Abstract
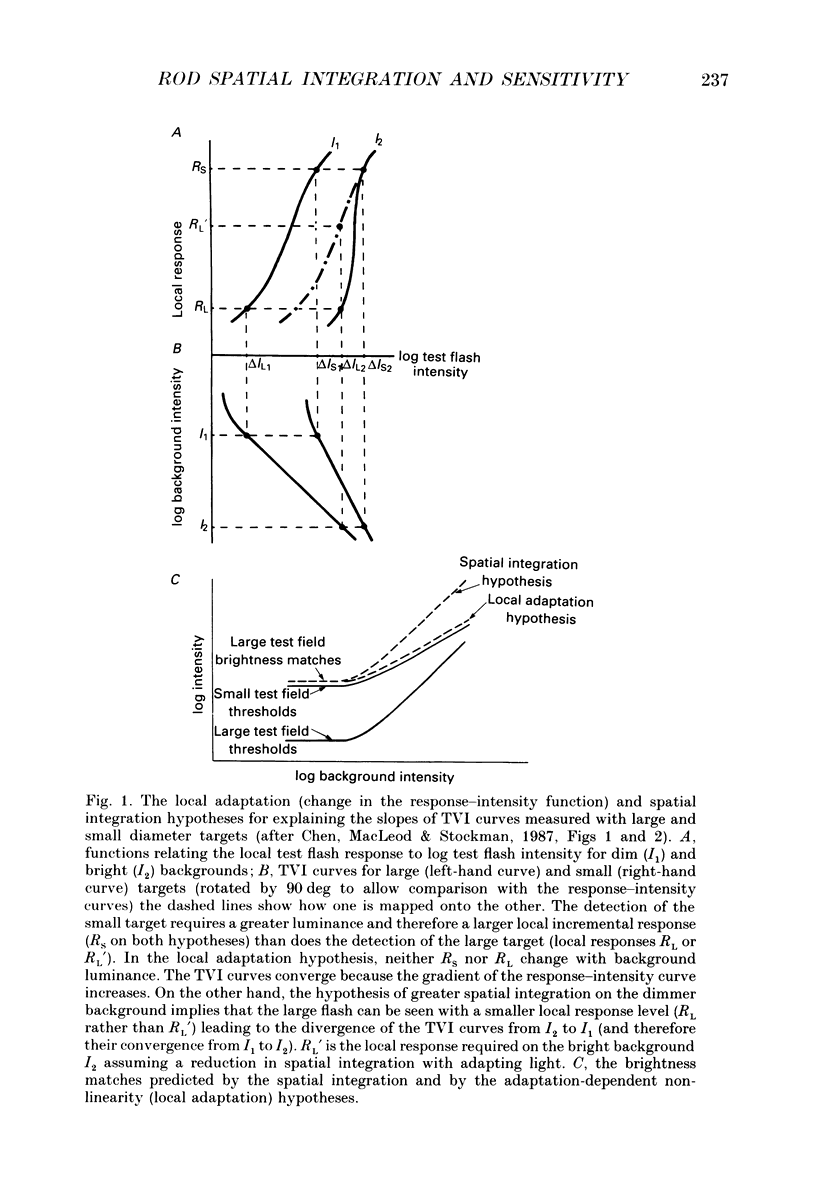
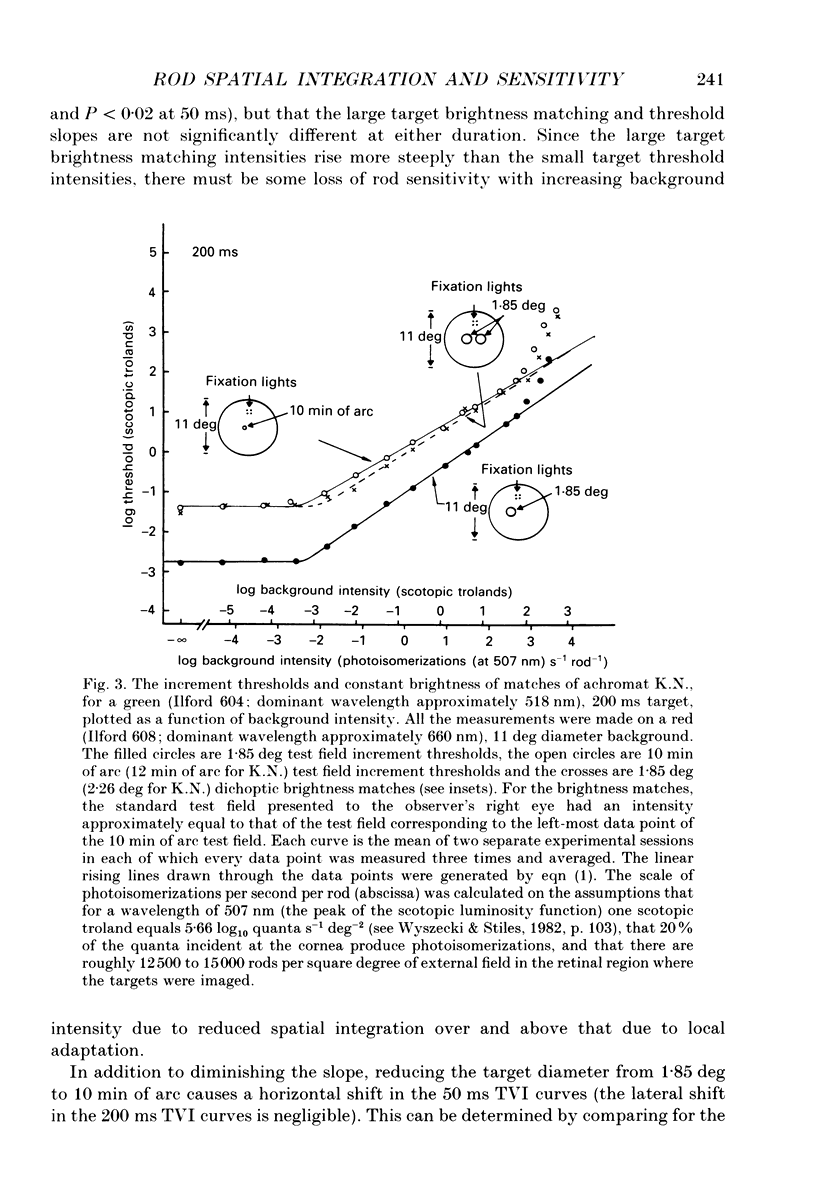
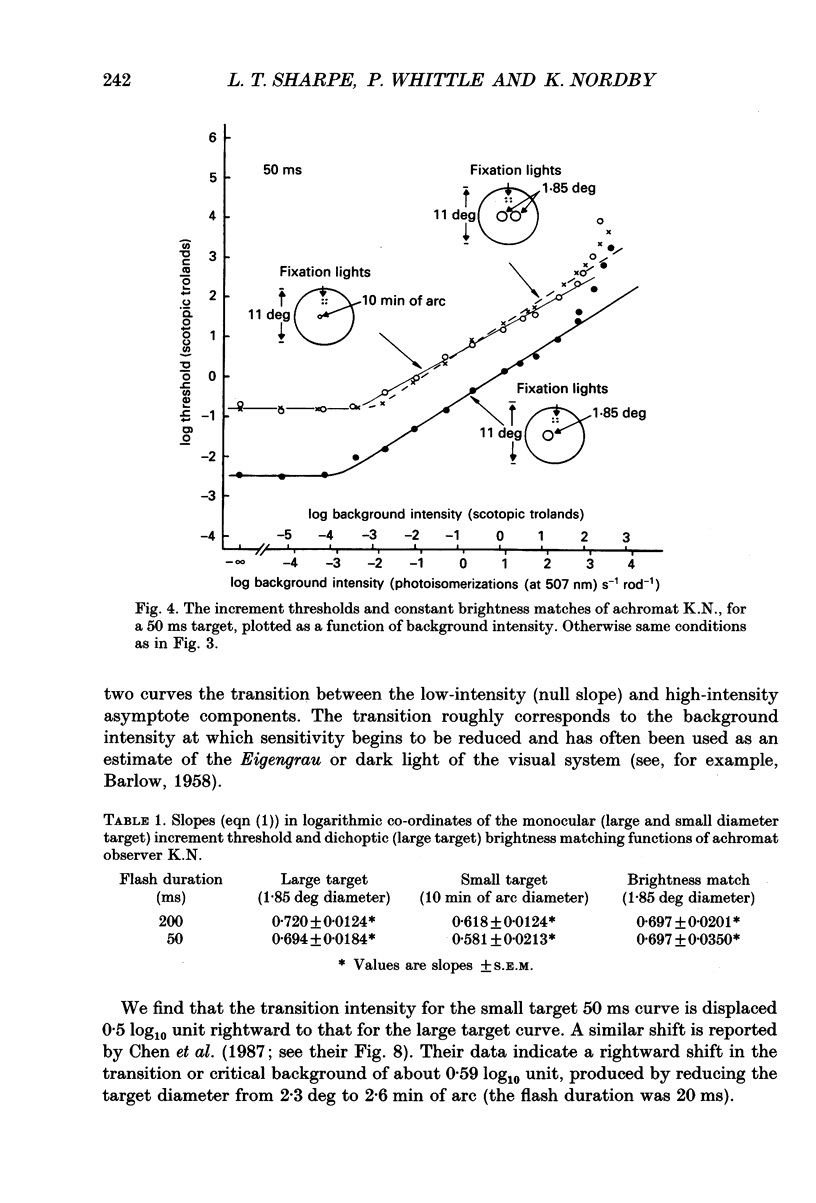
1. The factor by which increment threshold rises with increasing background intensity is less if the target is small than if it is large. The difference is usually attributed to a reduction in the area over which visual signals are integrated as the visual system light adapts. Recently, however, it has been argued that the difference in slope may instead be caused by an increase in the gain of the local response function with light adaptation. 2. To test this hypothesis in the rod-driven visual system, we compared monoptic, small and large target increment thresholds, and dichoptic, large target brightness matches, measured as a function of background intensity in a typical, complete achromat, who has no cone vision. 3. The dichoptic brightness matches were made using a large target of a similar intensity to the threshold intensity of the small target. If local intensity is important, the large target brightness matching curve should be more similar to the shallow, small target threshold curve. But, if changes in spatial integration are important, the brightness matching curve should be similar to the steeper, large target threshold curve. 4. The slope of the large (1.85 deg) target increment threshold functions measured with either 200 or 50 ms test flashes were steeper than those of the small (10 min of arc) target functions by 0.10 (on logarithmic co-ordinates) or about 15%. 