Abstract
This study investigates the synergistic effects of zinc oxide nanoparticles (ZnO NPs) and melatonin (MT) on Fragaria × ananassa (strawberry) plants under drought stress, focusing on growth, fruit biomass, and stress tolerance. ZnO NPs enhance nutrient uptake and stress resistance, while MT regulates growth hormones and boosts photosynthetic efficiency. Seven treatments were evaluated: T1 (no stress, 0.5 g/L ZnO NPs + 0.1 g/L MT), T2 (no stress, 0.5 g/L ZnO NPs), T3 (no stress, 0.1 g/L MT), T4 (drought stress, no application), T5 (drought stress, 0.5 g/L ZnO NPs + 0.1 g/L MT), T6 (drought stress, 0.5 g/L ZnO NPs), and T7 (drought stress, 0.1 g/L MT). Growth and stress parameters included shoot/root length, fruit biomass, bud number, chlorophyll content, oxidative stress markers (H₂O₂, MDA), and antioxidant enzyme activities in the leaves of Fragaria × ananassa. The combined treatment (ZnO NPs + MT) consistently outperformed others, achieving the highest growth metrics under both conditions: shoot length (22.33 ± 1.53 cm non-stress, 15.00 ± 1.53 cm drought), root length (18.67 ± 1.53 cm non-stress, 12.00 ± 1.53 cm drought), and fruit biomass (9.55 ± 0.31 g non-stress, 5.02 ± 0.23 g drought). Bud formation peaked at 3.33 ± 0.58 buds/plant non-stress and 2.00 ± 0.00 buds/plant drought. Under drought, the combined treatment also enhanced chlorophyll content (2.47 ± 0.20 mg/g FW) and significantly reduced H₂O₂ (28.67 ± 2.52 µmol/g FW) and MDA (4.21 ± 0.10 µmol/g FW) levels, while maximizing antioxidant enzyme activities (SOD: 121.67 ± 7.64 U/g FW, POD: 206.33 ± 14.84 U/g FW, CAT: 48.00 ± 3.61 U/g FW). These findings highlight the combined application of ZnO NPs and MT as a promising strategy to enhance growth and stress tolerance in strawberry plants, warranting further research on optimized concentrations, delivery methods, and molecular mechanisms.
Keywords: ZnO nanoparticles, Melatonin, Strawberry plants, Drought stress, Growth enhancement
Introduction
Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier is a fruit crop, renowned for its rich nutritional profile, which includes vitamins, antioxidants, and phenolic compounds [1, 2]. It plays a vital role in the global horticultural economy, with major producers like the USA, Mexico, and China [3]. However, the crop is susceptible to environmental stresses, particularly drought, due to its shallow root system, high water demand, and susceptibility to oxidative damage [4]. Drought stress disrupts key physiological processes, including stomatal conductance, photosynthesis, nutrient uptake, and leads to the excessive accumulation of reactive oxygen species (ROS), which severely impairs plant growth and yield. These challenges are exacerbated by the increasing frequency of extreme climatic events, necessitating sustainable solutions to enhance strawberry drought resilience [5–7].
Nanotechnology offers innovative strategies to mitigate the effects of abiotic stress on crops [8]. Among various nanomaterials, zinc oxide nanoparticles (ZnO NPs) have garnered attention for their ability to modulate antioxidant enzyme activities, enhance nutrient assimilation, and improve photosynthetic efficiency [9]. The green synthesis of ZnO NPs using plant extracts represents an eco-friendly alternative to conventional methods, aligning with sustainable agricultural practices. However, while ZnO NPs have shown promise in improving drought tolerance in other crops [10–12], their potential application in strawberries under water-deficit conditions remains unexplored.
Melatonin, a naturally occurring indoleamine, has also emerged as a powerful agent for alleviating abiotic stress in plants [13]. It enhances ROS scavenging, stabilizes cellular structures, regulates gene expression related to stress responses, and maintains physiological processes such as photosynthesis and osmotic adjustment under drought conditions [14]. Research on various crops has shown that the exogenous application of melatonin enhances root architecture, maintains chlorophyll content, and strengthens antioxidant defenses [15–17]. Despite these promising benefits, the synergistic potential of combining melatonin with ZnO nanoparticles to mitigate drought stress in strawberries remains unexplored. This research gap offers an opportunity to investigate innovative strategies for enhancing the resilience of high-value crops such as strawberries.
Despite advancements in understanding the individual effects of ZnO NPs and melatonin, their integrated role in mitigating drought stress remains underexplored. Additionally, while sustainable, green synthesis of ZnO NPs has yet to be fully utilized in high-value crops like strawberries under adverse environmental conditions. We hypothesize that the combined application of green-synthesized ZnO NPs and melatonin can synergistically enhance drought tolerance in strawberries by improving physiological processes, reducing oxidative stress, and sustaining growth and productivity. This study aims to evaluate the impact of these treatments on key growth parameters, oxidative stress markers, and physiological processes under drought conditions, contributing to the development of sustainable strategies for strawberry cultivation in drought-prone regions.
Materials and methods
Experimental location
The study was conducted in 2024 at the Department of Botany research area at The Islamia University of Bahawalpur, Punjab, Pakistan (29°24’0” N, 71°41’0” E, 117 m above sea level). This site, located in the southern part of Punjab near the Cholistan Desert, provided an ideal environment for assessing the impact of drought stress on Fragaria × ananassa, due to the region’s semi-arid conditions and extreme temperature fluctuations.
Green synthesis and characterization of ZnO nanoparticles
ZnO nanoparticles (ZnO NPs) were synthesized using a green chemistry approach involving Vachellia nilotica (L.) P.J.H.Hurter & Mabb. leaf extract. V. nilotica was selected for nanoparticle synthesis due to its wide availability, high antioxidant potential, and eco-friendly properties. This plant is known for its rich content of polyphenolic compounds, flavonoids, and tannins, which act as reducing agents and stabilize the nanoparticles [18]. Fresh, healthy leaves of V. nilotica were harvested from local trees in Bahawalpur, washed thoroughly to remove any contaminants, and shade-dried at room temperature for 48 h. The dried leaves were then ground into a fine powder, which was used as a reducing agent in nanoparticle synthesis. ZnO nanoparticles were synthesized by first preparing a mixture of 20 mM tetrabutyl orthotitanate in 50 mL ethanol. After stirring this solution for 20 min, a few drops of nitric acid were added to adjust the pH, followed by the addition of 1 mM zinc nitrate and 1 mM V. nilotica leaf extract. The mixture was continuously stirred for an hour, after which deionized water was added to form a gel. The gel was allowed to mature for 24 h, then dried at 80 °C and annealed at 400 °C for 3 h to obtain grayish ZnO nanoparticles [19].
To evaluate the physical and chemical properties of the synthesized ZnO nanoparticles, several characterization techniques were employed. The optical properties were analyzed using a UV-visible spectrometer (Cary Series, Agilent Technology, USA) over a range of 200–800 nm [20]. The morphology of the nanoparticles was examined using a Nano SEM 450 analyzer with an acceleration voltage of 10 kV, which enabled detailed visualization of the surface structure. Crystallinity was assessed using X-ray diffraction (XRD) with a Bruker AXS D8 Advance X-Ray Diffractometer, employing Cu K-alpha radiation (1.5402 Å).
Soil analysis and sowing method
Soil samples were taken from the experimental pots and analyzed for various physicochemical properties, such as pH, electrical conductivity (EC), organic matter content, and nutrient concentrations. An accredited analytical laboratory performed the analysis. The soil was classified as sandy loam with the following properties: pH 8.1, EC 2.3 dS/m, organic matter content 0.49%, phosphorus 6.5 mg/kg, and potassium 112 ppm (Table 1).
Table 1.
