the successful incorporation of curcumin into the proniosome formulation. In the CMPNs spectrum (Figure 1d), a broad absorption peak at 3507.16 cm–1 can be attributed to the stretching vibrations of the phenolic OH groups present in curcumin. The absorption peaks at 1601.42 and 1507.96 cm–1 are indicative of the symmetric stretching vibrations of the aromatic ring (C=Cring) and the carbonyl group (C=O) group, respectively. The peak at 1627.48 cm–1 corresponds to the C=C and the C=O moieties in curcumin.42,43 These peaks are absent in the BMPNs spectrum (Figure 1e), confirming the absence of curcumin in the blank sample. The peaks observed in the range of 1279.18–1021.01 cm–1 are likely due to the enol (C–O) stretching vibrations and the C–O–C groups present in curcumin. Finally, the absorption peaks in the range of 963.98–710.73 cm–1 can be attributed to the CH vibration of the aromatic rings of curcumin.42,43 The other peaks observed in the CMPNs spectrum are likely associated with the functional groups present in Span 60, cholesterol, and maltodextrin, which are the other components of the proniosome formulation. This result provides strong evidence for the successful incorporation of curcumin into the maltodextrin-based proniosome system.
3.3. Morphology of CMPNs
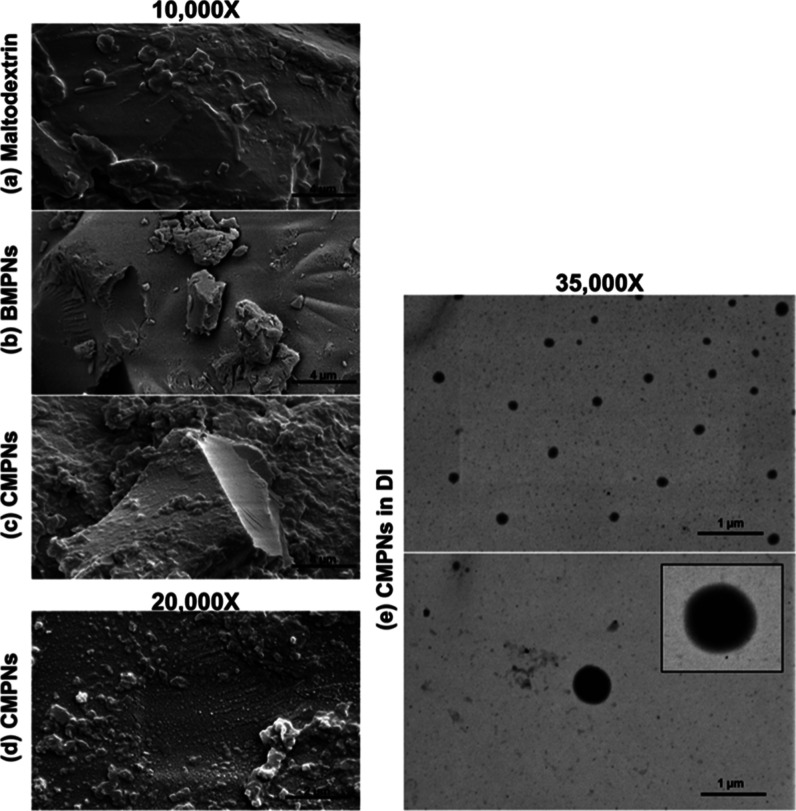
The E SEM photos reflect the surface morphology of CMPNs or BMPNs in powder form and its carrier maltodextrin. The significant difference between the CMPNs surface and BMPNs surface of maltodextrin can be clearly observed. BMPNs and maltodextrin showed a smooth surface morphology (Figure 2a,b) while the CMPNs coating had a granular surface (Figure 2c,d). The topography seen when maltodextrin was used as a carrier has been previously described.44 In addition, CMPNs after dispersal in deionized water, appear as homogeneous and spherical vesicles ranging from 104 to 644 nm, average size 215 nm (Figure 2e). The obtained results are similar to the DLS results.
Figure 2.
E SEM images of CMPNs before and after being dissolved in water. (a) Maltodextrin powder, (b) BMPNs powder, (c,d) CMPNs powder, (e) CMPNs dissolved in DI water.
3.4. Stability and Permeation Ability of CMPNs
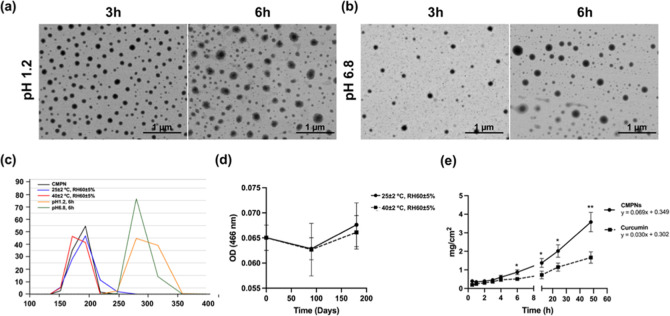
To further assess physical stability, CMPNs formulations were exposed to simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 6.8) at 37 °C. Upon exposure to acidic conditions, which is the first physiological environment encountered after oral administration, the nanoparticles exhibited dynamic changes in their physical characteristics. E SEM results were consistent with DLS findings, showing that the particle size of CMPNs remained relatively stable after 3 and 6 h incubation under acidic conditions (Figure 3a,b). Notably, the initial increase in particle size to 228.8 ± 10.0 nm with a PDI ranging 0.277 ± 0.109 during the first 3 h of acid exposure (pH 1.2) could be attributed to particle swelling due to the protonation of surface groups in acidic media, a phenomenon commonly observed in niosomal systems.45,46 A slight decrease in particle size to 212.8 ± 10.5 nm at 6 h incubation was observed along with an increase with a PDI ranging 0.441 ± 0.143. In simulated intestinal fluid (pH 6.8), the particle size and PDI exhibited similar stability patterns. After 3 h, the particle size was 212.7 ± 10.4 nm, with a PDI ranging 0.472 ± 0.077 while the particle size was slightly increased to 219.2 ± 9.1 nm with a PDI ranging 0.442 ± 0.027 at 6 h incubation (Figure 3c). These findings suggest that CMPNs formulations are stable in both gastric and intestinal conditions. Notably, these particle sizes remain within the optimal range (<500 nm) for uptake by the intestine to reach the systemic circulation.47 E SEM observations confirmed the maintained particle morphology but with a wider size distribution (100–300 nm) after prolonged acid exposure, suggesting that while some structural changes occur, the basic niosome architecture remains intact. These stability characteristics indicate that CMPNs could effectively protect its curcumin payload during typical gastric transit time. The combination of excellent storage stability and moderate acid resistance highlights CMPNs as a promising nanocarrier platform for oral curcumin delivery. However, the observed increase in PDI values following prolonged exposure to simulated gastric fluid necessitates the development of additional protective strategies to enhance stability during gastrointestinal transit and to optimize therapeutic efficacy. Additionally, the long-term stability of CMPNs were evaluated after storage at 25 ± 2 °C, RH 60 ± 5% and 40 ± 2 °C, RH 75 ± 5% for 6 months. The results showed that the curcumin content of CMPNs did not change significantly during storage under either condition (Figure 3d). Moreover, the particle size and PDI of CMPNs also remained stable, with a size of 163.1 ± 16.5 nm and PDI of 0.251 ± 0.124 at 25 ± 2 °C, RH 60 ± 5% after 6 months, and a size of 161.57 ± 5.59 nm and PDI of 0.287 ± 0.057 at 40 ± 2 °C, RH 75 ± 5% after 6 months. Furthermore, the dilution stability of CMPNs demonstrated good solubility in deionized water after 6 months under both conditions (Figure S1d,e). These results indicate that CMPNs exhibits excellent long-term stability.
