Abstract
Objective
To explore the mechanism of hyperbaric oxygen therapy in inhibiting subchondral bone angiogenesis and delaying the progression of osteoarthritis through the PHD2/HIF-1α signaling pathway.
Methods
Mice were randomly divided into three groups (control group, osteoarthritis group, and hyperbaric oxygen treatment group). The effect of hyperbaric oxygen therapy on osteoarthritis was evaluated using Micro-CT, Safranin O-Fast Green staining, and detection of osteoarthritis inflammation markers (MMP-13, ADAMTS-5, Col2a1, and Aggrecan). The activation relationship between PHD2 and downstream signaling pathways was investigated through gene knockout and overexpression experiments. Finally, cell scratch assays, tube formation assays, and chondrogenic differentiation experiments were conducted to verify the mechanism of the PHD2/HIF-1α signaling pathway under hyperbaric oxygen stimulation.
Results
Hyperbaric oxygen therapy delayed the progression of osteoarthritis in mice. It promoted chondrogenic differentiation of mesenchymal stem cells, inhibited angiogenesis, enhanced PHD2 expression, and suppressed the production of HIF-1α and VEGFA. Silencing/overexpression of PHD2 resulted in increased/decreased production of HIF-1α and VEGFA, respectively.
Conclusion
The hyperbaric oxygen environment promotes the expression of PHD2, accelerates the degradation of HIF-1α, and inhibits the production of VEGFA, thereby reducing the generation of type H vessels in subchondral bone.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-025-05514-8.
Keywords: Hyperbaric oxygen therapy, Osteoarthritis, PHD2, HIF-1α, Angiogenesis
Introduction
Osteoarthritis (OA) is a prevalent joint disorder characterized by joint pain, stiffness, and functional impairment. Among its various pathological features, alterations in angiogenesis within the subchondral bone significantly contribute to the progression of OA [1].
Studies have shown that H-type blood vessels (high-perfusion, high-permeability vessels) increase in the subchondral bone of OA, closely associated with disease progression [1]. These vessels may accelerate cartilage destruction by promoting the release of inflammatory cytokines and increasing oxidative stress levels. Therefore, downregulating H-type angiogenesis may be an effective strategy for treating OA. Studies have indicated that hypoxia-inducible factor-1α (HIF-1α) plays a pivotal role in regulating angiogenesis and cellular metabolism [2]. Under hypoxic conditions, the expression of HIF-1α is upregulated [3], promoting the release of angiogenesis-related factors such as vascular endothelial growth factor A (VEGFA), which subsequently enhances angiogenesis. Therefore, in osteoarthritis, excessive activation of HIF-1α may lead to abnormal proliferation of type H-type blood vessels.
Recently, hyperbaric oxygen (HBO) therapy has demonstrated potential in treating OA, particularly through its downregulation of H-type angiogenesis via the HIF-1α pathway [4]. In subchondral bone, the activity of HIF-1α is negatively regulated by PHD2 (HIF prolyl hydroxylase 2). PHD2 hydroxylates HIF-1α, reducing its protein stability and subsequently modulating the expression of its downstream genes [5]. The hyperbaric oxygen environment typically suppresses the expression and production of HIF-1α, influencing downstream signaling pathways and inhibiting the generation of VEGFA. This, in turn, inhibits the formation of H-type blood vessels in subchondral bone, thereby delaying the progression of osteoarthritis.
In summary, research on the mechanism of hyperbaric oxygen therapy (HBOT) downregulating H-type angiogenesis in subchondral bone of knee OA through the PHD2/HIF-1α pathway provides new insights into OA treatment. Future studies should further explore the specific mechanisms of HBOT and its clinical application prospects in OA treatment.
Materials and methods
Study approval
All the animal experiments were complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and received approval from the Institution of Animal Care and Use Committee (IACUC) of the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (approval no. SH9H-2023-A197-SB).
Animal experiments
15 male mice on the C57BL/6J background were acquired from the Central Laboratory of Shanghai Ninth People’s Hospital. All mice were maintained in a specific pathogen-free environment with food and water ad libitum. 15 mice of 8-week-old were divided randomly into 3 groups (n = 5 per group). 10 OA mice was induced through surgical DMM as previously described [6]. Briefly, the mice were anesthetized with 2% isoflurane anesthesia, and the medial meniscotibial ligament in the right knee joint was transected. Other 5 mice were subjected to sham operation. One day after surgery, 5 of the OA mice were treated with HBO (1 h/per day, lasting 4 weeks, 80% oxygen concentration), which were taken as DMM + HBO group. Then all the mice sacrificed 8 weeks after surgery, and the right knee joints were harvested for histological analyses.