5. The logarithmic slopes of the dichoptic brightness curves were also slightly steeper than the small target increment functions. This is contrary to the local response (only) hypothesis, which predicts that the brightness curve should have the same slope as the small target function because the luminance of the targets in the two cases is the same. 6. We conclude that there must be a change in spatial integration in the rod visual system during light adaptation, over and above that due to local gain changes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Chen B., MacLeod D. I., Stockman A. Improvement in human vision under bright light: grain or gain? J Physiol. 1987 Dec;394:41–66. doi: 10.1113/jphysiol.1987.sp016859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone C. M., Hayhoe M. M., MacLeod D. I. The spread of adaptation in human foveal and parafoveal cone vision. Vision Res. 1990;30(11):1603–1615. doi: 10.1016/0042-6989(90)90147-d. [DOI] [PubMed] [Google Scholar]
- Cicerone C. M., Hayhoe M. M. The size of the pool for bleaching adaptation in human rod vision. Vision Res. 1990;30(5):693–697. doi: 10.1016/0042-6989(90)90095-3. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. The influence of temporal frequency and adaptation level on receptive field organization of retinal ganglion cells in cat. J Physiol. 1982 Dec;333:343–366. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U., Martin P. R. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J Neurosci. 1991 Sep;11(9):2742–2758. doi: 10.1523/JNEUROSCI.11-09-02742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. I., Chen B., Crognale M. Spatial organization of sensitivity regulation in rod vision. Vision Res. 1989;29(8):965–978. doi: 10.1016/0042-6989(89)90111-9. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I. Visual sensitivity. Annu Rev Psychol. 