Figure 3.
Stability and permeability analysis of CMPNs. E SEM images showing morphology and size at (a) pH 1.2 and (b) pH6.8 after 3 and 6 h incubation. (c) Particle size under different conditions using DLS. (d) The curcumin content at OD 466 nm of CMPNs after storage periods of 0, 3, and 6 months at 25 ± 2 °C, RH 60 ± 5% (●) and 60 ± 2 °C, RH 75 ± 5% (■). (e) The permeation time of curcumin in CMPNs or curcumin suspension. Cumulative permeation of curcumin across the barrier membrane using the Franz cell diffusion method: CMPNs (equivalent to 1% curcumin, ●) or 1% curcumin (■) in PBS (pH 6.8) as donor; PBS at pH 7.4 as receptor medium; controlled at 37 ± 1 °C, stirring speed 600 rpm. All data are expressed as mean ± SD from three replicates experiments; *p < 0.05 and **p < 0.01.
The porcine esophagus was employed as a model for mucosal permeation due to its structural similarities to the human oral mucosa.27 Curcumin, whether in CMPNs or in the native form, exhibited consistent linear patterns of penetration through the esophageal mucosa of pigs over time, with correlation coefficients greater than 0.99 and 0.98 for CMPNs and curcumin, respectively. The amount of CMPNs that permeated through the barrier membrane was significantly greater than native curcumin at 6, 12, 24, and 48 h (Figure 3e). The transdermal flux of CMPNs (0.069 mg/cm2/h) at steady state was 2.3 times that of curcumin suspension (0.030 mg/cm2/h). The results suggest that niosomes can enhance transmucosal drug delivery of curcumin.
Our CMPNs formula used a well-proportioned combination of nonionic surfactants, specifically Span 60 and Tween 20, known for their stability, biocompatibility, and reduced toxicity. In addition, cholesterol increases the stiffness of vesicles and stabilizes niosomes, protecting them from the disruptive effects of plasma and serum components.14,48 Maltodextrin is the coating carrier, which is safe and has excellent aqueous solubility and is easy to reconstitute.49 These results suggest that the limitations of administering native curcumin can be overcome by employing the proniosome technology.
3.5. CMPNs Enhance Drug Uptake in KKU-213BGemR Cells via Endocytosis
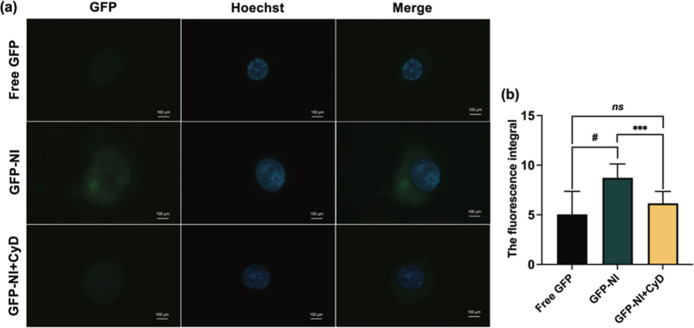
The cellular uptake of CMPNs was investigated using fluorescently labeled GFP-niosomes (GFP-NI) in KKU-213BGemR cells, with cytochalasin D (CyD) employed as an endocytosis inhibitor.50 CyD, which disrupts the actin cytoskeleton and cellular internalization mechanisms, enables precise examination of endocytic pathways.51 Our results demonstrated significantly enhanced cellular uptake of GFP-NI compared to free GFP (p < 0.0001) and GFP-NI + CyD treatments (p < 0.001, Figure 4a,b). Niosome accumulation was observed in both cytoplasm and nucleus, indicating efficient cellular penetration. However, further investigation using additional endocytic inhibitors that can comprehensively block multiple pathways is recommended to conclusively determine the precise cellular entry mechanism.50 Typically, the cellular uptake of nanoparticles is a complex process that involves a variety of mechanisms including endocytosis, phagocytosis, and pinocytosis.52 The proniosomal formulation significantly enhanced curcumin uptake, suggesting a promising strategy for improved drug delivery. The release mechanism of drugs from niosomes typically occurs through multiple pathways, including membrane fusion, endocytosis, and passive diffusion.53 While specific release mechanisms for CMPNs-encapsulated curcumin were not investigated in this study, understanding these pathways is crucial for optimizing drug delivery. Future studies should focus on examining release kinetics under various physiological conditions, conducting endocytosis inhibition studies, and employing real-time imaging techniques to track intracellular drug release. Additionally, further studies investigating the impact of pH and temperature on drug release patterns would enhance our understanding of the system’s behavior under physiological conditions.
Figure 4.
Cellular uptake of CMPNs in KKU-213BGemR cells. (a) Representative fluorescence microscopy images showing cellular uptake of free GFP, GFP-labeled niosomes (GFP-NI), and GFP-NI with cytochalasin D (CyD) treatment. (b) Quantification of cellular fluorescence intensity. Data represent mean ± SD from three independent experiments; ns = not significant, ***p < 0.001, #p < 0.0001. CyD was used as an endocytosis inhibitor to confirm the uptake mechanism.
3.6. Anti-CCA Efficacy of CMPNs on KKU-213BGemR
Anti-CCA activity in gemcitabine-resistant CCA cells (KKU-213BGemR) was investigated using MTT and colony-formation assays. CMPNs and native curcumin inhibited cell proliferation in KKU-213BGemR cells in a dose- and time-dependent manner, whereas BMPNs failed to inhibit cell proliferation (Figure 5a). Accordingly, 23.00 and 34.48 μg/mL (equivalent to 10 and 15 μM curcumin, respectively) of CMPNs or 10 and 15 μM of curcumin significantly suppressed colony formation compared with controls (p < 0.0001), while the BMPNs treatment was not significantly different from controls (Figure 5b,c). These results suggest that CMPNs exhibit antiproliferative effects in alignment with the properties of native curcumin itself.
Figure 5.
CMPNs treatment inhibited cell proliferation and colony formation in the gemcitabine-resistant CCA cell line (KKU-213BGemR). (a) Curcumin (CUR), BMPNs, CMPNs versus KKU-213BGemR using the MTT assay. Cell proliferation was determined by comparison with untreated controls (0 μM) in each case, and the results are expressed as a percentage of relative cell viability. (b,c) Examples of the colony formation assays of the CCA cell line treated with curcumin, BMPNs or CMPNs. All data are expressed as mean ± SD of three replicate experiments; *p < 0.05 and #p < 0.0001 compared to the untreated group.
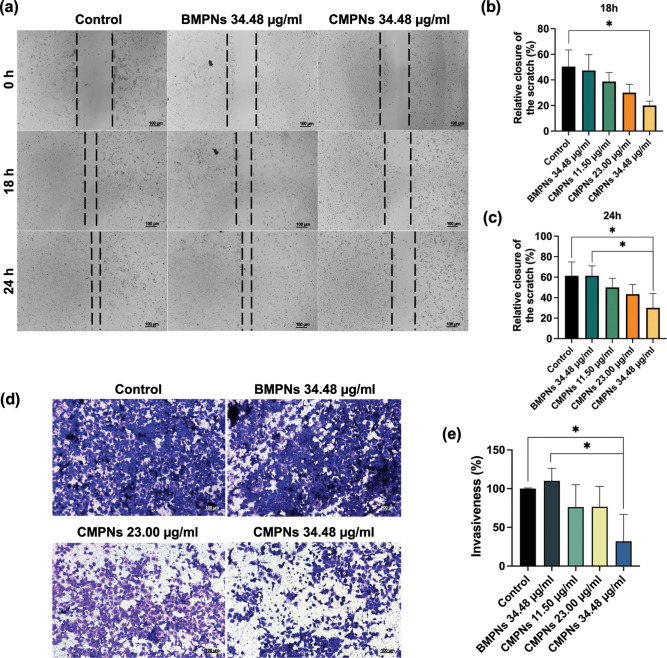
Figure 6a–e demonstrate that the wound space (between the black dashed lines) in the control or BMPNs group visible at 0 h had narrowed by 18 and 24 h. Consistent with this, high values for cell invasiveness were observed in the cell invasion study. In contrast, the CMPNs-treated group (34.48 μg/mL, corresponding to 15 μM curcumin) showed significantly lower cell migration and invasiveness than the control or BMPNs groups (p < 0.05).