Micro-CT evaluation
The knees of mice in different groups were subjected to radiographical evaluation at the indicated time points. In this study, we utilized the mCT-40 micro-CT system from Scanco Medical (Switzer- land) to scan the region of interest in the knee-joint tissue and analyze its growth. The scanning parameters employed were as follows: power current (385 µA), power voltage (65 kV), pixel size (9 μm), filter (AI 1.0 mm), and rotation step (0.4°). The reconstructed images were generated using Bruker’s NRecon software, and the data were analyzed using the CTAn program [7]. The micro-CT parameters assessed included: (a) bone volume fraction (BV/ TV), which represents the ratio of bone surface area to tissue volume; (b) trabecular separation (Tb/Sp), indicating the distance between trabeculae within the bone; (c) trabecular thickness (Tb.Th), reflecting the average thickness of trabeculae and describing their structural changes; (d) trabecular number (Tb.N), which counts the intersections between bone and nonbone tissue within a given length; (e) bone mineral density (BMD), a measure of bone quantity and distribution density.
Histochemical, immunofluorescence, and histomorphometric analyses
Mouse cartilage samples were fixed with 4% paraformaldehyde overnight and then decalcified in 10% EDTA solution for 30 days. After paraffin embedding, each sample was sectioned (5 μm thickness) and stained with safranin-O/fast green. For mouse samples stained with safranin-O/fast green, OARSI scores were evaluated by two independent scorers as previously reported [8]. For immunohistochemistry, slides were incubated with a primary antibody against CD31, or Aggrecan at 4 °C overnight and treated with biotinylated secondary antibodies at 37 °C for 2 h.
Flow cytometry
After dissecting the soft tissue, the knee joints and blood were collected for further processing. To analyze subchondral endothelial cells and mesenchymal stem cells (MSCs), 5 subchondral bone specimens were obtained from 5 knee joints, each containing tibial and femoral subchondral bone. These specimens were utilized for cell isolation and analysis in each sample.
Initially, the outer surface of the knee joint specimens was removed by immersing them in a protease solution (containing 2.5 mg/mL trypsin and 2 mg/mL collagenase A) for 20 min. Subsequently, the specimens were digested for 60 min to isolate the desired cells. Following the lysis of red blood cells using BD FACSTM (BD Biosciences, San Jose, CA), the cells present in the supernatant were collected and utilized for various analyses. These analyses included determining the number of total endothelial cells (ECs), CD31+ Endomucin+ (Emcn+) cells, and CD31−Emcn− cells, as well as assessing alterations in MSCs [9].
Flow cytometry analysis was conducted using antibodies against CD31-APC (RD, FAB3628A-025), Endomucin-FITC (Santa Cruz, sc-65495), and Sca-1-PE (BioLegend, 108107). CD31 + Emcn + cells were plotted and sorted by initially setting standard quadrant gates. The cells were then resuspended in staining buffer and counted using an LSR II flow cytometer (BD Biosciences). Data collection was performed using Cell Quest software on a FACS Calibur flow cytometer (Becton Dickinson). The collected data were analyzed and contour plots were created using Flow-Jo software.
Chondrocyte culture and treatment
Chondrocytes were isolated from the knee joints of newborn mice as previously described41 and cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS). Once they reached 80% confluence, the cells were subjected to com treatment, hypoxia treatment, or HBO treatment (hyperbaric oxygen therapy, HBO). For HBO, chondrocytes were cultured in the cell incubator with 60% oxygen concentration, and hypoxia treatment means 5% oxygen concentration, com treatment means 20% oxygen concentration. To preserve the chondrocyte phenotype, cells were used for in vitro experiments after fewer than two passages. Chondrocytes were stained with Alcian Blue kit(Thermo fisher, J60122.09).
Cell transfection
PDH2 overexpression plasmid vectors and silence plasmid vectors were designed and constructed by Gene Chem (Shanghai, China). Mouse chondrocytes were transfected with 50 nM overexpression/silence plasmid vectors or negative control vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cells were used for subsequent experiments 48 h after transfection. Fluorescence assay was used to confirm the transcription efficiency of the plasmids.
RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The RNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, DE, USA), and RNA integrity was assessed with an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). RNA samples (1 µg) were then used for cDNA synthesis with a RevertAid First Strand cDNA Synthesis Kit (TaKaRa, Dalian, China), and cDNA was amplified by qRT-PCR using the SYBR Premix Ex Tag Kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. All reactions were performed in triplicate on an ABI 7500 Sequencing Detection System (Applied Biosystems, Foster City, CA, USA). GAPDH was used as endogenous control. The data were analyzed by calculating the relative fold change using the formula 2 − ΔΔCt. The primers used in this study are shown in Table S1.
Western blot analysis
Western blot analysis was performed following standard methods. Briefly, total protein was extracted using RIPA buffer (Beyotime, Jiangsu, China). After being quantified by the BCA assay (Beyotime, Jiangsu, China), the protein samples were separated by SDS-PAGE and transferred onto PVDF membranes. Then, the PVDF membranes were incubated with primary and secondary antibodies at the indicated dilution. The primary antibodies used in this study were as follows: rabbit anti-MMP-13 (1:3000, Abcam, ab219620), rabbit anti-ADAMTS-5 (1:250, Abcam, ab41037), rabbit anti-Col2a1 (1:5000, CST, #2789), and rabbit anti-Aggrecan (1:1000, Abcam, ab216965), rabbit anti-PHD2 (1:1000, DF6285, Affinity), rabbit anti-HIF-1α (1:1000, BF8002, Affinity), rabbit anti-VEGFA (1:1000, 66828-1-Ig, Proteintech). A rabbit anti-GAPDH antibody (1:10000, Abcam, ab37168) was used as an internal control, and an HRP-conjugated goat anti-rabbit antibody (1:10000, ASPEN, AS1107) was used as the secondary antibody. The proteins bands were detected using the ECL method and processed using ImageJ software (NIH, Bethesda, MD, USA).
Angiogenesis assay
To evaluate angiogenesis, a tube formation assay was performed. Initially, 1 × 10 MSCs were plated onto a 96-well plate pre-coated with a 50 µl layer of Matrigel matrix (Corning). Various cell supernatants, along with 100 µM/ml α-KG (MCE) and 10 nM/ml IOX4 (a competitive inhibitor of α-KG, MCE), were added to different experimental groups. The MSCs were then incubated at 37 °C for 16 h, followed by the observation of tube formation. To quantify angiogenesis, the number of nodes formed in the tubes was assessed using ImageJ software. For this analysis, four randomly selected fields were chosen for each sample to determine the number of nodes within these areas.
Wound healing assay
Once the MSCs reached 90% confluence, a controlled scratch was created in the central region of the cell monolayer using a pipette tip. Subsequently, cell supernatants from various treatment groups, including 100 µM/ml α-KG and 10 nM/ml IOX4, were added to the respective experimental groups. After a 16-hour incubation period, the relative area covered by migrating cells was carefully observed. To quantify the extent of cell migration, the relative area of cell migration was assessed using ImageJ software.
Statistical analysis
All data are expressed as the mean ± SD, and all statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL USA). The Kolmogorov-Smirnov test was used to confirm the normal distribution of the data. Statistical significance was evaluated by Student’s paired and unpaired t-test for parametric data or the Mann-Whitney U test and Wilcoxon matched-pairs signed rank test for nonparametric data. ANOVA was used to compare more than two groups. For all analyses, p < 0.05 was considered significantly different.