Physico-chemical properties of soil
| Physic-Chemical Properties | Values |
|---|---|
| Electrical Conductivity (EC) | 2.3 dS/m |
| pH | 8.1 |
| Organic Matter | 0.49% |
| Phosphorus (P) | 6.5 mg/kg |
| Potassium (K) | 112 ppm |
| Soil Texture | Sandy Loam |
Fragaria × ananassa seeds were purchased from a local agricultural supplier in Bahawalpur during December 2023. Before sowing, the seeds were sterilized with a 1% sodium hypochlorite solution for 5 min to eliminate any surface contaminants. After sterilization, the seeds were thoroughly washed with distilled water to remove any residual chemicals before being sown [21]. The seeds were sown in mid-December (9–12-2023) in pots filled with prepared soil, which had been pre-moistened with water the day before sowing to ensure optimal germination conditions.
Experimental setup and drought stress induction
The experiment followed a completely randomized design (CRD) with three replications per treatment, and a total of 24 pots were used. Each pot contained three Fragaria × ananassa plants. Drought stress was induced by withholding irrigation at the vegetative stage (45 days after sowing). The drought group (T4-T7) received 50% of the required moisture for the control treatment (T0-T3), causing water deficit and simulating drought conditions. The drought stress was maintained throughout the experiment by providing limited irrigation to drought-stressed plants.
Application of ZnO nanoparticles and melatonin
ZnO nanoparticles were applied to the plants in the drought-stressed group (T4-T7) at a concentration of 0.5 g/L, mixed in water. The solution was applied as a foliar spray to ensure direct contact with the leaves. For melatonin, a 0.1 g/L concentration was applied to the plants in the same group (T4-T7) through root drenching. The melatonin solution was poured around the base of plants, ensuring uptake through the root system. The treatments and their corresponding details are summarized in Table 2:
Table 2.
Experimental treatments and conditions for drought stress induction, ZNO nanoparticle application, and melatonin treatment in Fragaria × ananassa
| Treatment | Drought Stress | ZnO Nanoparticles | Melatonin Application |
|---|---|---|---|
| T0 | No stress | No application | No application |
| T1 | No stress | 0.5 g/L foliar spray | 0.1 g/L root drench |
| T2 | No stress | 0.5 g/L foliar spray | No application |
| T3 | No stress | No application | 0.1 g/L root drench |
| T4 | Drought stress | No application | No application |
| T5 | Drought stress | 0.5 g/L foliar spray | 0.1 g/L root drench |
| T6 | Drought stress | 0.5 g/L foliar spray | No application |
| T7 | Drought stress | No application | 0.1 g/L root drench |
Irrigation practices and fertilizer application
During the experimental period, plants were irrigated with tap water to maintain soil moisture at 60% field capacity. Irrigation was carried out 3–4 times per week. Organic fertilizers, obtained from a regional supplier, were used in the experiment. These fertilizers contained 10–25% organic carbon, 0.5–4.0% nitrogen, 0.5–1.5% phosphorus, 0.5–1.0% potassium.
Crop harvest
At the end of the 90-day experimental period, plants were harvested for analysis. Growth parameters including germination rate, root length, shoot length, leaf number, leaf area, and biomass (shoot and root) were measured to evaluate the overall plant health and vigor.
Growth parameters
Several growth parameters were assessed to determine the overall health and development of the plants. This included root length, shoot length, leaf number, leaf area, and biomass of both shoots and roots. The values for each of these parameters were recorded and used to evaluate the effect of ZnO nanoparticles and melatonin on the plant’s growth under drought conditions.
Physiological parameters
Measurement of chlorophyll contents
Chlorophyll content, which reflects the plant’s photosynthetic capacity, was quantified using the method described by Arnon [22]. Fresh leaves were homogenized in 80% acetone (v/v), and the absorbance was measured at 663 nm for chlorophyll-a and at 645 nm for chlorophyll-b. Total chlorophyll content was calculated by summing the values for chlorophyll-a and chlorophyll-b.
Where: A663 = absorbance at 663 nm, A645 = absorbance at 645 nm, V = volume of extract (ml), W = weight of sample (g).
Electrolyte leakage
Electrolyte leakage was determined by placing the 1 g sample in 10 mL of distilled water in test tubes, which were incubated at room temperature for 24 h. The initial electrical conductivity (EC1) was recorded using a conductivity meter. The samples were then boiled for 20–30 min to fully disrupt the cell membranes, and the final electrical conductivity (EC2) was measured after cooling to room temperature. Electrolyte leakage was calculated as the percentage of EC1 relative to EC2, with higher leakage indicating greater membrane damage due to stress.
Biochemical parameters
Assessment of antioxidant enzyme activity
To evaluate the biochemical responses of the plants under drought stress, the activities of key antioxidant enzymes—superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT)—were measured [23, 24]. Enzyme extracts were prepared by homogenizing frozen plant tissue in a sodium phosphate buffer. CAT activity was measured by monitoring the decomposition of H₂O₂ at 240 nm. POD activity was determined using 4-methyl catechol as the substrate, measuring the increase in absorbance at 420 nm. SOD activity was assayed by measuring the inhibition of nitroblue tetrazolium (NBT) photoreduction, with absorbance recorded at 560 nm.
Determination of oxidative stress markers
The levels of oxidative stress were assessed by measuring malondialdehyde (MDA) and hydrogen peroxide (H₂O₂) content in the leaves of Fragaria × ananassa. Both markers were measured spectrophotometrically, with absorbance recorded at appropriate wavelengths [25, 26].
Data analysis
The data collected from the experiment were analyzed using SPSS 20.0 software. Statistical analysis included the use of ANOVA to evaluate the treatment effects, with significance determined by the Least Significant Difference (LSD) test at p < 0.05. Additionally, correlation and regression analyses were performed to investigate the relationships between various parameters.
Results
Characterization of ZnO nanoparticles
The XRD pattern of the green-synthesized ZnO NPs, produced using zinc acetate dihydrate and the aqueous extract of V. nilotica leaves, is shown in Fig. 1. The observed peaks confirm that the nanoparticles are highly crystalline, with all peaks aligning well with the hexagonal structure of ZnO, as indicated by the reference pattern (ZnO, 04–016-6648). The sharp and distinct peaks demonstrate the high purity and crystallinity of the synthesized ZnO NPs, with no evidence of additional zinc oxide phases or impurities, further affirming the quality of the synthesized nanoparticles.
Fig. 1.
XRD spectra of (a) green and (b) chemically synthesized ZnO nanoparticles
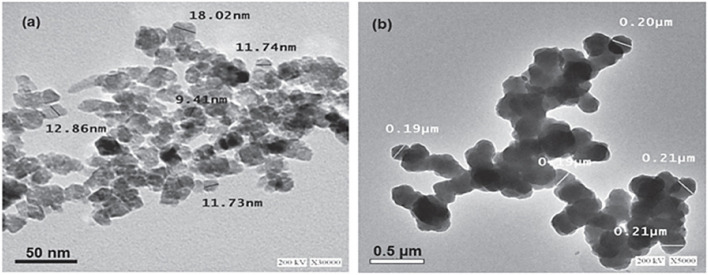
HR-TEM analysis reveals that the green-synthesized ZnO nanoparticles have a particle size range from 9 nm to 18 nm (Fig. 2a). The high-resolution TEM images provide detailed insights into the internal structure, offering a precise measurement of particle sizes. In contrast, Fig. 2b illustrates that the particle size of chemically synthesized zinc oxide nanoparticles ranges from 190 nm to 210 nm, highlighting a significant difference in size between the two synthesis methods.
Fig. 2.
HR-TEM image of (a) green and (b) chemically synthesized ZnO nanoparticles
The optical properties of the green-synthesized ZnO NPs, as measured by UV–vis spectrometry, show a strong absorption band in the range of 340–390 nm (Fig. 3). This absorption is characteristic of ZnO, with a prominent peak observed around 368 nm, which is consistent with the typical absorption wavelength for ZnO nanoparticles.
Fig. 3.