Figure 6.
Inhibition of migration and invasion of KKU-213BGemR cells by CMPNs. (a–c) Wound-healing assay and (d,e) Transwell invasion assay, treatment with 34.48 μg/mL BMPNs and 11.50, 23.00, and 34.48 μg/mL CMPNs (equivalent to 5, 10, and 15 μM of curcumin, respectively). Average number of migrating and invading cells plotted as a bar graph with mean ± SD from three replicate experiments; *p < 0.05 compared with the untreated group.
Our in vitro studies show that CMPNs exhibit the same anticancer effects as curcumin in a drug-resistant cell line, including inhibition of cell proliferation, colony-forming ability, and migration and invasion properties. This result is consistent with previous reports on the anticancer effects of curcumin in CCA cells, including drug-resistant CCA cells.8,54,55 Notably, recent advances in curcumin delivery systems, such as albumin-mediated supramolecular prodrug approaches, have demonstrated enhanced therapeutic efficacy through improved targeting and controlled release mechanisms.56 CMPNs and curcumin exhibited the similar effect on cell proliferation in vitro study due to direct exposure to the cells; however, the distinctive feature of CMPNs is their superior properties compared to native curcumin. In particular, the improvement in solubility, stability and permeability properties may be crucial for an effective drug-delivery system and lead to increased treatment efficacy in vivo study.
3.7. CMPNs Induce Cell Apoptosis and G2/M Phase Arrest in Gemcitabine-Resistant CCA Cells (KKU-213BGemR)
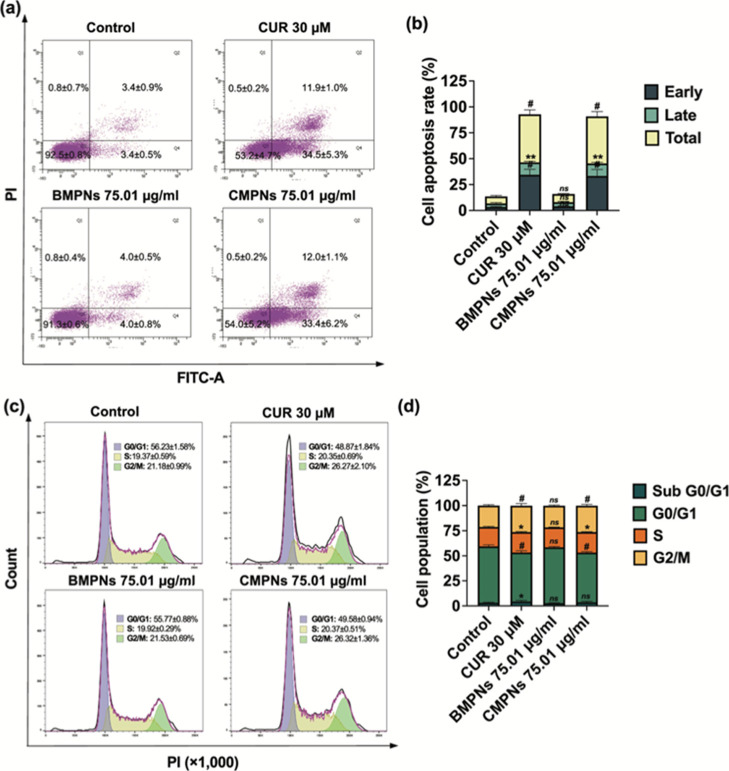
Analysis of apoptosis in KKU-213BGemR cells showed significant increases in both early and late apoptotic stages for curcumin and CMPNs treatments compared to the untreated control (Figure 7a,b). Curcumin (30 μM) induced substantial early (34.5±5.3%, p < 0.0001) and late (11.9±1.0%, p < 0.01) apoptosis, leading to a total apoptosis rate of 46.4% (p < 0.0001). Similarly, CMPNs treatment significantly enhanced early (33.4±6.2%, p < 0.0001) and late (12.0±1.1%, p < 0.01) apoptosis, resulting in a 45.4% (p < 0.0001) overall apoptosis rate. In contrast, BMPNs treatment did not significantly alter apoptosis rates compared to untreated controls, with early and late apoptosis at 4.0% each, totaling 8.0%. These findings demonstrate that CMPNs treatments effectively induced apoptosis at rates comparable to those observed with curcumin treatment.
Figure 7.
Effects of curcumin and CMPNs on cell apoptosis (a,b) and cell cycle arrest (c,d). Flow cytometry analysis using (a,c) Annexin V-FITC/PI staining, (b) the apoptosis rate (%) and (d) percentages of cell populations (%) at each phrase (sub G0/G1, G0/G1, S and G2M) of KKU-213BGemR cells under curcumin (CUR), BMPNs and CMPNs treatment as means ± SD of three biological and two technical replicate experiments; ns = not significant, *p < 0.05, **p < 0.01 and #p < 0.0001 compared to control groups.
Regarding cell-cycle analysis, KKU-213BGemR cells treated with curcumin showed significantly increased percentages in sub G0/G1 (4.40 ± 1.14%, p < 0.05), G0/G1 (48.87 ± 1.84%, p < 0.0001), S (20.35 ± 0.69%, p < 0.05), and G2M (26.27 ± 2.10%, p < 0.0001) phases compared to untreated controls (3.17 ± 0.52%, 56.23 ± 1.58%, 19.37 ± 0.59%, and 21.18 ± 0.99%, respectively). CMPNs treatment significantly induced G0/G1 (49.58 ± 0.94%, p < 0.0001), S (20.37 ± 0.51%, p < 0.05), and particularly G2/M (26.32 ± 1.36%, p < 0.0001) phase arrest compared to untreated controls. BMPNs treatment, however, did not significantly alter cell-cycle distribution (sub G0/G1: 2.70 ± 0.46%, G0/G1: 55.77 ± 0.88%, S: 19.92 ± 0.29%, G2M: 21.53 ± 0.69%) compared to untreated controls (Figure 7c,d).