Results
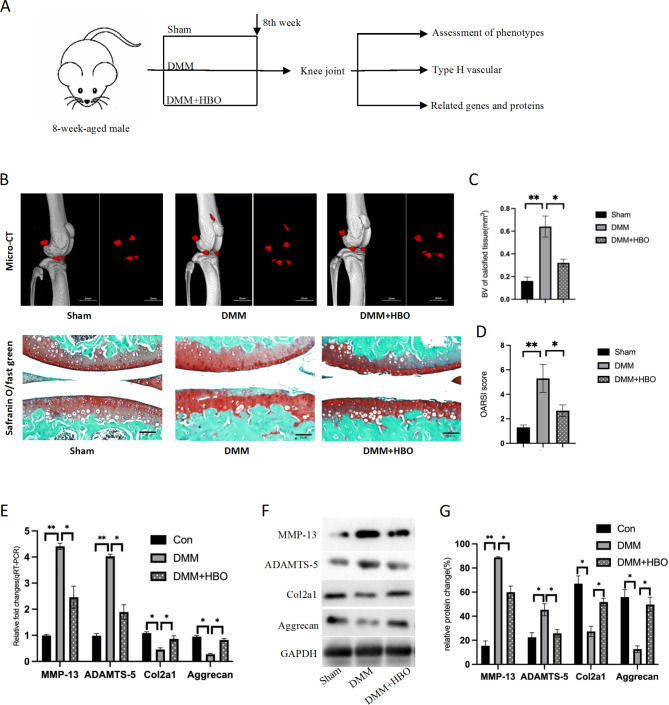
Hyperbaric oxygen therapy significantly delays the progression of osteoarthritis in mice
Fifteen mice were randomly assigned to different treatment groups. After eight weeks of treatment, their knee joints were harvested for therapeutic effect evaluation. MicroCT imaging and Safranin O-Fast Green staining revealed that mice treated with hyperbaric oxygen therapy showed reduced osteophyte formation, decreased cartilage matrix damage, and reduced chondrocyte apoptosis, resulting in lower OARSI scores. Additionally, inflammatory markers in chondrocytes were reduced, while there was a significant increase in chondrocyte marker proteins and matrix proteins. These findings suggest that hyperbaric oxygen therapy can effectively delay the progression of osteoarthritis in mice.
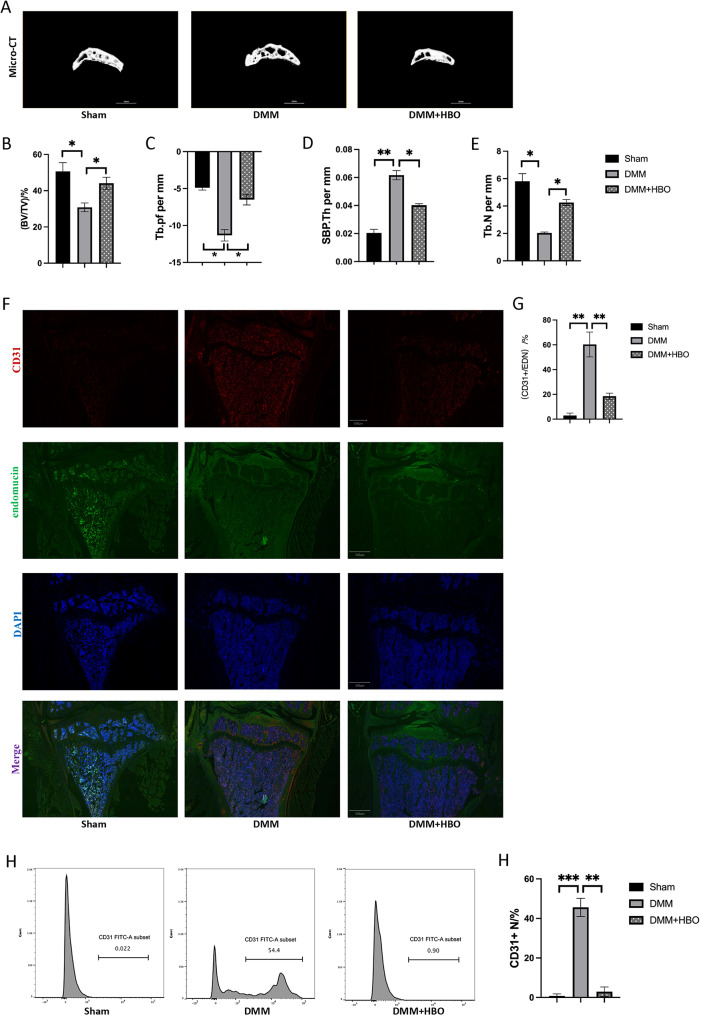
Hyperbaric oxygen therapy significantly inhibits the proliferation of type H blood vessels in subchondral bone
MicroCT analysis of mouse knee joints revealed that the indicators of subchondral bone in mice with osteoarthritis were alleviated after hyperbaric oxygen therapy. Immunofluorescence results suggested an increased proportion of type H vascular endothelial cells (CD31+EMCN+) among endothelial cells in the knee joints of mice with osteoarthritis, which was significantly reduced by hyperbaric oxygen therapy. Flow cytometry analysis yielded similar results, indicating that hyperbaric oxygen therapy can significantly inhibit the proliferation of type H vascular endothelial cells (CD31+EMCN+) among endothelial cells.