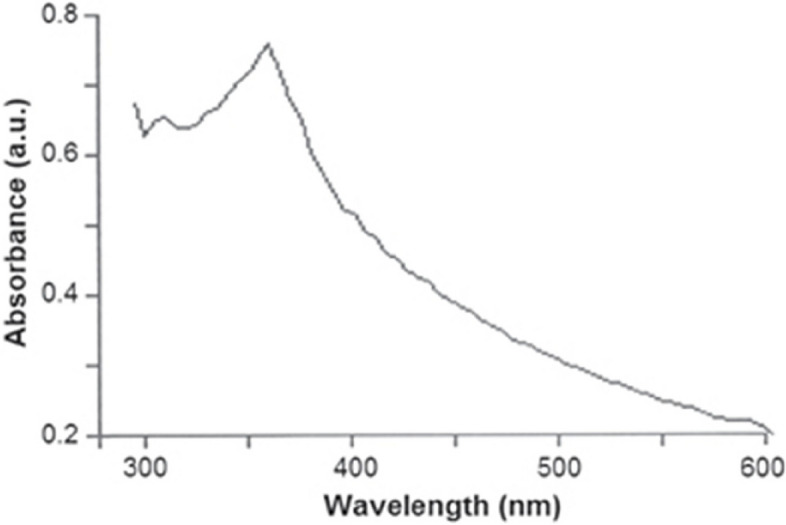
UV–vis spectrum of green synthesized ZnO nanoparticles
Effect of ZnO nanoparticles and melatonin on shoot and root growth parameters
Shoot length (cm)
Under non-stressed conditions, control plants exhibited a mean shoot length of 5.9 ± 0.01 cm. MT application slightly increased shoot length to 6.30 ± 0.10 cm, while ZnO NPs showed a more substantial improvement, with a mean shoot length of 7.02 ± 0.16 cm. Notably, the combination of NPs and MT yielded the greatest shoot length, measuring 7.60 ± 0.10 cm, highlighting a synergistic effect (Fig. 4a). Drought stress significantly reduced shoot length to 3.79 ± 0.22 cm. However, individual applications of MT (4.87 ± 0.07 cm) and NPs (5.03 ± 0.04 cm) mitigated this reduction, with NPs performing slightly better. The combination of NPs and MT under drought stress demonstrated the highest effectiveness in alleviating drought-induced reductions, resulting in a mean shoot length of 5.17 ± 0.03 cm. This treatment not only outperformed individual applications but also approached the shoot length of non-stressed control plants.
Fig. 4.
Bar plot of multiple comparisons of means (a) shoot length (cm), (b) root length (cm), (c) shoot biomass (g), (d) root biomass (g). Error bars represent the standard deviation of the mean of the data. Means with different letters are significantly different at alpha 0.05 from each other. Ctrl (control), MT (0.1 g/L melatonin), NPs (0.5 g/L ZnO NPs), NPs + MT (0.5 g/L ZnO NPs and 0.1 g/L Melatonin combined), drought is drought stress, MT + DT (0.1 g/L melatonin and drought), NPs + DT (0.5 g/L ZnO NPs and drought) and NPS + MT + DT (0.5 g/L ZnO NPs and 0.1 g/L melatonin and drought)
Root length (cm)
Under non-stressed conditions, control plants had a mean root length of 3.17 ± 0.21 cm. MT application increased root length to 3.65 ± 0.09 cm, while ZnO NPs had a modest effect, yielding roots of 3.28 ± 0.06 cm. The combined application of NPs and MT resulted in the longest roots under non-stressed conditions, measuring 4.18 ± 0.08 cm, indicating a synergistic benefit (Fig. 4b). Drought stress reduced root length significantly to 2.33 ± 0.07 cm. MT and NPs individually improved root length under drought conditions to 2.97 ± 0.07 cm and 3.19 ± 0.04 cm, respectively. The combination of NPs and MT proved the most effective under drought stress, achieving a root length of 3.32 ± 0.04 cm. This length exceeded that of control plants and highlighted the potential of combined treatments to mitigate drought effects.
Shoot biomass (g)
In the non-stressed condition, the application of MT resulted in a 5.64% increase in shoot biomass compared to the control, while ZnO NPs treatment provided a 13.14% improvement. The combined treatment of ZnO NPs and MT exhibited the most significant effect, with a 44.68% increase in shoot biomass. Under drought stress, the MT treatment led to a 31.21% improvement in shoot biomass, while ZnO NPs treatment enhanced shoot biomass by 47.41%. The combined application of ZnO NPs and MT under drought conditions demonstrated the highest increase in shoot biomass, with a 53.24% improvement compared to the control (Fig. 4c).
Root biomass (g)
Under drought stress, biomass reductions were substantial (Fig. 4d). Shoot biomass declined to 2.63 ± 0.54 g, and root biomass dropped to 0.03 ± 0.01 g. MT treatment under drought conditions increased shoot biomass to 3.45 ± 0.06 g and root biomass to 0.07 ± 0.02 g. NPs application provided further improvements, with shoot biomass reaching 3.88 ± 0.07 g and root biomass 0.74 ± 0.56 g.
The combined application of NPs and MT under drought stress demonstrated the most effective mitigation, with shoot biomass improving to 4.03 ± 0.06 g and root biomass increasing significantly to 1.12 ± 0.03 g. The combined treatment consistently outperformed individual applications and restored biomass values close to or exceeding those of non-stressed controls.
Effect of ZnO nanoparticles and melatonin on leaf growth parameters
Number of leaves
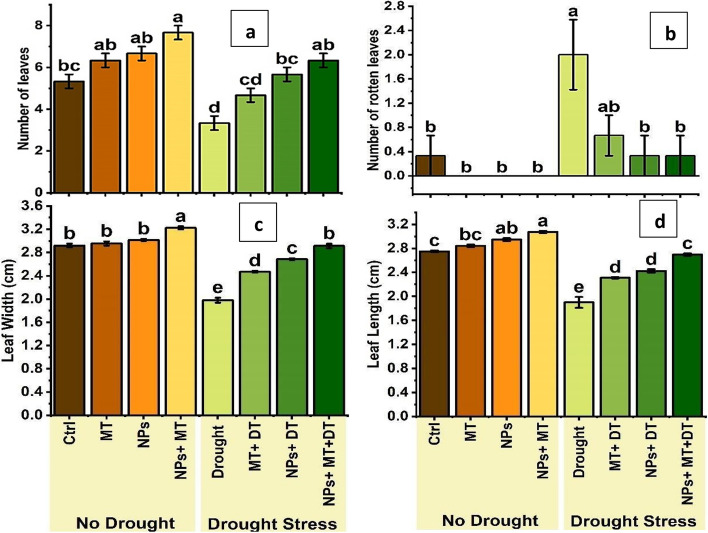
Control plants had 5.33 ± 0.58 leaves. MT increased this to 6.33 ± 0.58, and ZnO NPs to 6.67 ± 0.58. The combination of NPs and MT resulted in the highest leaf number at 7.67 ± 0.58 (Fig. 5a). Drought stress reduced leaf number to 3.33 ± 0.58. MT, ZnO NPs, and their combination mitigated this, with MT improving leaf count to 4.67 ± 0.58, NPs to 5.67 ± 0.58, and the combined treatment to 6.33 ± 0.58. The combination of NPs and MT was the most effective, nearly restoring leaf number to control levels.
Fig. 5.
Bar plot of multiple comparison of means (a) number of leaves, (b) number of rotten leaves, (c) leaf width (cm), (d) leaf length (cm). Error bars represent the standard deviation of the mean of the data. Means with different letters are significantly different at alpha 0.05 from each other. Ctrl (control), MT (0.1 g/L melatonin), NPs (0.5 g/L ZnO NPs), NPs + MT (0.5 g/L ZnO NPs and 0.1 g/L Melatonin combined), drought is drought stress, MT + DT (0.1 g/L melatonin and drought), NPs + DT (0.5 g/L ZnO NPs and drought) and NPS + MT + DT (0.5 g/L ZnO NPs and 0.1 g/L melatonin and drought)
Number of rotten leaves
Control plants had 0.33 ± 0.58 rotten leaves. MT, ZnO NPs, and their combination completely prevented rotting under non-stressed conditions (Fig. 5b). Drought increased rotten leaves to 2.00 ± 1.00. MT reduced this to 0.67 ± 0.58, while both NPs alone and combined with MT reduced it to 0.33 ± 0.58. The combination of NPs and MT was most effective in preventing leaf rot, both under normal and drought conditions.