These results demonstrate that curcumin and CMPNs treatments effectively induce apoptosis and alter cell-cycle distribution in KKU-213BGemR cells, with CMPNs showing comparable efficacy to curcumin, while BMPNs treatment had minimal effects on both apoptosis and cell cycle progression. These findings are consistent with previous studies that have reported the apoptosis and cell cycle-modulating effects of curcumin in various cancer cell lines.57,58
3.8. CMPNs Induce Apoptosis-Related Proteins via CHOP-Mediated Pathway
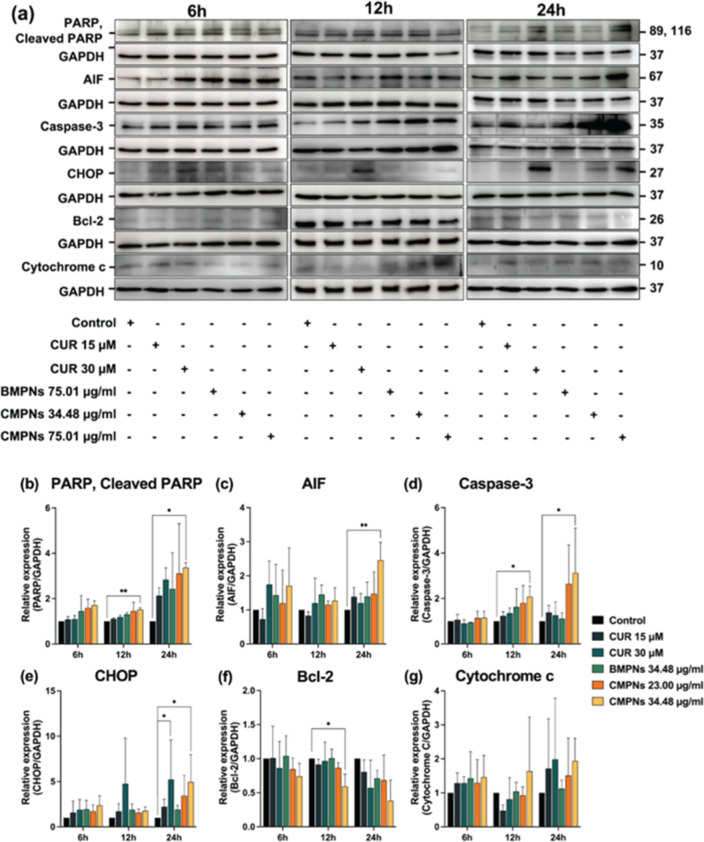
Western blot analysis was performed on KKU-213BGemR cells treated with 15 or 30 μM curcumin, or 75.01 μg/mL BMPNs, or 34.43 or 75.01 μg/mL CMPNs for 6, 12, and 24 h. The expression of apoptosis-related proteins including PARP/cleaved PARP, AIF, caspase-3, Bcl-2, CHOP, and cytochrome c was examined (Figure 8a).
Figure 8.
Western blot analysis of apoptosis-related proteins in KKU-213BGemR cells treated with curcumin, BMPNs, or CMPNs. (a) Representative Western blot images showing protein expression in KKU-213BGemR cells treated with curcumin (CUR), BMPNs, or CMPNs for 6, 12, and 24 h. Quantitative analysis of protein expression levels: (b) PARP, (c) AIF, (d) caspase-3, (e) CHOP, (f) Bcl-2, and (g) cytochrome c. Protein levels were normalized to GAPDH. Data are presented as mean ± SD from three independent experiments. *p < 0.05 and **p < 0.01 compared to untreated control.
Our results revealed that CMPNs at 75.01 μg/mL significantly induced several apoptosis-related proteins (Figure 8b–g). Specifically, PARP/cleaved PARP was significantly upregulated at 12 and 24 h (p < 0.01 and p < 0.05, respectively). AIF showed significant induction at 24 h (p < 0.05), while caspase-3 was significantly increased at both 12 and 24 h (p < 0.05 for both time points). CHOP expression was significantly elevated at 24 h (p < 0.05). Conversely, the antiapoptotic protein Bcl-2 was significantly inhibited at 12 h (p < 0.05). Although cytochrome c levels showed an increasing trend in CMPNs-treated cells, the difference was not statistically significant compared to the untreated control. Curcumin at 30 μM showed similar trends to CMPNs treatment, but only CHOP expression was significantly different from the untreated control. It is worth noting that some proteins exhibited expression changes at different time points, suggesting that shorter and extended treatment durations may be necessary for a more comprehensive understanding of the temporal dynamics of protein expression.59
These results suggest that CMPNs induce apoptosis in KKU-213BGemR cells through a CHOP-regulated pathway. The significant upregulation of CHOP, along with the induction of apoptotic proteins (PARP/cleaved PARP, AIF, caspase-3) and the inhibition of antiapoptotic Bcl-2, indicates the activation of both intrinsic and extrinsic apoptotic pathways. The CHOP-mediated apoptotic pathway is often associated with ER stress.60,61 The observed increase in CHOP expression suggests that CMPNs may trigger ER stress, leading to apoptosis. This is consistent with previous studies showing that curcumin can induce ER stress-mediated apoptosis in various cancer cell lines.62,63 The differential timing of protein expression changes between CMPNs and curcumin treatments suggests that CMPNs may have improved bioavailability or cellular uptake compared to free curcumin.64 This could explain the more pronounced and earlier effects observed with CMPNs treatment. The trend toward increased cytochrome c levels, although not statistically significant, may indicate the involvement of the mitochondrial apoptotic pathway.64 Further studies with extended time points or higher doses might reveal a significant change in cytochrome c levels.
Overall, our results demonstrate that CMPNs effectively induce apoptosis in KKU-213BGemR cells through a CHOP-regulated pathway, potentially involving ER stress. These findings suggest CMPNs as a promising therapeutic agent for CCA, warranting further investigation into the mechanism of action and potential clinical applications of this proniosome.
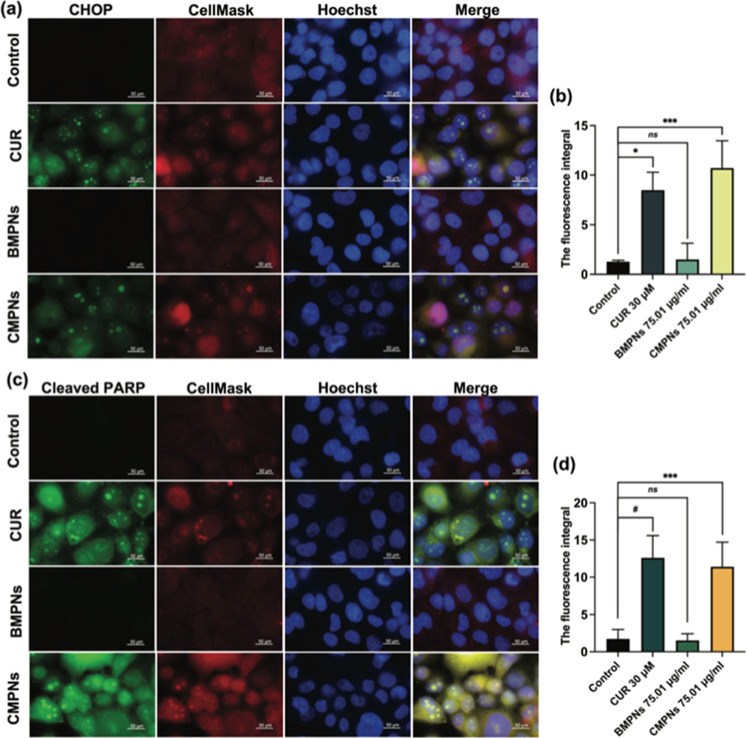
3.9. CMPNs Induce CHOP and Cleaved-PARP Expression in KKU-213BGemR Cells
To further corroborate the Western blot findings, we performed immunofluorescence assays to visualize and quantify CHOP and cleaved-PARP expression in KKU-213BGemR cells treated with curcumin, BMPNs, or CMPNs (Figure 9). The results showed that 30 μM curcumin and 75.01 μg/mL CMPNs significantly induced CHOP expression (p < 0.05 and p < 0.001, respectively) compared to untreated controls (Figure 9a,b). Similarly, cleaved-PARP levels were significantly increased in cells treated with 30 μM curcumin (p < 0.0001) and 75.01 μg/mL CMPNs (p < 0.001) (Figure 9c,d). In contrast, 75.01 μg/mL BMPNs showed no significant difference in CHOP or cleaved-PARP expression compared to untreated controls.
Figure 9.
Immunofluorescence staining of KKU-213BGemR cells treated with curcumin (CUR), BMPNs, or CMPNs. (a) CHOP expression (green), (b) the fluorescence integral of CHOP, (c) cleaved-PARP expression (green) and (d) the fluorescence integral of cleaved PARP. In both panels, cell membranes are stained with CellMask (red) and nuclei with Hoechst (blue). Images were captured at 10× magnification. Data are presented as mean ± SD; *p < 0.05, ***p < 0.001 and #p < 0.0001 compared to untreated control.
