Abstract
1. The dense system of horizontal connections that arise and course within the striate cortex are thought to inform single cells about stimuli arising in disparate points in visual space and to modulate responses evoked from within the receptive field. To learn whether or not the strength of the horizontal connections could vary over the long term, and if such changes could affect the integration of vertical, interlaminar inputs, we have recorded intracellularly from the superficial layers in slices of the adult cat's visual cortex. 2. The monosynaptic EPSP evoked by stimulating horizontal fibres showed long-term facilitation in twelve of the twenty cells that were conditioned by repetitively pairing synaptic responses with depolarizing pulses of current; the maximum increase observed was 200%. Strong inhibition present in the postsynaptic response usually indicated that facilitation would not occur. 3. In instances where horizontal input evoked both mono- and polysynaptic EPSPs, both early and late events showed facilitation, with the most dramatic enhancement contributed by the polysynaptic components. 4. For the twenty-eight cells whose responses to stimulation of interlaminar as well as horizontal pathways were assessed, all were found to receive non-overlapping inputs from each source. Conditioning produced long-term changes in the strength of the interlaminar inputs. 5. Changes in synaptic strength were usually confined to the conditioned pathway, though in four out of twenty-six times we observed heterosynaptic facilitation of polysynaptic EPSPs. 6. The conditioning protocol led to lasting depression rather than facilitation in three out of eleven instances; the reduction was only observed in the multisynaptic components. 7. We suggest that the synaptic changes observed here may be related to certain dynamic changes in receptive field properties that have been characterized in vivo.
Full text
PDF


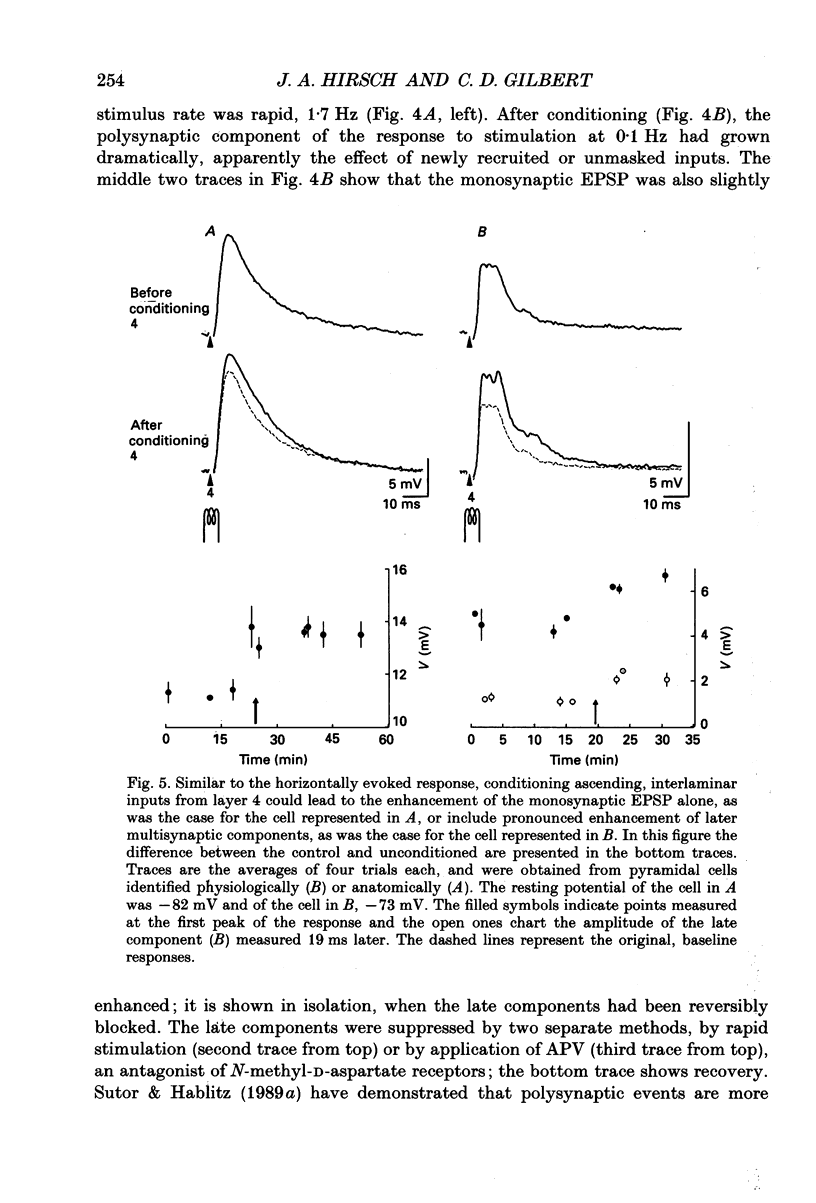

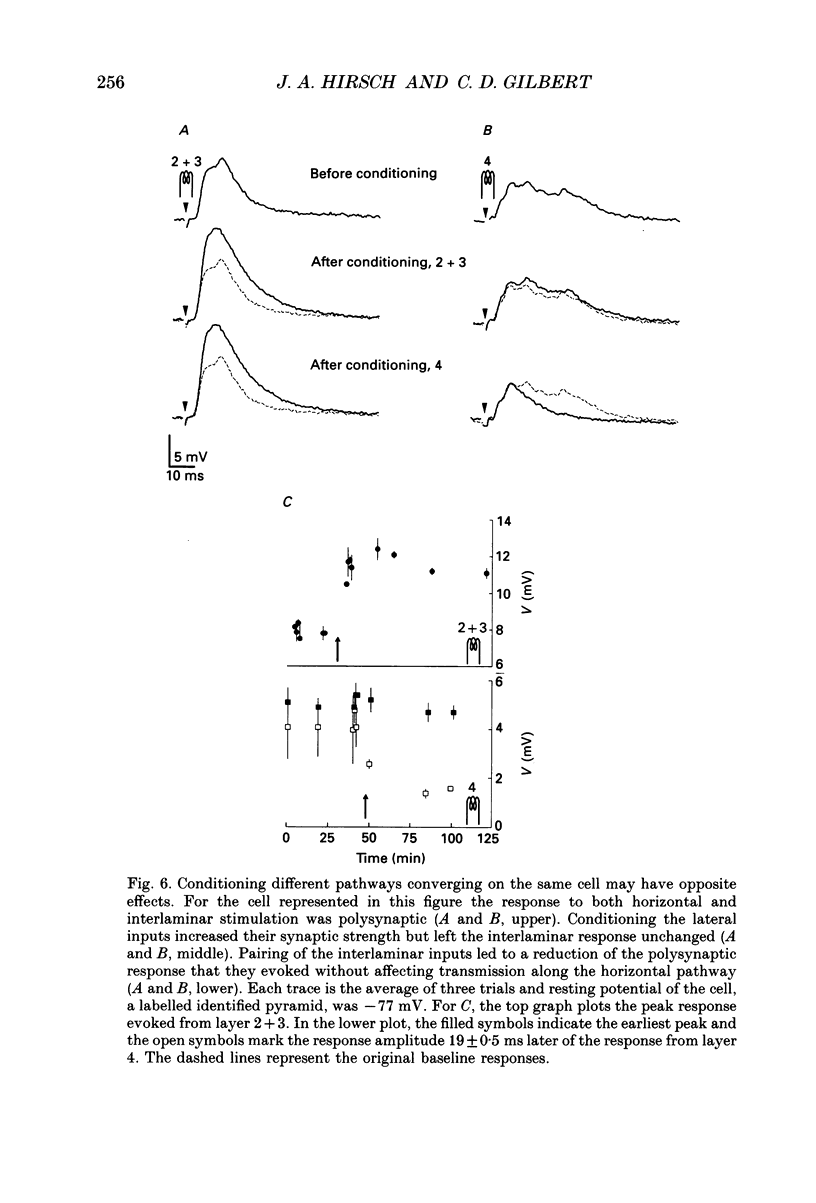
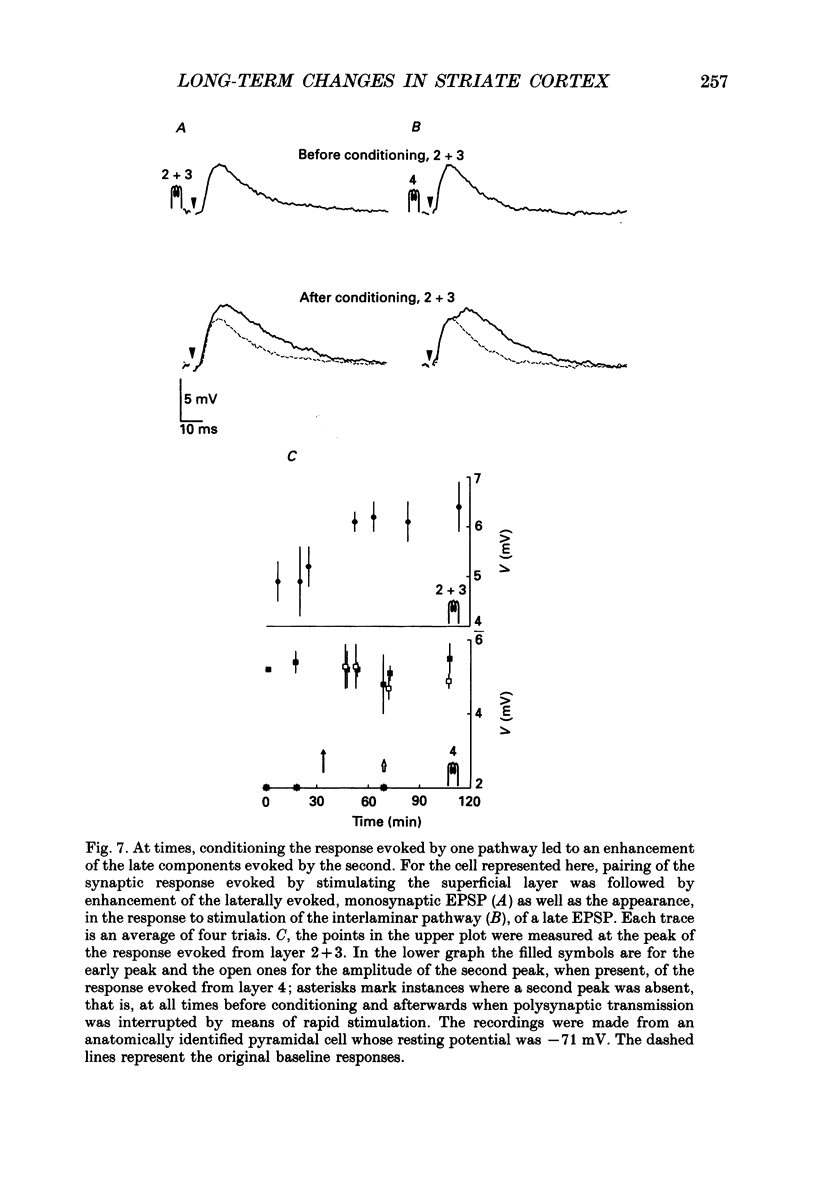