Leaf width (cm)
Control plants had a width of 2.92 ± 0.06 cm. MT increased slightly to 2.96 ± 0.06 cm, NPs to 3.02 ± 0.03 cm, and the combination to 3.22 ± 0.05 cm (Fig. 5c). Drought stress reduced width to 1.98 ± 0.08 cm. MT increased it to 2.47 ± 0.03 cm, NPs to 2.69 ± 0.03 cm, and the combined treatment to 2.91 ± 0.07 cm. The combined application of NPs and MT was most effective in maintaining leaf width under drought conditions.
Leaf length (cm)
Control plants had a length of 2.75 ± 0.03 cm. MT increased this to 2.84 ± 0.04 cm, NPs to 2.95 ± 0.04 cm, and the combination to 3.07 ± 0.03 cm (Fig. 5d). Drought reduced leaf length to 1.90 ± 0.16 cm. MT increased it to 2.31 ± 0.02 cm, NPs to 2.42 ± 0.05 cm, and the combination to 2.70 ± 0.04 cm. The combination of NPs and MT was most effective in maintaining leaf length under drought stress.
Effect of ZnO nanoparticles and melatonin on photosynthetic pigments
Chlorophyll-a mg/g FW
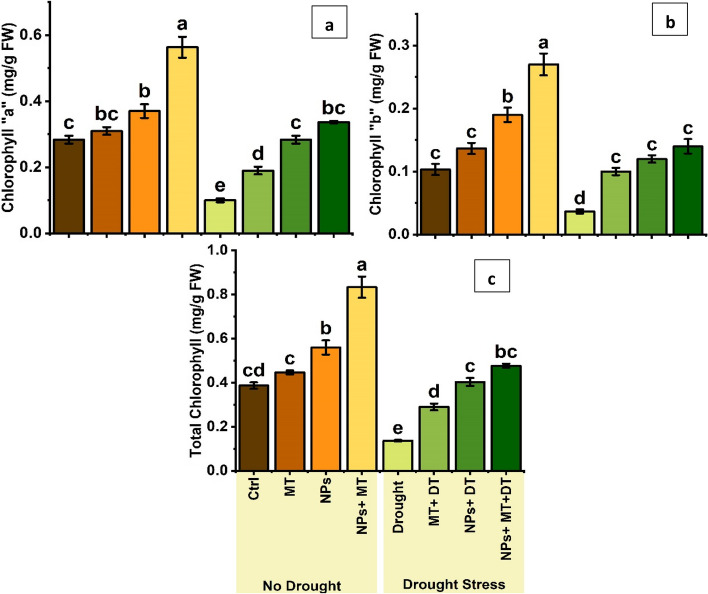
Control plants had 0.28 ± 0.02 mg/g FW. MT increased this to 0.31 ± 0.02 mg/g FW, while ZnO NPs raised it to 0.37 ± 0.04 mg/g FW. The combination of NPs and MT yielded the highest value of 0.56 ± 0.06 mg/g FW (Fig. 6a). Drought stress reduced chlorophyll “a” content to 0.10 ± 0.01 mg/g FW. However, treatments mitigated this effect: MT improved it to 0.19 ± 0.02 mg/g FW, NPs to 0.28 ± 0.02 mg/g FW (equivalent to control plants), and the combination of NPs and MT resulted in 0.34 ± 0.01 mg/g FW, the highest among drought-stressed treatments.
Fig. 6.
Bar plot of multiple comparisons of means (a) chlorophyll a content (mg/g FW), (b) chlorophyll b content (mg/g FW), (c) total chlorophyll content (mg/g FW). Error bars represent the standard deviation of the mean of the data. Means with different letters are significantly different at alpha 0.05 from each other. Ctrl (control), MT (0.1 g/L melatonin), NPs (0.5 g/L ZnO NPs), NPs + MT (0.5 g/L ZnO NPs and 0.1 g/L Melatonin combined), drought is drought stress, MT + DT (0.1 g/L melatonin and drought), NPs + DT (0.5 g/L ZnO NPs and drought) and NPS + MT + DT (0.5 g/L ZnO NPs and 0.1 g/L melatonin and drought)
Chlorophyll-b mg/g FW
Control plants showed 0.10 ± 0.02 mg/g FW. MT increased this to 0.14 ± 0.02 mg/g FW, while NPs raised it to 0.19 ± 0.02 mg/g FW. The combination of NPs and MT achieved the highest chlorophyll “b” content of 0.27 ± 0.03 mg/g FW (Fig. 6b). Drought stress reduced chlorophyll “b” to 0.04 ± 0.01 mg/g FW. Treatments alleviated this, with MT improving it to 0.10 ± 0.01 mg/g FW and NPs to 0.12 ± 0.01 mg/g FW. The combination of NPs and MT under drought stress showed the most significant effect, with 0.14 ± 0.02 mg/g FW, surpassing the chlorophyll “b” content of control plants.
Total chlorophyll mg/g FW
Control plants had 0.39 ± 0.03 mg/g FW. MT increased this to 0.45 ± 0.02 mg/g FW, while NPs raised it to 0.56 ± 0.06 mg/g FW. The combination of NPs and MT resulted in the highest total chlorophyll content of 0.83 ± 0.08 mg/g FW (Fig. 6c). Drought stress reduced total chlorophyll content to 0.14 ± 0.01 mg/g FW. MT improved it to 0.29 ± 0.03 mg/g FW, NPs to 0.40 ± 0.03 mg/g FW, and the combination of NPs and MT achieved 0.48 ± 0.02 mg/g FW, surpassing the chlorophyll content of control plants.
Effect of ZnO nanoparticles and melatonin on oxidative stress markers
H2O2 content (µmol/g FW)
Control plants exhibited a baseline H2O2 content of 23.67 ± 1.53 µmol/g FW (Fig. 7a). Application of MT alone did not significantly alter H2O2 content, which was 23.33 ± 1.53 µmol/g FW, while ZnO NPs treatment caused a more pronounced decrease, reducing the content to 18.33 ± 3.51 µmol/g FW. The combination of NPs and MT led to the lowest H2O2 content of 14.33 ± 1.53 µmol/g FW under non-stressed conditions. Drought stress significantly increased H2O2 content to 80.33 ± 4.16 µmol/g FW. However, both MT and NPs treatments under drought stress effectively mitigated this increase. MT-treated plants under drought stress showed a reduced H2O2 level (54.67 ± 3.51 µmol/g FW), and NPs treatment lowered H2O2 content further to 37.67 ± 3.51 µmol/g FW. Notably, the combination of NPs and MT under drought stress resulted in the lowest H2O2 accumulation (28.67 ± 2.52 µmol/g FW), which was comparable to the non-stressed conditions.
Fig. 7.