The immunofluorescence assay results provide strong visual and quantitative evidence supporting our Western blot findings, confirming that both curcumin and CMPNs induce the expression of CHOP and cleaved PARP in KKU-213BGemR cells. These results reinforce the hypothesis that CMPNs triggers apoptosis through a CHOP-regulated pathway, likely involving ER stress.62 The observed increase in cleaved PARP further supports the activation of apoptotic pathways. PARP cleavage is a hallmark of apoptosis, often executed by activated caspases, particularly caspase-3.65 This aligns with our Western blot results showing increased caspase-3 levels, indicating that both curcumin and CMPNs are triggering the execution phase of apoptosis.
3.10. CMPNs Inhibit Tumor Growth of KKU-213BGemR in the Xenograft Model
We established the xenograft tumor model of KKU-213BGemR cells using BALB/cAJcl-nu mice (Figure 10a). We observed that gemcitabine did not significantly decrease tumor growth compared with the control group but showed a tendency to increase the tumor volume (Figure S2a,b). Two mice had tumor masses greater than 2.0 cm in largest diameter at 3 weeks and at the end point after receiving 50 mg/kg BW of gemcitabine, resulting in a low survival rate, which correlated with a decrease in body weight observed at 3 weeks after treatment (Figure S2c,d).
Figure 10.
Antitumor effect of curcumin (CUR), blank maltodextrin-based proniosomes (BMPNs) and curcumin-loaded maltodextrin-based proniosome (CMPNs) on a gemcitabine-resistant CCA xenograft model. (a) Xenograft tumors in mice initiated using KKU-213BGemR (1 × 106 cells) were treated with curcumin (20 mg/kg BW), BMPNs (125 mg/kg BW) and CMPNs (62.5 and 125 mg/kg BW) by oral administration. (b) The effect of curcumin, BMPNs and CMPNs on the body weight of mice and (c) survival rate. (d) Representative images of tumor tissue (e) tumor volume (mm3). All data are expressed as mean ± SD, N = 5; **p < 0.01 compared with the untreated group, N = 3.
The body weight of mice used in our experiments showed no significant changes in the five groups (Figure 10b). The CMPNs group, at a dose of 125 mg/kg BW (equivalent to 20 mg/kg of curcumin) showed the lowest survival rate (Figure 10c). At the baseline and 1 week after treatment, there were no significant differences in tumor volumes between all groups. However, tumor growth rate began to show changes from the second week after treatment. The CMPNs group, at a dose of 62.5 mg/kg BW (equivalent to 10 mg/kg of curcumin), showed significantly inhibited tumor growth at 2 weeks (p < 0.001), 3 weeks (p < 0.01), and 4 weeks (p < 0.001) after the start of experimental treatment compared with the control group. The curcumin group showed significant inhibition of tumor growth compared to the control group only at 3 and 4 weeks after treatment (p < 0.5) (Figure S3). By the end point of the study, treatment with 62.5 mg/kg BW CMPNs had significantly suppressed tumor growth compared to the control group (p < 0.01). In contrast, the groups treated with the curcumin, BMPNs and 125 mg/kg BW CMPNs (equivalent to 20 mg/kg of curcumin), showed no significant difference from the control group (Figure 10d,e).
Our results show that a low dose of CMPNs (62.5 mg/kg BW, equivalent to 10 mg/kg of curcumin) significantly reduced tumor volume relative to the control group, while the high-dose (125 mg/kg BW, equivalent to 20 mg/kg of curcumin) treatment group did not significantly differ from the control group. The anticancer effect of curcumin alone was only apparent in the 2–4 weeks after treatment, with a significant difference from the control group. However, at the end point, there was no significant difference of curcumin-only treatment relative to the control group. These results are consistent with a previous study demonstrating the efficacy of daily administration of curcumin-loaded nanocomplexes (CNCs) against CCA induced in hamsters by a combination of liver fluke infection and carcinogen. CNCs at a dose equivalent to 10 mg/kg curcumin increased survival and decreased the incidence of CCA. Conversely, CNCs administered at a dose equivalent to 20 mg/kg curcumin showed adverse effects due to curcumin overdose, as evidenced by a higher incidence of cholangiofibroma and increased tumor mass compared with the lower dose.30 These findings underscore the critical need for dose optimization, and further studies utilizing diverse animal models are warranted to refine dosing strategies before considering clinical applications.
While both curcumin and CMPNs exhibited comparable antitumor effects, CMPNs offered significant advantages, requiring lower doses and less frequent administration. Animals treated with CMPNs for one month, administered three times a week, received a total dose of 120 mg, whereas curcumin required daily administration, resulting in a total accumulated dose of 600 mg—approximately five times higher than that of CMPNs to get a similar anti-CCA activity. The enhanced tumor suppression efficacy of CMPNs compared to native curcumin is likely due to its higher permeation rate, which improves intestinal absorption. These findings highlight the superior therapeutic potential of CMPNs, enabled by the ability of niosomes to overcome curcumin’s inherent limitations. Moreover, using maltodextrin as a carrier enhances oral bioavailability, higher absorption and leads to increased plasma concentration.18 These results suggest that CMPNs can improve therapeutic efficacy and mitigate the prevailing challenge of drug resistance in CCA.
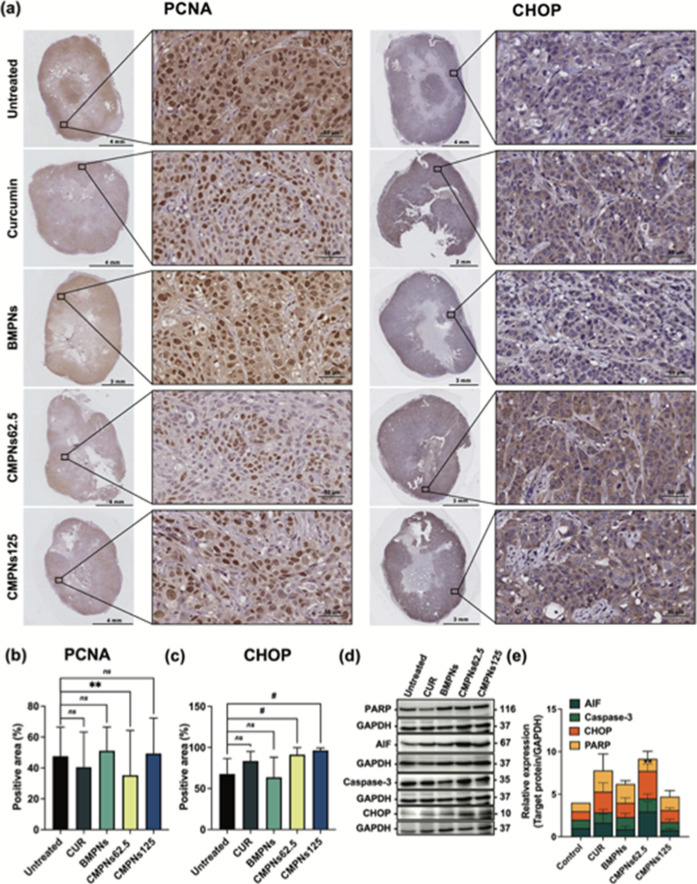
3.11. CMPNs Suppress Cell Proliferation and Induce CHOP Expression by KKU-213BGemR Cells in Tumor Tissues
The proliferation status of tumor tissue was evaluated by immunohistochemical analysis. The results showed that PCNA-positive areas were present in all examined areas of the tumor tissue in both the control and treated groups (Figure 11a,b). The highest percentage of PCNA-positive areas was observed in the control and 125 mg/kg BW BMPNs groups with 32.38 ± 10.66% and 33.80 ± 10.12%, respectively, followed by the 125 mg/kg BW CMPNs group (29.57 ± 8.98%), the 20 mg/kg BW curcumin group (27.55 ± 4.50%). The lowest PCNA expression was in the 62.5 mg/kg BW CMPNs group (20.97 ± 6.81%), which also showed significantly suppressed cell proliferation in tumor tissue compared to the control group (p < 0.01). Moreover, CHOP-positive areas were significantly greater in both the 62.5 mg/kg BW (91.25 ± 7.77%) and 125 mg/kg BW (96.38 ± 2.36%) CMPNs groups compared to the control (p < 0.0001). In contrast, the 20 mg/kg BW curcumin and 125 mg/kg BW BMPNs groups showed no significant difference from the untreated group (Figure 11a,c).
