Abstract
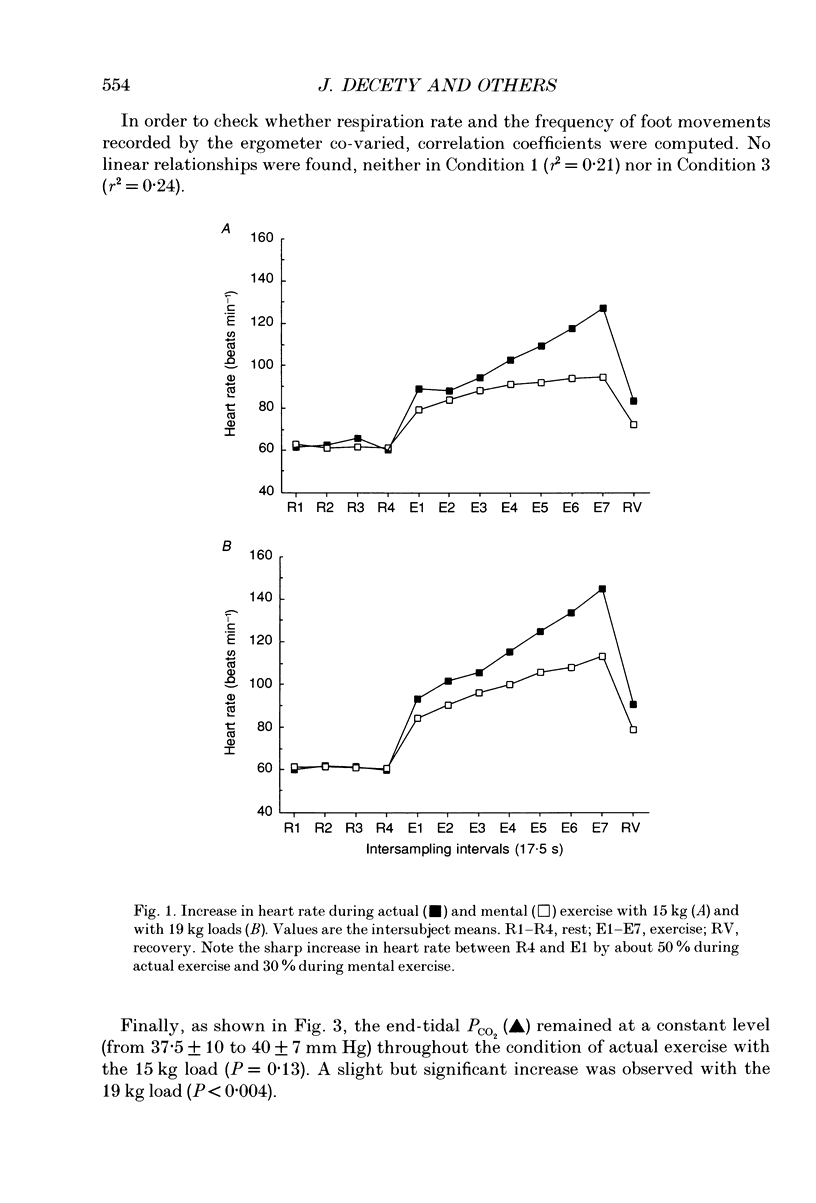
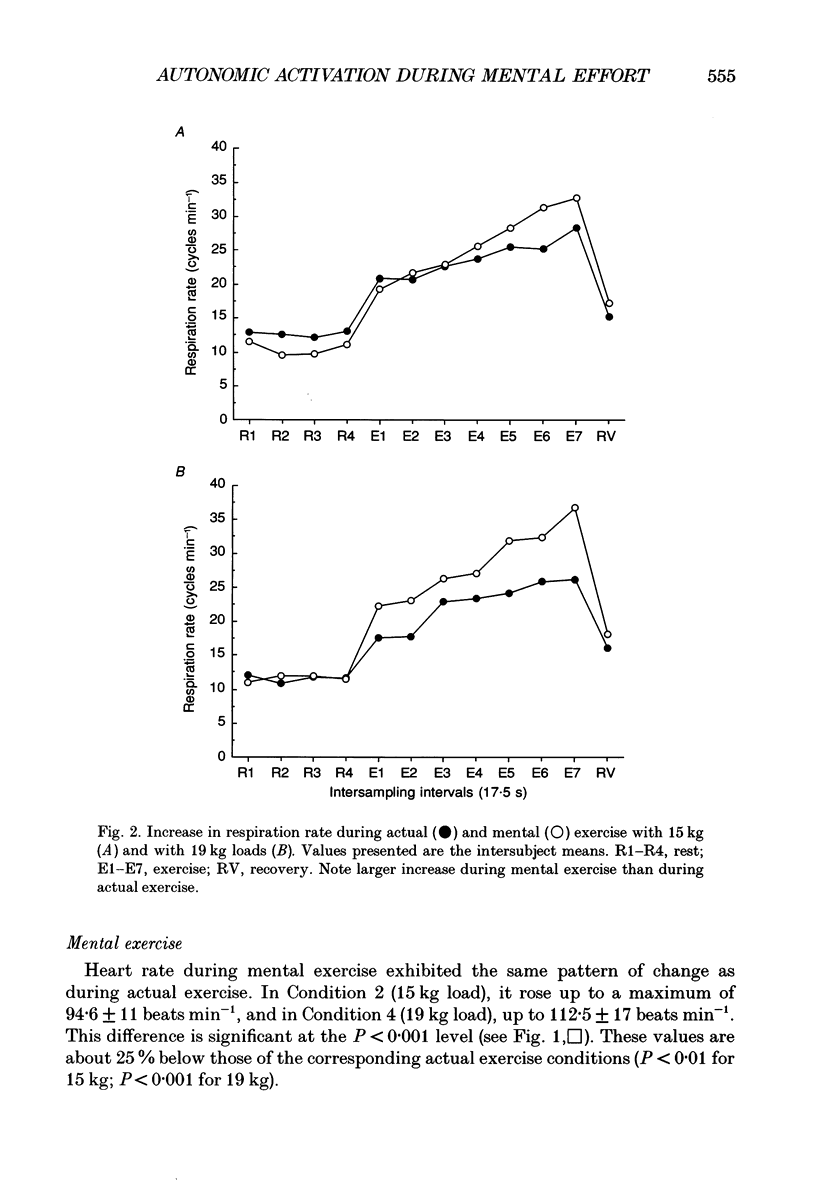
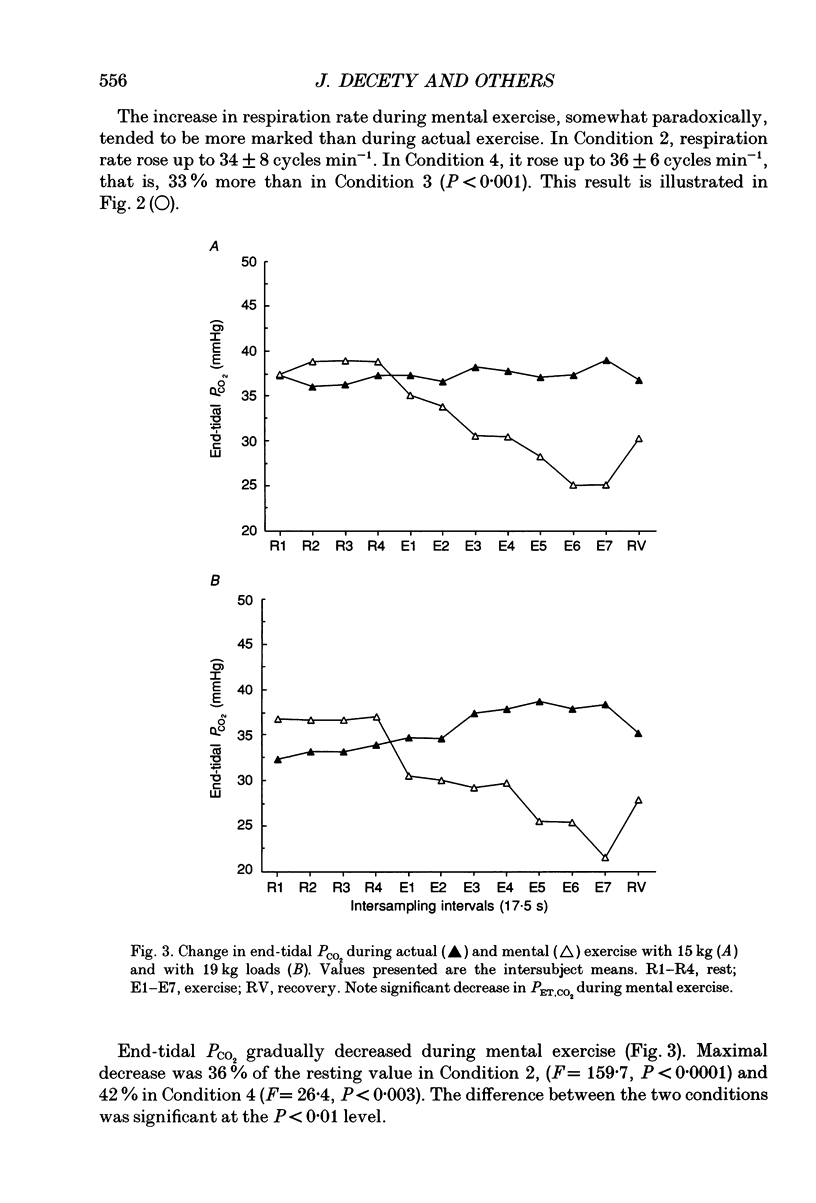
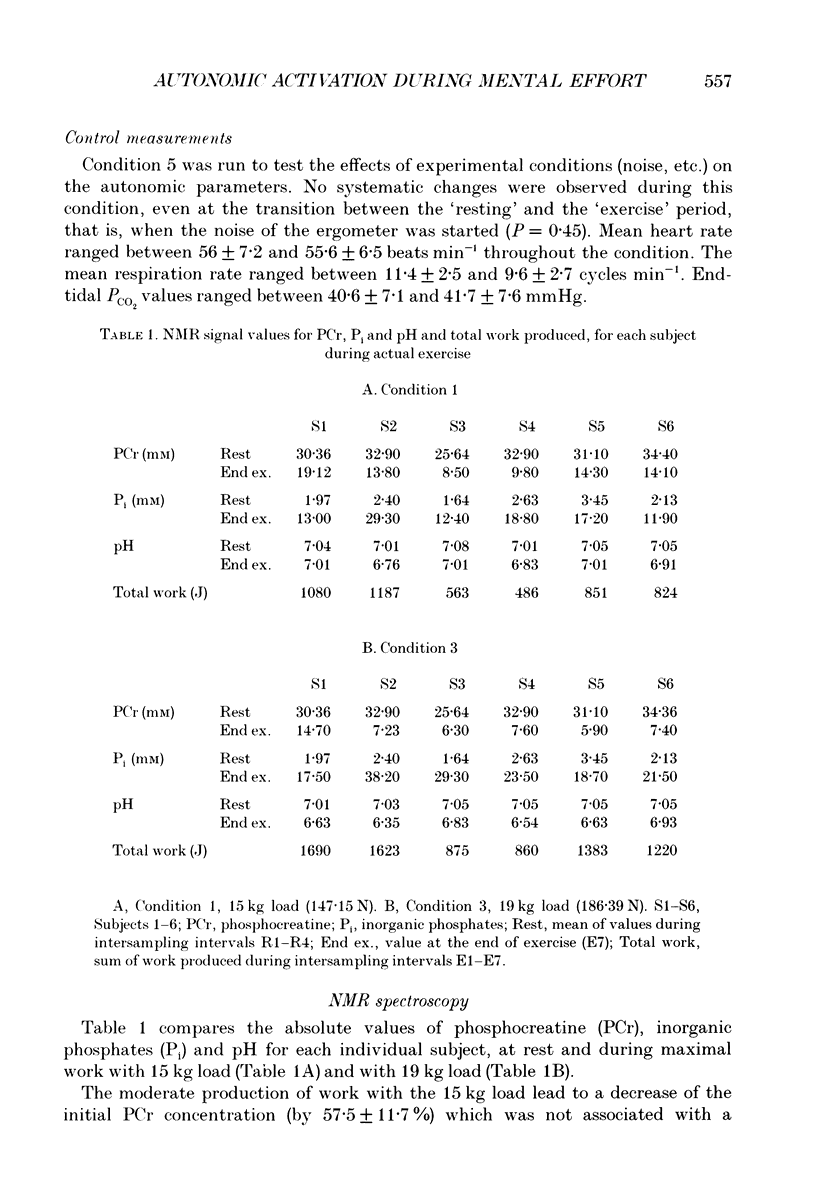
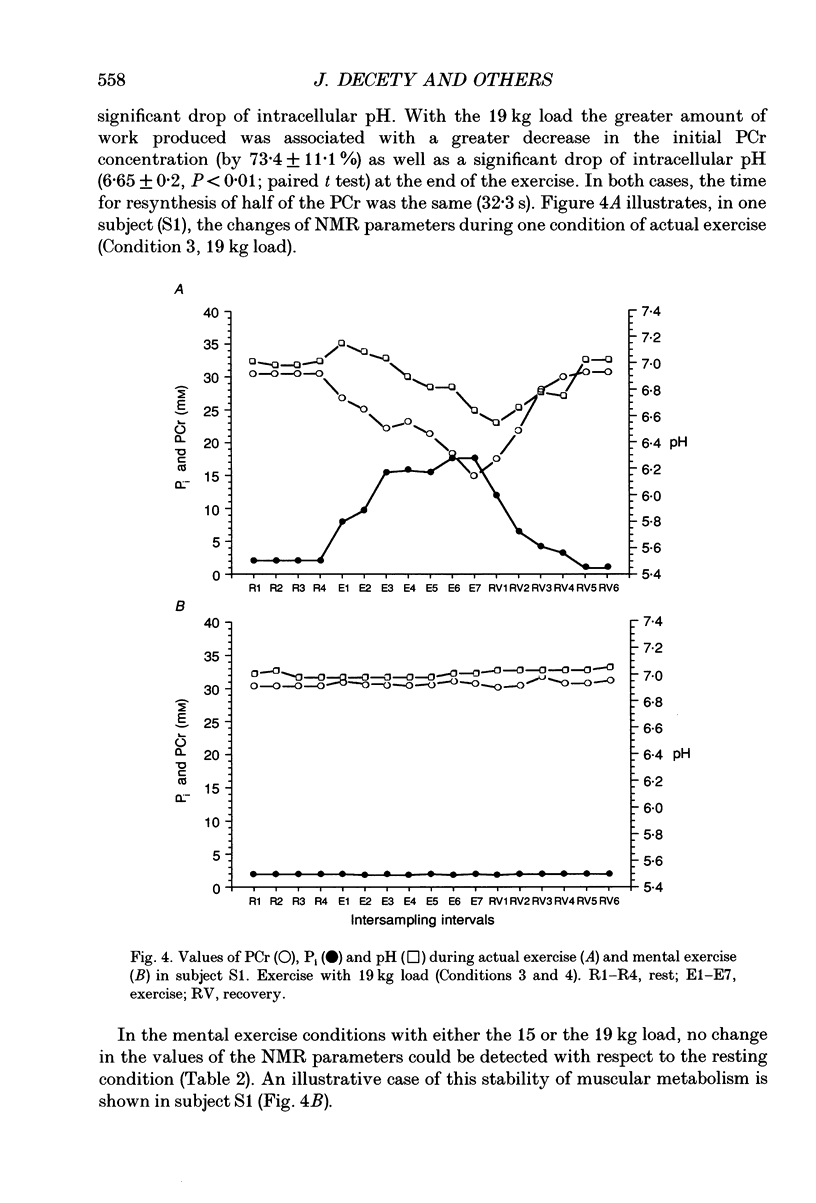
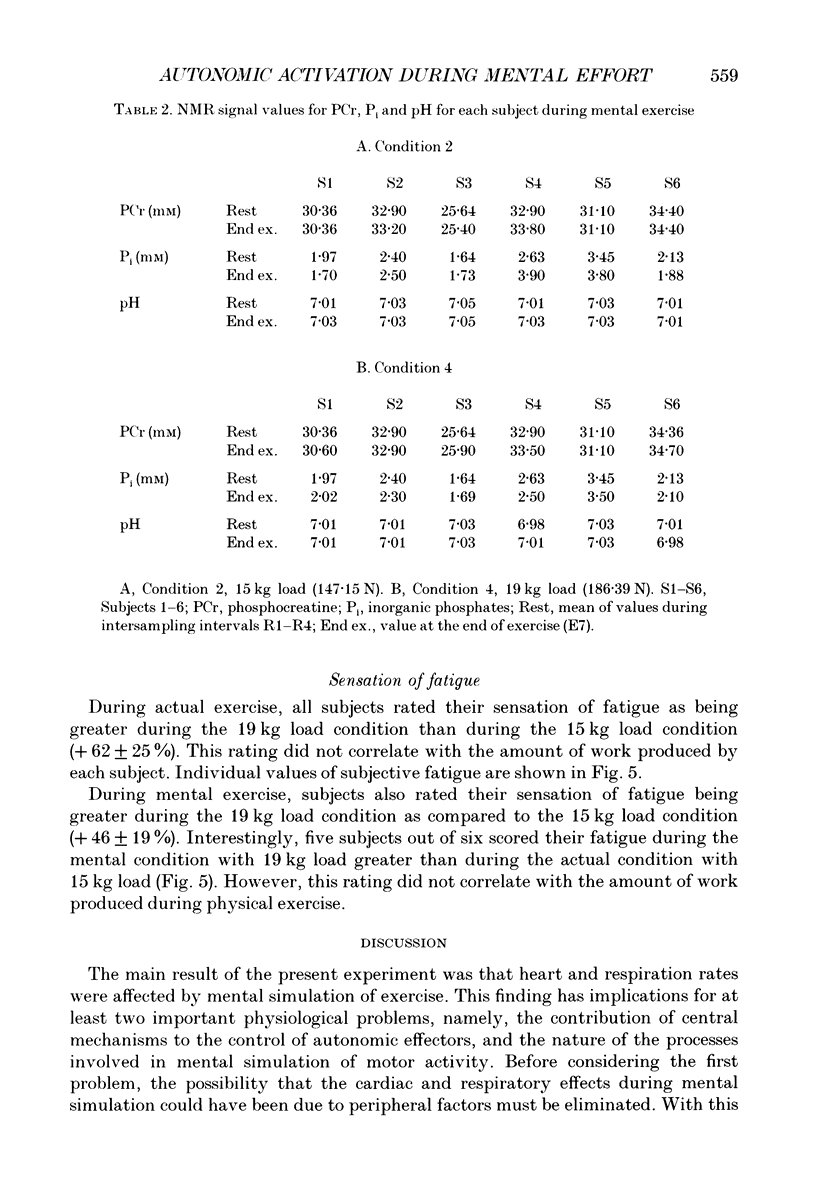
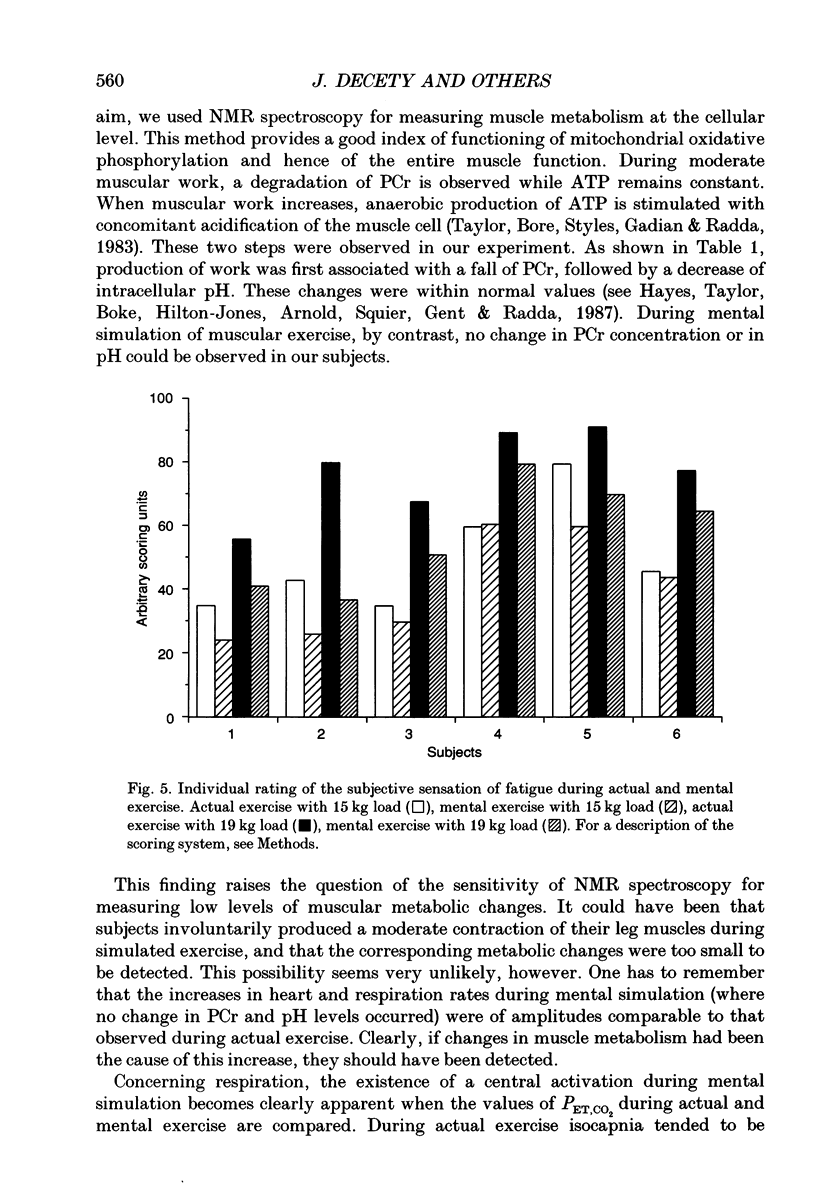
1. Healthy subjects actually performed and mentally simulated a leg exercise at two levels of work (15 and 19 kg loads). Heart rate, respiration rate and end-tidal PCO2 were measured in both conditions. In addition, muscular metabolism was simultaneously measured using 31P nuclear magnetic resonance (NMR) spectroscopy. 2. During actual exercise, heart and respiration rates increased, first abruptly and then gradually in relation to