Bar plot of multiple comparisons of means (a) H2O2 (µmol/g FW), (b) MDA (µmol /g FW), (c) electrolyte leakage (%), (d) SOD, (e) POD, (f) CAT activity. Error bars represent the standard deviation of the mean of the data. Means with different letters are significantly different at alpha 0.05 from each other. Ctrl (control), MT (0.1 g/L melatonin), NPs (0.5 g/L ZnO NPs), NPs + MT (0.5 g/L ZnO NPs and 0.1 g/L Melatonin combined), drought is drought stress, MT + DT (0.1 g/L melatonin and drought), NPs + DT (0.5 g/L ZnO NPs and drought) and NPS + MT + DT (0.5 g/L ZnO NPs and 0.1 g/L melatonin and drought)
Malondialdehyde content (µmol/g FW)
MDA content in strawberry plants, a marker for lipid peroxidation, was significantly affected by the factors under study (Fig. 7b). Control plants had a mean MDA content of 4.07 ± 0.21 µmol/g FW. Application of MT reduced MDA content to 3.12 ± 0.33 µmol/g FW, and ZnO NPs further reduced it to 2.23 ± 0.08 µmol/g FW. The combination of MT and NPs resulted in the lowest MDA content under non-stressed conditions (2.01 ± 0.10 µmol/g FW). Drought stress significantly elevated MDA content to 8.80 ± 0.56 µmol/g FW, indicating enhanced lipid peroxidation. However, treatment with MT or NPs under drought conditions mitigated this stress-induced increase. Plants treated with MT showed reduced MDA content (4.96 ± 0.12 µmol/g FW), while NPs treatment resulted in 4.64 ± 0.07 µmol/g FW. The combined treatment of NPs and MT under drought stress was most effective, reducing MDA to 4.21 ± 0.10 µmol/g FW.
Electrolyte leakage (%)
Electrolyte leakage, a key indicator of membrane damage, was also significantly affected by the treatments (Fig. 7c). Control plants exhibited a mean electrolyte leakage of 25.33 ± 3.21%. MT application alone reduced leakage to 20.33 ± 1.53%, while NPs treatment decreased it further to 16.33 ± 1.15%. The combination of MT and NPs resulted in the lowest leakage (13.00 ± 2.65%) under non-stressed conditions. Drought stress markedly increased electrolyte leakage to 62.67 ± 4.73%, indicating severe membrane damage. However, MT or NPs application under drought conditions reduced electrolyte leakage. MT-treated plants showed leakage of 44.67 ± 4.04%, and NPs treatment reduced leakage to 35.00 ± 2.00%. The combination of MT and NPs under drought stress resulted in the lowest leakage of 27.33 ± 2.08%, which was close to the levels seen in non-stressed conditions.
Effect of ZnO nanoparticles and melatonin on antioxidant enzyme activities
The antioxidant enzyme activities, such as superoxide dismutase, peroxidase, and catalase were significantly affected by the treatments.
Superoxide dismutase (U/g FW)
SOD activity in control plants was 39.33 ± 5.03 U/g FW (Fig. 7d). MT application increased SOD activity to 60.00 ± 5.00 U/g FW, and ZnO NPs treatment further increased it to 82.33 ± 6.81 U/g FW. The combination of NPs and MT resulted in the highest SOD activity (87.67 ± 10.50 U/g FW) under non-stressed conditions. Drought stress, however, significantly reduced SOD activity to 24.67 ± 5.51 U/g FW. MT or NPs application under drought conditions effectively counteracted this decrease, with MT treatment elevating SOD activity to 66.00 ± 11.00 U/g FW and NPs treatment further enhancing it to 96.00 ± 7.00 U/g FW. The combination of MT and NPs under drought stress resulted in the highest SOD activity (121.67 ± 7.64 U/g FW), which was significantly higher than all other treatments.
Peroxidase (U/g FW)
The POD activity in control plants was 63.00 ± 9.85 U/g FW (Fig. 7e). MT treatment increased POD activity to 87.00 ± 9.17 U/g FW, while ZnO NPs treatment showed a more pronounced effect, increasing POD activity to 119.33 ± 9.50 U/g FW. The combination of NPs and MT resulted in the highest POD activity (143.33 ± 10.41 U/g FW) under non-stressed conditions. Drought stress reduced POD activity to 43.33 ± 4.04 U/g FW, but both MT and NPs treatments under drought stress counteracted this decrease. MT-treated plants showed improved POD activity (85.67 ± 8.08 U/g FW), while NPs treatment increased POD activity to 157.67 ± 18.15 U/g FW. The combination of MT and NPs under drought stress showed the highest POD activity (206.33 ± 14.84 U/g FW), significantly surpassing all other treatments.
Catalase (U/g FW)
CAT activity in control plants was 16.33 ± 2.52 U/g FW (Fig. 7f). MT treatment increased CAT activity to 21.67 ± 2.52 U/g FW, while NPs treatment further elevated it to 29.00 ± 2.00 U/g FW.
The combination of NPs and MT resulted in the highest CAT activity (42.00 ± 3.61 U/g FW) under non-stressed conditions. Drought stress reduced CAT activity to 11.00 ± 2.65 U/g FW. MT application under drought conditions increased CAT activity to 21.33 ± 2.08 U/g FW, and NPs treatment elevated it to 30.67 ± 2.52 U/g FW. The combination of MT and NPs under drought stress showed the highest CAT activity (48.00 ± 3.61 U/g FW), surpassing all other treatments, including those under non-stressed conditions.
Impact of ZnO nanoparticles and melatonin on bud formation and Fruit Biomass
Number of buds
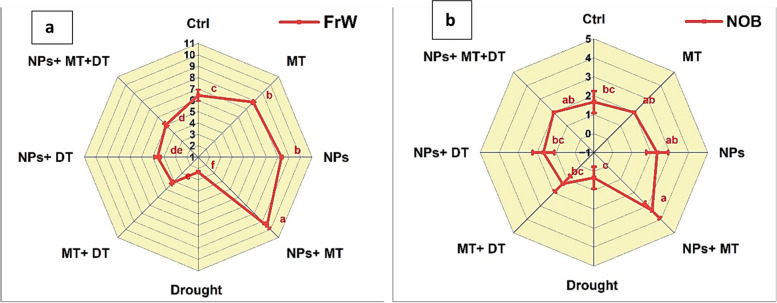
Control plants had 1.67 ± 0.58 buds, while MT increased it to 2.00 ± 0.00 buds, and ZnO NPs raised it to 2.33 ± 0.58. The combination of NPs and MT under non-stressed conditions led to the highest number of buds, 3.33 ± 0.58. Drought stress reduced bud formation to 0.33 ± 0.58, but both MT (1.33 ± 0.58) and NPs (1.67 ± 0.58) improved bud count. The combination of NPs and MT under drought conditions achieved 2.00 ± 0.00 buds, matching the performance of MT-treated plants under non-stressed conditions (Fig. 8a).
Fig. 8.
Radar chart showing fruit biomass (FrW) (a) and number of buds (NOB) (b) under different treatments studied in the experiment. Ctrl (control), MT (0.1 g/L melatonin), NPs (0.5 g/L ZnO NPs), NPs + MT (0.5 g/L ZnO NPs and 0.1 g/L Melatonin combined), drought is drought stress, MT + DT (0.1 g/L melatonin and drought), NPs + DT (0.5 g/L ZnO NPs and drought) and NPS + MT + DT (0.5 g/L ZnO NPs and 0.1 g/L melatonin and drought)
Fruit biomass (g)
Control plants showed 6.43 ± 0.50 g of fruit biomass, while MT increased it to 7.83 ± 0.15 g, and ZnO NPs raised it to 8.32 ± 0.11 g (Fig. 8b). The combination of NPs and MT under non-stressed conditions resulted in the highest fruit biomass, 9.55 ± 0.31 g. Drought stress significantly reduced fruit biomass to 2.31 ± 0.09 g. However, MT (4.20 ± 0.20 g) and NPs (4.57 ± 0.28 g) individually alleviated the reduction. The combination of NPs and MT under drought conditions resulted in 5.02 ± 0.23 g of fruit biomass, which was the highest among drought-stressed treatments and close to the control plants’ biomass.
Correlation analysis of growth and physiological attributes in strawberry plants under ZnO nanoparticles and Melatonin treatments
Figure 9 illustrates the Pearson correlation matrix for growth and physiological attributes of strawberry plants treated with ZnO NPs and MT under drought stress. Positive correlations (red intensities) were observed among vegetative growth parameters, including the number of leaves, leaf area, and leaf length, with root length, shoot length, plant biomass and total chlorophyll content, indicating enhanced growth under treatments. Conversely, oxidative stress markers such as electrolyte leakage, H2O2, and malondialdehyde exhibited strong negative correlations with chlorophyll content, fruit weight, and plant biomass, suggesting reduced oxidative damage. Antioxidant enzyme activities (SOD, POD, CAT) positively correlated with chlorophyll content and fruit biomass, emphasizing their role in stress mitigation.