Figure 11.
Immunohistochemical and Western blot analyses of KKU-213BGemR xenograft tumors treated with curcumin, BMPNs, and CMPNs. (a) Representative immunohistochemistry images of PCNA and CHOP staining in tumor tissues (20× magnification). Quantification of (b) PCNA-positive areas and (c) CHOP-positive areas in tumor tissues. (d) Representative Western blot images and (e) densitometric analysis of apoptosis-related proteins in tumor tissues. Data are presented as mean ± SD ns = not significant, **p < 0.01 and #p < 0.0001 compared to untreated group.
Western blot analysis of tumor tissue supported the activation of the CHOP-regulated apoptosis pathway (Figure 11d). The 62.5 mg/kg BW CMPNs group showed significantly increased CHOP expression compared to the untreated group (p < 0.01), while other groups showed no significant difference. This result corroborates findings from both in vitro and in vivo studies. Although not statistically significant, other molecules including PARP/cleaved-PARP, AIF and caspase-3 showed a trend toward increased expression (Figure 11e). Further validation with a larger sample size may be necessary to confirm these observations.
Our in vivo study provides valuable insights into the effects of CMPNs on CCA in a physiological context. The immunohistochemical and Western blot analyses reveal a complex interplay between proliferative and apoptotic signals in response to different treatments. The 62.5 mg/kg BW CMPNs treatment emerged as the most effective in reducing tumor cell proliferation, as evidenced by the lowest PCNA expression. This group also showed significantly increased CHOP expression, both in immunohistochemistry and Western blot analyses, suggesting the activation of ER stress-mediated apoptotic pathways.65 These findings align with our in vitro results and support the potential of CMPNs as an effective treatment for CCA. Interestingly, the 125 mg/kg BW CMPNs group presented a paradoxical response. Despite showing the highest CHOP expression in immunohistochemistry, indicating strong activation of ER stress pathways, this group maintained relatively high PCNA levels. This suggests that at higher doses, CMPNs might trigger compensatory proliferative responses that counteract its pro-apoptotic effects.66 Further research is needed to understand this dose-dependent response and optimize the therapeutic potential of CMPNs. Additionally, the trend toward increased expression of apoptosis-related proteins in the Western blot analysis, although not statistically significant, supports the pro-apoptotic potential of CMPNs. The lack of statistical significance might be due to the limited sample size of in vivo systems, where multiple factors can influence protein expression.67 The ineffectiveness of the 20 mg/kg BW curcumin treatment in significantly altering CHOP or PCNA expression underscores the potential benefits of the CMPNs formulation in improving bioavailability and efficacy of curcumin.68
In brief, our study demonstrates that CMPNs at 62.5 mg/kg BW effectively reduce tumor cell proliferation and induce CHOP expression in a CCA xenograft model, likely through ER stress-mediated apoptosis. However, the unexpected results at the higher dose of CMPNs emphasize the need for careful dose optimization and further investigation into the mechanisms governing the balance between apoptosis and proliferation in response to CMPNs treatment. To further validate these findings and better mimic the tumor microenvironment, future studies should consider the use of an orthotopic CCA model, which would provide a more physiologically relevant context for understanding the therapeutic effects of CMPNs.
4. Conclusions
Our study demonstrates that CMPNs effectively overcome the limitations of native curcumin while maintaining its potent anticancer activity in gemcitabine-resistant CCA models. CMPNs treatment induced apoptosis through the CHOP-mediated pathway, showing superior efficacy compared to native curcumin and BMPNs. In vivo studies revealed that 62.5 mg/kg BW CMPNs significantly reduced tumor proliferation, while higher doses yielded unexpected results with increased CHOP expression but maintained PCNA levels. This research provides a promising preclinical platform for developing novel chemotherapeutic strategies for gemcitabine-resistant CCA and potentially other cancers. The proniosome technology demonstrates potential in enhancing curcumin’s therapeutic efficacy, warranting further investigation into optimizing dosing regimens and exploring combination therapies to maximize its anticancer effects.
Acknowledgments
We gratefully acknowledge Prof. David Blair for invaluable suggestions and editing the manuscript via the Publication Clinic, Khon Kaen University, Thailand. We also thank Yutthaya Khemjeen of the Research Instrument Center, Khon Kaen University, for providing technical expertise with E SEM characterization. This research was supported by the RGJ-PhD program through National Research Council of Thailand (NRCT; NRCT5-RGJ63003-063) and the Fundamental Fund of Khon Kaen University, under the National Science, Research and Innovation Fund (NSRF).
Data Availability Statement
The graphical abstract was created in BioRender (2024), available at https://BioRender.com/t34n743.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.4c01832.