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Goddard G. V. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature. 1983 Oct 20;305(5936):717–719. doi: 10.1038/305717a0. [DOI] [PubMed] [Google Scholar]
- Abraham W. C., Gustafsson B., Wigström H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987 Dec;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A., Bröcher S., Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990 Sep 6;347(6288):69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Baranyi A., Szente M. B. Long-lasting potentiation of synaptic transmission requires postsynaptic modifications in the neocortex. Brain Res. 1987 Oct 13;423(1-2):378–384. doi: 10.1016/0006-8993(87)90867-5. [DOI] [PubMed] [Google Scholar]
- Bindman L. J., Murphy K. P., Pockett S. Postsynaptic control of the induction of long-term changes in efficacy of transmission at neocortical synapses in slices of rat brain. J Neurophysiol. 1988 Sep;60(3):1053–1065. doi: 10.1152/jn.1988.60.3.1053. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T., Staiger V., Aertsen A. Synaptic plasticity in rat hippocampal slice cultures: local "Hebbian" conjunction of pre- and postsynaptic stimulation leads to distributed synaptic enhancement. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Garey L. J., Kuroda R., Wolff J. R. The distribution of degenerating axons after small lesions in the intact and isolated visual cortex of the cat. Exp Brain Res. 1977 Mar 30;27(3-4):419–440. doi: 10.1007/BF00235514. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Starkey S. J., Pozza M. F., Collingridge G. L. GABA autoreceptors regulate the induction of LTP. Nature. 1991 Feb 14;349(6310):609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Hirsch J. A., Wiesel T. N. Lateral interactions in visual cortex. Cold Spring Harb Symp Quant Biol. 1990;55:663–677. doi: 10.1101/sqb.1990.055.01.063. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vision Res. 1990;30(11):1689–1701. doi: 10.1016/0042-6989(90)90153-c. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen S. J., Skavenski A. A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83(3):670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Gilbert C. D. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci. 1991 Jun;11(6):1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. C., Crepel F. Use-dependent changes in synaptic efficacy in rat prefrontal neurons in vitro. J Physiol. 1990 Aug;427:31–49. doi: 10.1113/jphysiol.1990.sp018159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Krubitzer L. A., Chino Y. M., Langston A. L., Polley E. H., Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Katz L. C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987 Apr;7(4):1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F., Nishigori A., Shirokawa T., Tsumoto T. Long-term potentiation and N-methyl-D-aspartate receptors in the visual cortex of young rats. J Physiol. 1989 Jul;414:125–144. doi: 10.1113/jphysiol.1989.sp017680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res. 1976;16(10):1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Nelson J. I., Frost B. J. Intracortical facilitation among co-oriented, co-axially aligned simple cells in cat striate cortex. Exp Brain Res. 1985;61(1):54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983 May 20;216(3):303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Sah P., Nicoll R. A. Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol. 1991 Feb;433:615–630. doi: 10.1113/jphysiol.1991.sp018446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-D-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989 Mar;61(3):621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. Long-term potentiation in frontal cortex: role of NMDA-modulated polysynaptic excitatory pathways. Neurosci Lett. 1989 Feb 13;97(1-2):111–117. doi: 10.1016/0304-3940(89)90148-1. [DOI] [PubMed] [Google Scholar]
- Toyama K., Kimura M., Tanaka K. Organization of cat visual cortex as investigated by cross-correlation technique. J Neurophysiol. 1981 Aug;46(2):202–214. doi: 10.1152/jn.1981.46.2.202. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985 Sep;125(1):159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]