Hyperbaric oxygen environment inhibits angiogenic differentiation of mesenchymal stem cells and promotes their chondrogenic differentiation
The hyperbaric oxygen environment suppresses the angiogenic differentiation of mesenchymal stem cells, with relevant indicators decreasing in the hyperbaric setting. Conversely, this environment enhances the chondrogenic differentiation of mesenchymal stem cells. Both Alcian blue staining and fluorescence staining for chondrogenic-specific markers suggest a higher degree of chondrogenic differentiation in the hyperbaric oxygen condition. Additionally, the hyperbaric oxygen environment significantly activates the PHD2 factor, promoting the degradation of HIF-1α and inhibiting the expression of VEGFA.
Regulation of tubulogenic differentiation of mesenchymal stem cells by the PHD2/HIF-1α signaling pathway
Silencing/overexpression of PHD2 in mesenchymal stem cells was confirmed by luciferase assay, demonstrating normal plasmid expression. Downstream expression products were verified using qRT-PCR and western blot analysis, revealing that PHD2 negatively regulates the production of HIF-1α and the expression of VEGFA. Functional experiments indicated that the PHD2 factor can delay cell migration (with cell migration area results suggesting that silencing PHD2 promotes cell migration, while overexpressing PHD2 inhibits it) and suppress blood vessel formation (silencing PHD2 enhances angiogenic indicators, while overexpressing PHD2 suppresses them.
Discussion
HBOT is a treatment method that addresses tissue ischemia and hypoxia by increasing oxygen partial pressure and enhancing oxygen solubility in the blood. In recent years, the application of HBOT has become increasingly widespread in the treatment of various diseases, particularly in OA, where its mechanism and effects have gradually gained attention [4].
In the progression of osteoarthritis, changes in subchondral bone, particularly the proliferation of type H vessels, emerge as a critical factor. Type H vessels (CD31+EMCN+), also known as highly invasive vessels [10], invade the subchondral bone region, leading to destruction of the bone-cartilage interface and accelerated cartilage degeneration [11]. Immunohistochemistry and in vitro cellular experiments have confirmed that the expression level of HIF-1α is higher in type H endothelial cells compared to type L endothelial cells [12]. In vivo knockout or inhibition of the HIF-1α gene in endothelial cells results in a significant reduction in the number of type H endothelial cells in the bone [13], while the number of type L endothelial cells remains largely unchanged [14]. This suggests that the high expression level of HIF-1α in endothelial cells plays a crucial role in the generation and maintenance of the type H endothelial cell phenotype. Furthermore, Langen et al. found that the expression level of vascular endothelial growth factor receptor (VEGFR) is significantly higher in type H endothelial cells compared to type L endothelial cells, making them more sensitive to VEGF signaling [10]. Ji et al. applied a VEGFR antagonist to mice and observed a significant decrease in the number of type H endothelial cells in the bone, demonstrating that, like HIF-1α, high expression of VEGFR in endothelial cells is closely related to the generation of the type H endothelial cell phenotype [15, 16].
The mechanism by which HBOT downregulates H-type angiogenesis through the PHD2/HIF-1α pathway may involve multiple aspects [17].
Firstly, HBOT may reduce the hydroxylation of HIF-1α, thereby decreasing the expression of its downstream angiogenesis-related genes. HBOT affects the stability and activity of HIF-1α by increasing tissue oxygen partial pressure. Under hyperbaric oxygen conditions, the hydroxylation of HIF-1α increases, leading to reduced stability and subsequent inhibition of downstream target gene expression, including angiogenic factors such as VEGFR [16]. This process aids in suppressing abnormal proliferation of H-type blood vessels and mitigating subchondral bone destruction, ultimately slowing the progression of osteoarthritis. PHD2 serves as a crucial negative regulatory protein for HIF-1α [18], capable of reducing the stability of HIF-1α protein through hydroxyl modification [19]. Consequently, modulating the PHD2/HIF-1α pathway can impact the regulation of angiogenesis.