Figures S1–S3. Solubility studies of CMPNs, including visual comparisons under various conditions and temperatures, gemcitabine-resistant xenograft model results, and tumor response to treatments. Table S1. Composition and particle size distribution of CMPNs formulations (PDF)
Author Contributions
Phonpilas Thongpon: Conceptualization, methodology, validation, investigation, formal analysis, writing—original draft, review and editing, K.I.,T.P.: Conceptualization, investigation, methodology, formal analysis, review and editing, A.P., S.C., Pakornkiat Tanasuka, P.M., U.S., K.V.: Investigation, formal analysis, review and editing, P.P., S.P.: Conceptualization, project administration, funding acquisition, writing, review and editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Banales J. M.; Marin J. J. G.; Lamarca A.; Rodrigues P. M.; Khan S. A.; Roberts L. R.; Cardinale V.; Carpino G.; Andersen J. B.; Braconi C.; et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17 (9), 557–588. 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuccio P.; Malvezzi M.; Carioli G.; Hashim D.; Boffetta P.; El-Serag H. B.; La Vecchia C.; Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71 (1), 104–114. 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Xia J.; Jiang S. C.; Peng H. J. Association between liver fluke infection and hepatobiliary pathological changes: a systematic review and meta-analysis. PLoS One 2015, 10 (7), e0132673 10.1371/journal.pone.0132673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty B.; Nambudiri V. E.; Palmer W. C. Update on the diagnosis and treatment of cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19 (1), 2. 10.1007/s11894-017-0542-4. [DOI] [PubMed] [Google Scholar]
- Jia Y.; Xie J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis. 2015, 2 (4), 299–306. 10.1016/j.gendis.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwattanakul M.; Hahnvajanawong C.; Tippayawat P.; Chio-Srichan S.; Leelayuwat C.; Limpaiboon T.; Jearanaikoon P.; Heraud P. Classification of gemcitabine resistant cholangiocarcinoma cell lines using synchrotron FTIR microspectroscopy. J. Biophotonics 2017, 10 (3), 367–376. 10.1002/jbio.201500253. [DOI] [PubMed] [Google Scholar]
- Giordano A.; Tommonaro G. Curcumin and Cancer. Nutrients 2019, 11 (10), 2376. 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobwong S.; Gupta S. C.; Kim J. H.; Sung B.; Pinlaor P.; Hiraku Y.; Wongkham S.; Sripa B.; Pinlaor S.; Aggarwal B. B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 2011, 32 (9), 1372–1380. 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongpon P.; Intuyod K.; Chomwong S.; Pongking T.; Klungsaeng S.; Muisuk K.; Charoenram N.; Sitthirach C.; Thanan R.; Pinlaor P.; et al. Curcumin synergistically enhances the efficacy of gemcitabine against gemcitabine-resistant cholangiocarcinoma via the targeting LAT2/glutamine pathway. Sci. Rep. 2024, 14 (1), 16059. 10.1038/s41598-024-66945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti A. L. The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects?. Adv. Nutr. 2018, 9 (1), 41–50. 10.1093/advances/nmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Zhai Y.; Heng X.; Che F. Y.; Chen W.; Sun D.; Zhai G. Oral bioavailability of curcumin: problems and advancements. J. Drug Targeting 2016, 24 (8), 694–702. 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- Prasad S.; Tyagi A. K.; Aggarwal B. B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014, 46 (1), 2–18. 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran T.; Haile T.; Nigusse T.; Dhanaraju M. D. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl 1), S1–S7. 10.12980/apjtb.4.2014c980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ag Seleci D.; Seleci M.; Walter J.-G.; Stahl F.; Scheper T. Niosomes as nanoparticular drug carriers: fundamentals and recent applications. J. Nanomater. 2016, 2016, 1–13. 10.1155/2016/7372306. [DOI] [Google Scholar]
- Hu C.; Rhodes D. G. Proniosomes: a novel drug carrier preparation. Int. J. Pharm. 1999, 185 (1), 23–35. 10.1016/S0378-5173(99)00122-2. [DOI] [PubMed] [Google Scholar]
- Khoee S.; Yaghoobian M.. Chapter 6 - Niosomes: a novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Andronescu E., Grumezescu A. M., Eds.; Elsevier, 2017; pp 207–237. [Google Scholar]
- Akbarzadeh I.; Shayan M.; Bourbour M.; Moghtaderi M.; Noorbazargan H.; Eshrati Yeganeh F.; Saffar S.; Tahriri M. Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology 2021, 10 (3), 173. 10.3390/biology10030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo R. K.; Biswas N.; Guha A.; Kuotsu K. Maltodextrin based proniosomes of nateglinide: bioavailability assessment. Int. J. Biol. Macromol. 2014, 69, 430–434. 10.1016/j.ijbiomac.2014.05.075. [DOI] [PubMed] [Google Scholar]
- Mousazadeh N.; Gharbavi M.; Rashidzadeh H.; Nosrati H.; Danafar H.; Johari B. Anticancer evaluation of methotrexate and curcumin-coencapsulated niosomes against colorectal cancer cell lines. Nanomedicine 2022, 17 (4), 201–217. 10.2217/nnm-2021-0334. [DOI] [PubMed] [Google Scholar]
- Kong Y.; Jiang J.; Huang Y.; Li L.; Liu X.; Jin Z.; Wei F.; Liu X.; Zhang S.; Duan X.; et al. Endoplasmic reticulum stress in melanoma pathogenesis and resistance. Biomed. Pharmacother. 2022, 155, 113741. 10.1016/j.biopha.2022.113741. [DOI] [PubMed] [Google Scholar]
- Piao M. J.; Han X.; Kang K. A.; Fernando P.; Herath H.; Hyun J. W. The endoplasmic reticulum stress response mediates shikonin-induced apoptosis of 5-fluorouracil-resistant colorectal cancer cells. Biomol. Ther. 2022, 30 (3), 265–273. 10.4062/biomolther.2021.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intuyod K.; Priprem A.; Pairojkul C.; Hahnvajanawong C.; Vaeteewoottacharn K.; Pinlaor P.; Pinlaor S. Anthocyanin complex exerts anti-cholangiocarcinoma activities and improves the efficacy of drug treatment in a gemcitabine-resistant cell line. Int. J. Oncol. 2018, 52 (5), 1715–1726. 10.3892/ijo.2018.4306. [DOI] [PubMed] [Google Scholar]
- Tabas I.; Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13 (3), 184–190. 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S.; Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11 (4), 381–389. 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Chaitanya G. V.; Alexander J. S.; Babu P. P. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun. Signaling 2010, 8, 31. 10.1186/1478-811x-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsarra I. A. Evaluation of proniosomes as an alternative strategy to optimize piroxicam transdermal delivery. J. Microencapsulation 2009, 26 (3), 272–278. 10.1080/02652040802305618. [DOI] [PubMed] [Google Scholar]
- Diaz del Consuelo I.; Pizzolato G.-P.; Falson F.; Guy R. H.; Jacques Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94 (12), 2777–2788. 10.1002/jps.20409. [DOI] [PubMed] [Google Scholar]
- Moghassemi S.; Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Controlled Release 2014, 185, 22–36. 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Sripa B.; Seubwai W.; Vaeteewoottacharn K.; Sawanyawisuth K.; Silsirivanit A.; Kaewkong W.; Muisuk K.; Dana P.; Phoomak C.; Lert-Itthiporn W.; et al. Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Hum. Cell 2020, 33 (3), 695–708. 10.1007/s13577-020-00334-w. [DOI] [PubMed] [Google Scholar]
- Jantawong C.; Chamgramol Y.; Intuyod K.; Priprem A.; Pairojkul C.; Klungsaeng S.; Dangtakot R.; Pongking T.; Sitthirach C.; Pinlaor P.; et al. Curcumin-loaded nanocomplexes alleviate the progression of fluke-related cholangiocarcinoma in hamsters. Cancer Nanotechnol. 2023, 14 (1), 5. 10.1186/s12645-023-00155-0. [DOI] [Google Scholar]
- Somjid S.; Shinsuphan N.; Temprom L.; Krongsuk S. Effects of cholesterol and temperature on structural properties and dynamic behavior of niosome bilayers with melatonin Inclusion: A Coarse-Grained simulation study. J. Mol. Liq. 2022, 368, 120686. 10.1016/j.molliq.2022.120686. [DOI] [Google Scholar]
- Khan D. H.; Bashir S.; Khan M. I.; Figueiredo P.; Santos H. A.; Peltonen L. Formulation optimization and in vitro characterization of rifampicin and ceftriaxone dual drug loaded niosomes with high energy probe sonication technique. J. Drug Delivery Sci. Technol. 2020, 58, 101763. 10.1016/j.jddst.2020.101763. [DOI] [Google Scholar]