the level of work. End-tidal PCO2 was unaltered. NMR spectra showed a drop in phosphocreatine (PCr) and an increase in inorganic phosphate (Pi) concentrations. Intracellular pH fell to 6.65 at maximal effort with a 19 kg load. 3. During mental simulation, both heart and ventilatory rate increased immediately after mental exercise was begun. This increase was proportional to the amount of simulated exercise. Heart rate remained about 25% below the level observed during actual exercise. The increase in respiration rate, by contrast, was more marked than during actual exercise. Finally, end-tidal PCO2 decreased progressively to about 18% of the resting value. 4. During mental simulation, NMR spectra were unchanged with respect to the resting values. 5. Subjects rated their sensation of fatigue using an analog rating scale, during both actual exercise and mental simulation. During mental exercise, the sensation of fatigue was greater with the 19 kg load than with the 15 kg load. 6. These results demonstrate that mental simulation of action can activate heart and respiration control mechanisms. They suggest that autonomic activation during imagined action pertains to the more general phenomenon of preparation for action.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L., Garlick J., Guz A., Murphy K., Semple S. J. Is the voluntary control of exercise in man necessary for the ventilatory response? J Physiol. 1984 Oct;355:71–83. doi: 10.1113/jphysiol.1984.sp015407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams L., Guz A., Innes J. A., Murphy K. The early circulatory and ventilatory response to voluntary and electrically induced exercise in man. J Physiol. 1987 Feb;383:19–30. doi: 10.1113/jphysiol.1987.sp016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Jeannerod M., Germain M., Pastene J. Vegetative response during imagined movement is proportional to mental effort. Behav Brain Res. 1991 Jan 31;42(1):1–5. doi: 10.1016/s0166-4328(05)80033-6. [DOI] [PubMed] [Google Scholar]
- Decety J., Lindgren M. Sensation of effort and duration of mentally executed actions. Scand J Psychol. 1991;32(2):97–104. doi: 10.1111/j.1467-9450.1991.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Decety J., Sjöholm H., Ryding E., Stenberg G., Ingvar D. H. The cerebellum participates in mental activity: tomographic measurements of regional cerebral blood flow. Brain Res. 1990 Dec 10;535(2):313–317. doi: 10.1016/0006-8993(90)91615-n. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E., Waldrop T. G. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981 Feb 20;211(4484):844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C. The perception of motor commands or effort during muscular paralysis. Brain. 1982 Mar;105(Pt 1):151–159. doi: 10.1093/brain/105.1.151. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Mitchell J. H. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972 Oct;226(1):173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D. J., Taylor D. J., Bore P. J., Hilton-Jones D., Arnold D. L., Squier M. V., Gent A. E., Radda G. K. An unusual metabolic myopathy: a malate-aspartate shuttle defect. J Neurol Sci. 1987 Dec;82(1-3):27–39. doi: 10.1016/0022-510x(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913 Oct 17;47(1-2):112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland P. E. Organization of motor control by the normal human brain. Hum Neurobiol. 1984;2(4):205–216. [PubMed] [Google Scholar]
- Sheehan P. W. A shortened form of Betts' questionnaire upon mental imagery. J Clin Psychol. 1967 Jul;23(3):386–389. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]