Secondly, HBOT may indirectly affect the angiogenesis process through other pathways, such as antioxidant and anti-inflammatory effects. HBOT, by providing a high-concentration oxygen environment, can reduce oxidative stress responses within the body, thereby exerting an antioxidant effect and protecting vascular endothelial cells from damage. Additionally, HBOT can inhibit inflammatory responses and reduce the adverse impact of inflammatory factors on angiogenesis. These effects collectively facilitate the smooth progression of angiogenesis, contributing to improved blood supply and oxygenation of tissues, and creating favorable conditions for the restoration and repair of the body.
In summary, HBOT offers a novel approach for the treatment of osteoarthritis by regulating the PHD2/HIF-1α pathway and inhibiting abnormal proliferation of H-type blood vessels in subchondral bone. However, further research and validation are needed to elucidate the specific mechanisms and long-term effects of HBOT, aiming to provide more effective treatment options for patients.
Fig. 1.
Hyperbaric oxygen therapy delays the progression of knee osteoarthritis in mice. (A) Schematic diagram of the animal experiment. (B) Micro-CT images of mouse knee joints (The red part is the newly grown osteophyte) and safranin O-fast green staining of pathological sections. (C) Quantitative statistics of proliferative osteophytes in mouse knee joints. (D) Quantitative scoring of safranin O-fast green staining in pathological sections of mouse knee joints. (E) Changes in inflammatory markers of chondrocytes and indicators of cartilage and cartilage matrix in mouse osteoarthritis. *p < 0.05, **p < 0.01; n = 3
Fig. 2.
Hyperbaric oxygen therapy reduces type H angiogenesis in subchondral bone of knee osteoarthritis in mice. (A) MicroCT exhibition of subchondral bone in mouse knee joints. (B-E) Quantitative comparison of subchondral bone in mouse knee joints suggests that hyperbaric oxygen therapy can alleviate the destruction of subchondral bone in mouse osteoarthritis and delay the progression of the disease. (F) Immunofluorescence staining of CD31+Endomucin+, a specific marker of type H vascular endothelium, in endothelial cells. (G) The proportion of CD31+ endothelial cells in the vascular endothelium indicates that hyperbaric oxygen therapy significantly inhibits the proliferation and proportion of CD31+ cells. (H) Flow cytometry detection of CD31+ cell proportion in mouse subchondral bone. (I) Quantitative comparison of flow cytometry results. *p < 0.05, **p < 0.01; n = 3
Fig. 3.
Hyperbaric oxygen environment inhibits angiogenic differentiation of mesenchymal stem cells but promotes chondrogenic differentiation. (A) The hyperbaric oxygen environment suppresses the angiogenic differentiation of mesenchymal stem cells. (B, C, D) Quantitative analysis of the angiogenesis experiments. (E) The hyperbaric oxygen environment promotes chondrogenic differentiation of mesenchymal stem cells, as indicated by Alcian blue staining. (F) The hyperbaric oxygen environment enhances chondrogenic differentiation of mesenchymal stem cells, demonstrated by immunofluorescence staining for the chondrocyte-specific protein col2a1. (G, H, I) The hyperbaric oxygen environment inhibits VEGFA expression and angiogenesis through the PDH2/HIF-1α signaling pathway. *p < 0.05, **p < 0.01; n = 3
Fig. 4.
PHD2 enhances the degradation of HIF-1α, thereby inhibiting the expression of VEGFA and the formation of type H blood vessels. (A) Schematic diagram of luciferase detection in mesenchymal stem cells after silencing/overexpressing the PHD2 gene. (B) Quantitative results of luciferase assay indicate normal plasmid expression without any differences. ns, P > 0.05. (C, D, E) The effect of the PHD2 gene on downstream HIF-1α and VEGFA, where C represents qRT-PCR, D represents western-blot, and E represents the quantitative statistics of western-blot. (F-K) The impact of the PHD2 gene on the migration ability and angiogenic function of mesenchymal stem cells. F shows the cell scratch assay, G represents the quantitative statistical comparison of cell migration area. H demonstrates the angiogenic experiment of mesenchymal stem cells, and IJK provides the quantitative statistical comparison. *p < 0.05, **p < 0.005; n = 3
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The study was supported by Jiangsu Provincial Health Commission, General Program (No.H2023006, Bo Chen), Jiangsu Provincial Sports Bureau, Key Project (No.ST231207, Zhaoxiang Meng), Yangzhou Municipal Health Commission, Key Project (No.2023-1-01, Zhaoxiang Meng).