- Bashkeran T.; Kamaruddin A. H.; Ngo T. X.; Suda K.; Umakoshi H.; Watanabe N.; Nadzir M. M. Niosomes in cancer treatment: A focus on curcumin encapsulation. Heliyon 2023, 9 (8), e18710 10.1016/j.heliyon.2023.e18710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata T.; Kono Y.; Higaki K.; Kimura T.; Ogawara K.-i. In vivo distribution characteristics and anti-tumor effects of doxorubicin encapsulated in PEG-modified niosomes in solid tumor-bearing mice. J. Drug Delivery Sci. Technol. 2023, 80, 104122. 10.1016/j.jddst.2022.104122. [DOI] [Google Scholar]
- Sezgin-Bayindir Z.; Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech 2012, 13 (3), 826–835. 10.1208/s12249-012-9805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judefeind A.; de Villiers M. M.. Drug loading into and in vitro release from nanosized drug delivery systems. Nanotechnology in Drug Delivery; de Villiers M. M., Aramwit P., Kwon G. S., Eds.; Springer: New York, 2009; pp 129–162. [Google Scholar]
- Witika B. A.; Bassey K. E.; Demana P. H.; Siwe-Noundou X.; Poka M. S. Current advances in specialised niosomal drug delivery: manufacture, characterization and drug delivery applications. Int. J. Mol. Sci. 2022, 23 (17), 9668. 10.3390/ijms23179668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchegbu I. F.; Vyas S. P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172 (1), 33–70. 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- Moammeri A.; Chegeni M. M.; Sahrayi H.; Ghafelehbashi R.; Memarzadeh F.; Mansouri A.; Akbarzadeh I.; Abtahi M. S.; Hejabi F.; Ren Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. 10.1016/j.mtbio.2023.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owodeha-Ashaka K.; Ilomuanya M. O.; Iyire A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Prog. Biomater. 2021, 10 (3), 207–220. 10.1007/s40204-021-00164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-T.; Kuo Y.-L.; Chen C.-H.; Wu H.-T.; Chen H.-W.; Fang W.-P. Improving the stability and bioactivity of curcumin using chitosan-coated liposomes through a combination mode of high-pressure processing. LWT--Food Sci. Technol. 2022, 168, 113946. 10.1016/j.lwt.2022.113946. [DOI] [Google Scholar]
- Bich V. T.; Thuy N. T.; Binh N. T.; Huong N. T. M.; Yen P. N. D.; Luong T. T.. Structural and spectral properties of curcumin and metal- curcumin complex derived from turmeric (Curcuma longa). Physics and Engineering of New Materials; Cat D. T., Pucci A., Wandelt K., Eds.; Springer: Berlin, Heidelberg, 2009; pp 271–278. [Google Scholar]
- Mohan P. R. K.; Sreelakshmi G.; Muraleedharan C. V.; Joseph R. Water soluble complexes of curcumin with cyclodextrins: characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. 10.1016/j.vibspec.2012.05.002. [DOI] [Google Scholar]
- Sammour R. M. F.; Taher M.; Chatterjee B.; Shahiwala A.; Mahmood S. Optimization of aceclofenac proniosomes by using different carriers, part 1: development and characterization. Pharmaceutics 2019, 11 (7), 350. 10.3390/pharmaceutics11070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copello F. d. R.; Lizarraga L.; Orsetti S.; Molina F. V. Swelling and aggregation of leonardite upon pH change and PbII binding: an AFM study. Environ. Chem. 2018, 15 (3), 162–170. 10.1071/EN17224. [DOI] [Google Scholar]
- Ponomareva N.; Brezgin S.; Karandashov I.; Kostyusheva A.; Demina P.; Slatinskaya O.; Bayurova E.; Silachev D.; Pokrovsky V. S.; Gegechkori V.; et al. Swelling, rupture and endosomal escape of biological nanoparticles Per Se and those fused with liposomes in acidic environment. Pharmaceutics 2024, 16 (5), 667. 10.3390/pharmaceutics16050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitiski C. B.; Carvalho R. A.; Ribeiro A. J.; Neufeld R. J.; Veiga F. Strategies toward the improved oral delivery of insulin nanoparticles via gastrointestinal uptake and translocation. BioDrugs 2008, 22 (4), 223–237. 10.2165/00063030-200822040-00002. [DOI] [PubMed] [Google Scholar]
- Kumar G. P.; Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm. Sin. B 2011, 1 (4), 208–219. 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- Badria F. A.; Abdelaziz A. E.; Hassan A. H.; Elgazar A. A.; Mazyed E. A. Development of provesicular nanodelivery system of curcumin as a safe and effective antiviral agent: statistical optimization, in vitro characterization, and antiviral effectiveness. Molecules 2020, 25 (23), 5668. 10.3390/molecules25235668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager I.; Langel K.; Lehto T.; Eiriksdottir E.; Langel U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818 (3), 502–511. 10.1016/j.bbamem.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Mortensen K.; Larsson L. I. Effects of cytochalasin D on the actin cytoskeleton: association of neoformed actin aggregates with proteins involved in signaling and endocytosis. Cell. Mol. Life Sci. 2003, 60 (5), 1007–1012. 10.1007/s00018-003-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad R.; Bilal S.; Khan A.; Shehzad A.; Al-Suhaimi E. A.. Chapter two - Emerging concept on cellular uptake mechanism of nanoparticles. In Molecular Impacts of Nanoparticles on Plants and Algae; Tombuloglu H., Tombuloglu G., Al-Suhaimi E., Baykal A., Hakeem K. R., Eds.; Academic Press, 2024; pp 31–40. [Google Scholar]
- Izhar M. P.; Hafeez A.; Kushwaha P.; Simrah Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications. J. Cluster Sci. 2023, 34 (5), 2257–2273. 10.1007/s10876-023-02423-w. [DOI] [Google Scholar]
- Chen M.; Qian C.; Jin B.; Hu C.; Zhang L.; Wang M.; Zhou B.; Zuo W.; Huang L.; Wang Y. Curcumin analog WZ26 induces ROS and cell death via inhibition of STAT3 in cholangiocarcinoma. Cancer Biol. Ther. 2023, 24 (1), 2162807. 10.1080/15384047.2022.2162807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San T. T.; Khaenam P.; Prachayasittikul V.; Sripa B.; Kunkeaw N.; Chan-On W. Curcumin enhances chemotherapeutic effects and suppresses ANGPTL4 in anoikis-resistant cholangiocarcinoma cells. Heliyon 2020, 6 (1), e03255 10.1016/j.heliyon.2020.e03255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Li H.; Zhao L.; Kim G.; Chen Y.; Yan X.; Yoon J. Albumin-mediated ″Unlocking″ of supramolecular prodrug-like nanozymes toward selective imaging-guided phototherapy. Chem. Sci. 2022, 13 (26), 7814–7820. 10.1039/D2SC02025D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S.; Xu Y.; Meng L.; Huang L.; Sun H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16 (2), 1266–1272. 10.3892/etm.2018.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Bu S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evidence-Based Complementary Altern. Med. 2017, 2017, 5787218. 10.1155/2017/5787218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007, 35 (4), 495–516. 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A.; Moskowitz M. A. PARP-1--a perpetrator of apoptotic cell death?. Science 2002, 297 (5579), 200–201. 10.1126/science.1074592. [DOI] [PubMed] [Google Scholar]
- Hu H.; Tian M.; Ding C.; Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Cheng X.; Xu S.; Bao J.; Yu H. Curcumin induces endoplasmic reticulum stress-associated apoptosis in human papillary thyroid carcinoma BCPAP cells via disruption of intracellular calcium homeostasis. Medicine 2018, 97 (24), e11095 10.1097/MD.0000000000011095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustapha A.; Pérétout P.; Rainey N. E.; Sureau F.; Geze M.; Petit J. M.; Dewailly E.; Slomianny C.; Petit P. X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discovery 2015, 1 (1), 15017. 10.1038/cddiscovery.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. Bioavailability of curcumin: problems and promises. Mol. Pharmaceutics 2007, 4 (6), 807–818. 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Farghadani R.; Naidu R. Curcumin: modulator of key molecular signaling pathways in hormone-independent breast cancer. Cancers 2021, 13 (14), 3427. 10.3390/cancers13143427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo H. D.; Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harbor Perspect. Biol. 2012, 4 (8), a008797. 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144 (5), 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Ergin A. D.; Üner B.; Balcı S. ¸.; Demirbağ C. ¸.; Benetti C.; Oltulu C. ¸. Improving the bioavailability and efficacy of coenzyme Q10 on Alzheimer’s disease through the arginine based proniosomes. J. Pharm. Sci. 2023, 112 (11), 2921–2932. 10.1016/j.xphs.2023.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The graphical abstract was created in BioRender (2024), available at https://BioRender.com/t34n743.