Fig. 9.
Pearson correlation for different growth attributes of strawberry plant in response to ZnO NPs and MT under drought stress. Intensity of blue color shows negative correlation while intensity of red color is showing positive correlation. NOL = number of leaves; LW = leaf area; LL = leaf length; NORL = number of rotten leaves; NOB = number of buds; H 2 O 2 = hydrogen peroxide content; EL = electrolyte leakage; Cha = chlorophyll a content; Chb = chlorophyll b content; Tchl = total chlorophyll; FrW = fruit biomass; SL = shoot length; RL = root length; PL = plant length; SW = shoot biomass; RW = root biomass; PW = plant biomass.
The number of rotten leaves negatively correlated with key growth parameters, highlighting the adverse effects of drought. These results demonstrate the synergistic impact of ZnO NPs and MT in promoting growth, mitigating oxidative stress, and improving drought resilience in strawberry plants.
Discussion
The green synthesis of ZnO nanoparticles using V. nilotica leaf extract exhibited superior characteristics compared to their chemically synthesized counterparts, as confirmed through XRD, HR-TEM, and UV-vis analyses. The smaller particle size (9–18 nm) of the green-synthesized ZnO NPs aligns with previous reports indicating that plant-mediated synthesis results in smaller and more uniformly distributed nanoparticles due to the biomolecules present in plant extracts that act as capping and stabilizing agents [27]. The high crystallinity and absence of impurities observed via XRD emphasize the suitability of the green synthesis approach for agricultural applications. Smaller particle sizes enhance the surface area-to-volume ratio, increasing interaction with plant tissues and improving stress mitigation. Furthermore, UV-vis analysis validated the successful synthesis of ZnO NPs, with an absorption peak around 368 nm, consistent with findings in similar studies [28].
The combined application of ZnO nanoparticles and melatonin significantly improved growth, physiological, and biochemical responses under drought stress, emphasizing the strong interrelationship between these parameters. Growth parameters, such as shoot and root lengths, exhibited positive correlations with chlorophyll content and antioxidant enzyme activities, indicating that the improved growth observed in treated plants was closely linked to enhanced photosynthetic efficiency and reduced oxidative stress. ZnO NPs likely facilitated nutrient uptake and supported auxin biosynthesis, promoting root elongation, while MT mitigated oxidative damage in meristematic tissues. These results align with Saleem et al. [29], who emphasized the role of Zn in auxin transport and cell wall flexibility. The best performance was achieved with the 20 ppm ZnO NPs + 100 µM MT treatment, which optimally balanced nutrient availability and cellular protection, leading to increased shoot and root lengths and biomass. ZnO NPs contributed to root architecture improvement and auxin dynamics, while MT stabilized cellular structures and reduced oxidative damage, facilitating cell division and elongation [30, 31]. Enhanced biomass under this treatment showed the importance of Zn in nutrient uptake and root proliferation [32].
Drought-induced reductions in biomass are often linked to oxidative stress and water deficits. However, treated plants exhibited significantly increased biomass due to cell membrane stabilization by ZnO NPs and osmoprotective effects of MT. Stabilized cell membranes reduce water loss, while MT maintains cellular hydration, enabling metabolic activities even under stress. These findings are consistent with Anik et al. [33], who reported enhanced biomass and photosynthesis in Zn-treated plants under drought conditions. Biomass improvements signify the plant’s ability to sustain energy production and growth despite stress, making these treatments vital for maintaining crop yield under water-deficit scenarios.
Leaf characteristics, including size and number, showed strong positive correlations with photosynthetic pigments. Retaining healthy, larger leaves under treatment ensured sustained photosynthesis and carbohydrate production, crucial for plant growth and resilience. ZnO NPs contributed to chlorophyll biosynthesis and structural integrity, while MT delayed leaf senescence by scavenging ROS and activating stress-responsive genes. These results corroborate Nazir et al. [34], who demonstrated that Zn improves the leaf area index, enhancing light absorption and energy conversion. Healthy leaf retention under stress highlights the importance of these treatments in maintaining energy production during drought.
Biochemical analyses revealed strong inverse correlations between oxidative stress markers (MDA and H₂O₂) and antioxidant enzyme activities. Treated plants showed elevated levels of SOD, CAT, and POD, coupled with reduced MDA and H₂O₂ levels, demonstrating effective oxidative stress mitigation. ZnO NPs served as cofactors for enzymatic antioxidants, while MT upregulated stress-responsive genes and directly scavenged ROS. These findings are consistent with Li et al. [35], who reported reduced lipid peroxidation and oxidative damage in Zn-treated plants. The highest antioxidant enzyme activities and the lowest oxidative stress marker levels were observed in the 20 ppm ZnO NPs + 100 µM MT treatment, reinforcing the synergy between ZnO NPs and MT in optimizing enzymatic defenses and cellular protection under drought conditions.
Chlorophyll contents, essential for photosynthetic efficiency, were positively correlated with growth and physiological parameters. ZnO NPs preserved chloroplast structures, while MT prevented pigment degradation by reducing ROS accumulation [36]. These findings are consistent with Ahmed et al. [37], who reported that Zn treatments stabilize pigment levels under stress, contributing to improved photosynthetic capacity. The observed correlations emphasize the central role of pigment stability in energy production, growth, and overall plant health during drought stress [38].
The integration of correlation analysis into this study underscores the interconnectivity of growth, physiological, and biochemical responses in drought-stressed plants treated with ZnO NPs and MT [39–44]. Strong positive correlations between antioxidant enzyme activities, water retention, and photosynthetic efficiency indicate that these treatments effectively mitigate drought-induced damage [45–49]. These findings highlight the potential of ZnO NPs and MT as sustainable agricultural tools for enhancing crop productivity under challenging environmental conditions. Future research should focus on optimizing application strategies and exploring the mechanisms underlying these correlations to maximize their practical applicability in diverse agro-ecological settings [50–53].
The best performance of the 20 ppm ZnO NPs + 100 µM MT treatment can be attributed to the synergistic mechanisms at play. ZnO NPs not only enhanced nutrient uptake but also acted as a catalyst in key enzymatic reactions, while MT improved cellular redox homeostasis and osmoprotective mechanisms. This balanced approach tackled both the nutrient deficiency aspect of drought stress and the oxidative damage, making it a comprehensive solution.
A few limitations linked to the study include the fact that it was conducted under controlled greenhouse conditions, which may not fully reflect the complexities of field environments, where temperature fluctuations, soil variability, and other environmental factors are present. Additionally, the study focused on a single plant variety and stress type, potentially limiting the applicability of the findings to other crops or stressors. Furthermore, the long-term impact of ZnO nanoparticle accumulation in the soil and potential ecological effects were not assessed. Future research should incorporate multi-seasonal field trials and explore the potential environmental impacts.
Conclusion
This study demonstrates the significant potential of ZnO NPs synthesized using Vachellia nilotica leaf extract and melatonin in improving growth and physiological performance of strawberry plants under both non-stressed and drought conditions. The green-synthesized ZnO NPs exhibited high crystallinity and small particle size, as confirmed by XRD and HR-TEM analysis, making them highly effective in enhancing plant growth. The application of ZnO NPs and MT individually improved shoot and root lengths, biomass production, and leaf growth, while their combined application yielded the most favorable outcomes. Specifically, under drought stress, the combination of NPs and MT effectively mitigated reductions in growth parameters, including shoot and root lengths, biomass, and leaf area. Furthermore, this combination enhanced photosynthetic pigments, such as chlorophyll a, chlorophyll b, and total chlorophyll, which are critical for maintaining plant health under stress. Overall, the synergistic effects of ZnO NPs and MT offer a promising strategy for alleviating drought stress and improving plant resilience, with potential applications in agricultural practices aimed at enhancing crop productivity in water-limited environments.