Author contributions
Jianjian Wang: Writing – original draft, Investigation, Formal analysis, Data curation. Wen Yu: Validation, Investigation. Yuxin Zhang: Project administration, Investigation, Data curation. Bo Chen: Validation, Supervision, Funding acquisition. Zhaoxiang Meng: Writing – review & editing, Supervision, Funding acquisition.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Conflict of interest
The authors have declared that no competing interest exists.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianjian Wang and Wen Yu contributed equally to this work.
Contributor Information
Bo Chen, Email: 18051060729@yzu.edu.cn.
Zhaoxiang Meng, Email: yzmzx001@163.com.
References
- 1.Xu J, He SJ, Xia TT, Shan Y, Wang L. Targeting type H vessels in bone-related diseases. J Cell Mol Med. 2024;28:e18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Li B, Yu R, et al. ETV2 regulating PHD2-HIF-1alpha axis controls metabolism reprogramming promotes vascularized bone regeneration. Bioact Mater. 2024;37:222–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu JJ, Shi XJ, Fu Q, Li YC, Zhu L, Lu N. MicroRNA-584-5p/RUNX family transcription factor 2 axis mediates hypoxia-induced osteogenic differentiation of periosteal stem cells. World J Stem Cells. 2023;15:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riegger J, Schoppa A, Ruths L, Haffner-Luntzer M, Ignatius A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. 2023;28:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaskiewicz M, Moszynska A, Kroliczewski J, et al. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol Biol Lett. 2022;27:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Shi X, Fu Q et al. Extracellular vesicles from inflammatory-stimulated BMSCs ameliorate osteoarthritis via Rpl14 mediated synovial macrophage polarization. Chem Eng J 2024;499.
- 7.Lu J, Shi X, Fu Q, et al. New mechanistic understanding of osteoclast differentiation and bone resorption mediated by P2X7 receptors and PI3K-Akt-GSK3beta signaling. Cell Mol Biol Lett. 2024;29:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. [DOI] [PubMed] [Google Scholar]
- 9.Cui Z, Wu H, Xiao Y, et al. Endothelial PDGF-BB/PDGFR-beta signaling promotes osteoarthritis by enhancing angiogenesis-dependent abnormal subchondral bone formation. Bone Res. 2022;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji G, Xu R, Niu Y, et al. Vascular endothelial growth factor pathway promotes osseointegration and CD31(hi)EMCN(hi) endothelium expansion in a mouse tibial implant model: an animal study. Bone Joint J. 2019;101–B:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Hu W, Zhang Y, et al. Cellular senescence in skeletal disease: mechanisms and treatment. Cell Mol Biol Lett. 2023;28:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Z, Crane J, Xie H, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R, Yallowitz A, Qin A, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji P, Zhang Z, Mingyao E, et al. Ginsenosides ameliorates high altitude-induced hypoxia injury in lung and kidney tissues by regulating PHD2/HIF-1alpha/EPO signaling pathway. Front Pharmacol. 2024;15:1396231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Yin H, Rao SS, et al. Harmine enhances type H vessel formation and prevents bone loss in ovariectomized mice. Theranostics. 2018;8:2435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Wang L, Guo H, Chen L, Huang X. Dapagliflozin-loaded Exosome Mimetics facilitate Diabetic Wound Healing by HIF-1alpha-Mediated enhancement of Angiogenesis. Adv Healthc Mater. 2023;12:e2202751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo BY, Cheng SY, Liao WZ, et al. PHD2 shRNA-Modified bone marrow mesenchymal stem cells facilitate Periodontal Bone Repair in Response to Inflammatory Condition. Chin J Dent Res. 2024;27:215–24. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Li YY, Xia K, et al. Harmine targets inhibitor of DNA binding-2 and activator protein-1 to promote preosteoclast PDGF-BB production. J Cell Mol Med. 2021;25:5525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muz B, Larsen H, Madden L, Kiriakidis S, Paleolog EM. Prolyl hydroxylase domain enzyme 2 is the major player in regulating hypoxic responses in rheumatoid arthritis. Arthritis Rheum. 2012;64:2856–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.