1978;29:613–645. doi: 10.1146/annurev.ps.29.020178.003145. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I., Williams D. R., Makous W. A visual nonlinearity fed by single cones. Vision Res. 1992 Feb;32(2):347–363. doi: 10.1016/0042-6989(92)90144-8. [DOI] [PubMed] [Google Scholar]
- Nordby K., Sharpe L. T. The directional sensitivity of the photoreceptors in the human achromat. J Physiol. 1988 May;399:267–281. doi: 10.1113/jphysiol.1988.sp017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. THE SENSITIVITY OF RODS UNDER ILLUMINATION. J Physiol. 1965 May;178:141–160. doi: 10.1113/jphysiol.1965.sp007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A., WESTHEIMER G. The effect upon the rod threshold of bleaching neighbouring rods. J Physiol. 1962 Nov;164:318–329. doi: 10.1113/jphysiol.1962.sp007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J. L., Nunn B. J., Meister M., Baylor D. A. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990 Aug;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeton J. M., van Norren D. Light adaptation of primate cones: an analysis based on extracellular data. Vision Res. 1983;23(12):1539–1547. doi: 10.1016/0042-6989(83)90167-0. [DOI] [PubMed] [Google Scholar]
- Whittle P., Challands P. D. The effect of background luminance on the brightness of flashes. Vision Res. 1969 Sep;9(9):1095–1110. doi: 10.1016/0042-6989(69)90050-9. [DOI] [PubMed] [Google Scholar]
- Whittle P. The brightness of coloured flashes on backgrounds of various colours and luminances. Vision Res. 1973 Mar;13(3):621–638. doi: 10.1016/0042-6989(73)90027-8. [DOI] [PubMed] [Google Scholar]