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project number (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Authors’ contributions
TA: Methodology, supervision, Writing and drafting, and research design; AS: Experimentation and data Curation HQ: Validation and Software; writing, Investigation, drafting, statistical analysis and validation; EHS: writing, Software, Resource, research design, validation; MTN: data collection, drafting, statistical analysis; WS: writing, funding, statistical analysis, validation, Review, and Editing. All authors have read and approved the final manuscript and declare that they have no competitive interest.
Funding
The authors extend their appreciation to Researchers Supporting Project number (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Data availability
The current study did not involve the generation of new sequencing data. Therefore, there are no datasets generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tauseef Anwar, Email: tauseef.anwar@iub.edu.pk.
Huma Qureshi, Email: huma.qureshi@uoc.edu.pk.
Muhammad Tahir Naseem, Email: nmtahir@yu.ac.kr.
References
- 1.Sharma S, Joshi VK, Abrol GS. An overview on strawberry [Fragaria × ananassa (Weston) Duchesne ex Rozier] wine production technology, composition, maturation and quality evaluation. Nat Prod Radiance. 2009;8(4):356–65. [Google Scholar]
- 2.Bezerra M, Ribeiro M, Cosme F, Nunes FM. Overview of the distinctive characteristics of strawberry, raspberry, and blueberry in berries, berry wines, and berry spirits. Compr Rev Food Sci Food Saf. 2024;23(3): e13354. 10.1111/1541-4337.13354. [DOI] [PubMed] [Google Scholar]
- 3.Simpson D. The economic importance of strawberry crops. In: Hytönen T, Graham J, Harrison R, editors. The genomes of rosaceous berries and their wild relatives. Compendium of plant genomes. Cham: Springer; 2018. 10.1007/978-3-319-76020-9_1. [Google Scholar]
- 4.Zahedi SM, Hosseini MS, Hoveizeh F, Kadkhodaei N, S., Vaculík M. Physiological and biochemical responses of Commercial Strawberry cultivars under Optimal and Drought stress conditions. Plants (Basel Switzerland). 2023;12(3):496. 10.3390/plants12030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yenni M, Ibrahim MH, Nulit R, Sakimin SZ. Influence of drought stress on growth, biochemical changes and leaf gas exchange of strawberry (Fragaria × ananassa Duch.) In Indonesia. AIMS Agric Food. 2022;7(1):37–60. 10.3934/agrfood.2022003. [Google Scholar]
- 6.Blanke MM, Cooke DT. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004;42:153–60. 10.1023/B:GROW.0000017489.21970.d4. [Google Scholar]
- 7.Sun C, Li X, Hu Y, Zhao P, Xu T, Sun J, Gao X. Proline, sugars, and antioxidant enzymes respond to drought stress in the leaves of strawberry plants. Korean J Hortic Sci Technol. 2015;33(5):625–32. 10.7235/hort.2015.15054. [Google Scholar]
- 8.Liu Z, Faizan M, Zheng L, Cui L, Han C, Chen H, Yu F. Nanoparticles Enhance Plant Resistance to Abiotic stresses: a bibliometric statistic. Agronomy. 2023;13(3):729. 10.3390/agronomy13030729. [Google Scholar]
- 9.Umair Hassan M, Huang G, Haider FU, Khan TA, Noor MA, Luo F, Zhou Q, Yang B, Haq U, Iqbal MM. Application of zinc oxide nanoparticles to mitigate cadmium toxicity: mechanisms and future prospects. Plants (Basel Switzerland). 2024;13(12):1706. 10.3390/plants13121706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raeisi Sadati SY, Godehkahriz J, Ebadi S, Sedghi M. Zinc oxide nanoparticles enhance drought tolerance in wheat via physio-biochemical changes and stress genes expression. Iran J Biotechnol. 2022;20(1):12–24. 10.30498/ijb.2021.280711.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimian Z, Samiei L. ZnO nanoparticles efficiently enhance drought tolerance in Dracocephalum kotschyi through altering physiological, biochemical and elemental contents. Front Plant Sci. 2023;14:1063618. 10.3389/fpls.2023.1063618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semida WM, Abdelkhalik A, Mohamed GF, El-Mageed A, El-Mageed TAA, Rady SA, Ali EF. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L). Plants. 2021;10(2): 421. 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad I, Song X, Hussein Ibrahim ME, Jamal Y, Younas MU, Zhu G, Zhou G, Adam Ali AY. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front Plant Sci. 2023;14:1108507. 10.3389/fpls.2023.1108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Zheng B. Melatonin mediated regulation of drought stress: physiological and molecular aspects. Plants (Basel Switzerland). 2019;8(7):190. 10.3390/plants8070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Wang J, Zhang T, Li M, Yan H, Liu Q, Wei Y, Ji X, Zhao Q. Exogenous melatonin positively regulates rice root growth through promoting the antioxidant system and mediating the auxin signaling under root-zone hypoxia stress. Agronomy. 2023;13(2): 386. 10.3390/agronomy13020386. [Google Scholar]
- 16.Gao T, Liu X, Tan K, Zhang D, Zhu B, Ma F, Li C. Introducing melatonin to the horticultural industry: physiological roles, potential applications, and challenges. Hortic Res. 2022;9: uhac094. 10.1093/hr/uhac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karumannil S, Khan TA, Kappachery S, Gururani MA. Impact of exogenous melatonin application on photosynthetic machinery under abiotic stress conditions. Plants (Basel). 2023;12(16): 2948. 10.3390/plants12162948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubair M, Azeem M, Mumtaz R, Younas M, Adrees M, Zubair E, Khalid A, Hafeez F, Rizwan M, Ali S. Green synthesis and characterization of silver nanoparticles from Acacia nilotica and their anticancer, antidiabetic and antioxidant efficacy. Environ Pollution (Barking Essex: 1987). 2022;304:119249. 10.1016/j.envpol.2022.119249. [DOI] [PubMed] [Google Scholar]
- 19.Jamjoum HAA, Umar K, Adnan R, Razali MR, Mohamad Ibrahim MN. Synthesis, characterization, and photocatalytic activities of Graphene Oxide/metal Oxides nanocomposites: a review. Front Chem. 2021;9:752276. 10.3389/fchem.2021.752276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karam ST, Abdulrahman AF. Green Synthesis and characterization of ZnO nanoparticles by using Thyme Plant Leaf Extract. Photonics. 2022;9(8):594. 10.3390/photonics9080594. [Google Scholar]
- 21.Sauer DB, Burroughs R. Disinfection of seed surfaces with Sodium Hypochlorite. Phytopathology. 1986;76:745–9. 10.1094/Phyto-76-745. [Google Scholar]
- 22.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1–15. 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarker U, Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep. 2018;8(1):16496. 10.1038/s41598-018-34944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alici EH, Arabaci G. Determination of SOD, POD, PPO, and CAT enzyme activities in Rumex obtusifolius L. Annual Res Rev Biology. 2016;11(3):1–7. [Google Scholar]
- 25.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Mihailova I, Krasovska M, Sledevskis E, Gerbreders V, Mizers V, Ogurcovs A. Assessment of oxidative stress by detection of H2O2 in Rye samples using a CuO- and Co3O4-Nanostructure-based Electrochemical Sensor. Chemosensors. 2023;11(10):532. 10.3390/chemosensors11100532. [Google Scholar]
- 27.Ying S, Guan Z, Ofoegbu PC, Clubb P, Rico C, He F, Hong J. Green synthesis of nanoparticles: current developments and limitations. Environ Technol Innov. 2022;26:102336. 10.1016/j.eti.2022.102336. [Google Scholar]
- 28.Al-Askar AA, Hashem AH, Elhussieny NI, Saied E. Green Biosynthesis of Zinc Oxide nanoparticles using Pluchea indica Leaf Extract: antimicrobial and photocatalytic activities. Molecules. 2023;28(12):4679. 10.3390/molecules28124679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleem MH, Usman K, Rizwan M, Jabri A, Alsafran M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front Plant Sci. 2022;13: 1033092. 10.3389/fpls.2022.1033092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabaghkar Y, Eghlima G, Babashpour-Asl M, et al. Exploring the impact of exogenous melatonin on agro-morphological characteristics, carvacrol, and rosmarinic acid production in Satureja Rechingeri Jamzad under drought stress. Chem Biol Technol Agric. 2024;11:112. 10.1186/s40538-024-00643-4. [Google Scholar]
- 31.Mohammadi M, Eghlim G, Aghamir F, Nezamdoost D, Bagnazari M, Shirani Bidabadi S. Melatonin alleviates drought stress and increases lutein and zeaxanthin industrial pigments in African marigold by some physiological regulations. Ind Crops Prod. 2024;222(5): 120089. 10.1016/j.indcrop.2024.120089. [Google Scholar]
- 32.Hassan MU, Aamer M, Umer Chattha M, Haiying T, Shahzad B, Barbanti L, Nawaz M, Rasheed A, Afzal A, Liu Y, Guoqin H. The critical role of zinc in plants facing the Drought stress. Agriculture. 2020;10(9): 396. 10.3390/agriculture10090396. [Google Scholar]
- 33.Anik TR, Mostofa MG, Rahman MM, Khan MAR, Ghosh PK, Sultana S, Das AK, Hossain MS, Keya SS, Rahman MA, Jahan N, Gupta A, Tran LP. Zn Supplementation mitigates Drought effects on Cotton by improving photosynthetic performance and antioxidant defense mechanisms. Antioxid (Basel Switzerland). 2023;12(4):854. 10.3390/antiox12040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazir MA, Hasan M, Mustafa G, et al. Zinc oxide nano-fertilizer differentially effect on morphological and physiological identity of redox-enzymes and biochemical attributes in wheat (Triticum aestivum L). Sci Rep. 2024;14:13091. 10.1038/s41598-024-63987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Yang Y, Jia L, Chen H, Wei X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf. 2013;89:150–7. 10.1016/j.ecoenv.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Raeisi Sadati SY, Godehkahriz J, Ebadi S, Sedghi M. Zinc oxide nanoparticles enhance Drought Tolerance in Wheat via Physio-biochemical changes and stress genes expression. Iran J Biotechnol. 2022;20(1):e3027. 10.30498/ijb.2021.280711.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed M, Marrez DA, Rizk R, Zedan M, Abdul-Hamid D, Decsi K, Kovács GP, Tóth Z. The influence of zinc oxide nanoparticles and salt stress on the morphological and some biochemical characteristics of Solanum lycopersicum L. plants. Plants (Basel). 2024;13(10):1418. 10.3390/plants13101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng G, Wu Z, Cao W, Wang Y, Deng X, Zhou Y. Identification of anti-nociceptive constituents from the pollen of Typha angustifolia L. using effect-directed fractionation. Nat Prod Res. 2020;34(7):1041–5. 10.1080/14786419.2018.1539979. [DOI] [PubMed] [Google Scholar]
- 39.Akpor OB, Ezekudo EO, Sobajo OA, Edoh PA, Mabayoje SO. Optimization and antimicrobial properties of biosurfactant production by four indigenous soil bacterial species. Asian J Agric Biol. 2023;2023:2022146. 10.35495/ajab.2022.146.
- 40.Bui VH, Nguyen HC, Ngo QH. Establishment of rice yield prediction model using soil compaction. Asian J Agric Biol. 2023;2023:202109327. 10.35495/ajab.2021.09.327.
- 41.Fatemi R, Yarnia M, Mohammadi S, Vand EK, Mirashkari B. Screening barley genotypes in terms of some quantitative and qualitative characteristics under normal and water deficit stress conditions. Asian J Agric Biol. 2023;2023:2022071. 10.35495/ajab.2022.071.
- 42.Fatmawati U, Sari DP, Santosa S, Wiraswati SM. IAA-producing and phosphate solubilizer of rhizosphere actinobacteria consortium to promote plant growth in soybean (Glycine max L.). Asian J Agric Biology. 2023;2023:2021402. 10.35495/ajab.2021.402. [Google Scholar]
- 43.Ijaz M, Afzal A, Shabbir G, Iqbal J, Rafique M. Breeding wheat for leaf rust resistance: past, present and future. Asian J Agric Biol. 2023;2023:2021426. 10.35495/ajab.2021.426.
- 44.Irawan AP, Rahmawati A, Fahmi UA, Budiman A, Maghfiroh KQ, Erfianti T, Andeska DP, Putri RAE, Nurafifah I, Sadewo BR, Suyono EA. Studies on bioflocculant exopolysaccharides (EPS) produced by Anabaena sp. and its application as bioflocculant for low-cost harvesting of Chlorella Sp. Asian J Agric Biol. 2023;2023:2022150. 10.35495/ajab.2022.150.
- 45.Ishaq L, Simamora AV, Bako PO, Benggu YI, Airthur MM, Roefaida E, Nguru ESO. Abundance of arbuscular mycorrhizal fungi in the rhizosphere of healthy and declining citrus in East Nusa Tenggara, Indonesia. Asian Journal of Agriculture and Biology. 2023;2023:2023011. 10.35495/ajab.2023.011. [Google Scholar]
- 46.Ismail HN, Noor NM, Ahmad Z, Wan Anuar WNH. Algal composition in the ecosystem of rice field under the application of herbicides and insecticides. Asian J Agric Biol. 2023;2023:202106254. 10.35495/ajab.2021.06.254.
- 47.Kupradit C, Ranok A, Mangkalanan S, Khongla C, Musika S. β-glucan and antioxidant activities of four edible mushroom extracts from Thailand. Asian J Agric Biol. 2023;2023:202107285. 10.35495/ajab.2021.07.285.
- 48.Le Thanh T, Hoang NH, Thumanu K, Saengchan C, Daddam JR, Sangpueak R, Papathoti NK, Buensanteai K. Expression and role of defense components in Bacillus subtilis-treated rice plants against Xanthomonas oryzae pv. oryzae. Asian Journal of Agriculture and Biology. 2023;2023:2022161. 10.35495/ajab.2022.161. [Google Scholar]
- 49.Li C, Ahmad S, Cao C. Mitigation of climate crisis from rice paddy field by tillage combination in central China. Asian J Agric Biol. 2023;2023:2022122. 10.35495/ajab.2022.122.
- 50.Osei AF, Jin X, Abdullah WZBW, Sidique SNM. Silicon improves strawberry plants’ nutrient uptake and epicuticular wax formation in a rhizosphere cooling system. Asian J Agric Biol. 2023;2023:2022060. 10.35495/ajab.2022.060.
- 51.Pangaribuan DH, Widagdo S, Hariri AM, Siregar S, Sardio MI. The effect of rice straw mulch and cow urine on growth, yield, quality on sweet corn and pest population density. Asian J Agric Biol. 2023;2023:202103123. 10.35495/ajab.2021.03.123.
- 52.Rejab MRM, Manam NKA, Fauzi NS, Mohamed S, Ngah N. The effectiveness of Furcraea plants in controlling golden apple snail and their effects on the non-target organism at the rice field. Asian J Agric Biol. 2023;2023:202104164. 10.35495/ajab.2021.04.164.
- 53.Safdar ME, Ehsan A, Maqbool R, Ali A, Qamar R, Ali H. Assessing critical period of weed competition in direct-seeded rice (Oryza sativa L.). Asian J Agric Biology. 2023;2023:2022190. 10.35495/ajab.2022.190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The current study did not involve the generation of new sequencing data. Therefore, there are no datasets generated or analyzed during the current study.