Abstract
Background
Osteosarcoma is the most common malignant bone tumor in children and adolescents, characterized by high disability and mortality rates. Over the past three decades, therapeutic outcomes have plateaued, underscoring the critical need for innovative therapeutic targets. Solute carrier (SLC) family transporters have been implicated in the malignant progression of a variety of tumors, however, their specific role in osteosarcoma remains poorly understood.
Methods
The single-cell sequencing data from GSE152048 and GSE162454, along with RNA-seq from the TARGET and GSE21257 cohorts, were utilized for the analysis in this study. LASSO regression analysis was conducted to identify prognostic genes and construct an SLC-related prognostic signature. Survival analysis and ROC analysis evaluated the validity of the prognostic signature. The ESTIMATE and CIBERSORT Packages were utilized to assess the immune infiltration status. Pseudotime and CellChat analyses were performed to investigate the relationship between SLC7A1, malignant phenotypes, and the immune microenvironment. CCK8 assays, EdU staining, colony formation assays, Transwell assays, and co-culture systems were used to assess the effects of SLC7A1 on cell proliferation, metastasis, and macrophage polarization. Finally, virtual docking identified potential drugs targeting SLC7A1.
Results
SLCs displayed distinct expression patterns across various cell types within the osteosarcoma microenvironment, with myeloid cells exhibiting a preference for amino acid uptake. A prognostic model comprising nine genes was constructed via LASSO regression, with SLC7A1 showing the highest hazard ratio. Multiple analytical algorithms indicated that SLCs were associated with immune cell infiltration and immune checkpoint gene expression. Single-cell analysis indicated that SLC7A1 was predominantly expressed in osteosarcoma cells and correlated with various malignant tumor characteristics. SLC7A1 also regulate interactions between tumor cells and macrophages, as well as modulate macrophage function through multiple pathways. In vitro assays and survival analysis demonstrated that inhibition of SLC7A1 suppressed the malignant phenotype of osteosarcoma cells, with SLC7A1 expression correlating with poor prognosis. Co-culture models confirmed the involvement of SLC7A1 in macrophage polarization. Finally, virtual screening and CETSA identified Cepharanthine as potential inhibitors of SLC7A1.
Conclusion
SLC-related prognostic signatures can be utilized for the prognostic evaluation of osteosarcoma. Pharmacological inhibition of SLC7A1 may be a feasible therapeutic approach for osteosarcoma.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06086-1.
Keywords: Osteosarcoma, Solute carrier (SLC) family transporters, Tumor immune microenvironment, SLC7A1, TAMs
Introduction
Osteosarcoma (OS) is the most prevalent primary malignant bone tumor in children and adolescents, with an annual incidence of ~ 4.5 per million worldwide [1, 2]. It is characterized by high malignancy and rapid progression, which often leads to disability and death. The standard treatment for osteosarcoma combines extensive surgical resection with chemotherapy, typically using the MAP regimen, which includes high-dose methotrexate, doxorubicin (Adriamycin), and cisplatin (Platinol) [3]. Despite advances in surgical techniques and the incorporation of neoadjuvant chemotherapy, the 5-year survival rate for patients with non-metastatic osteosarcoma has improved from 20–60% [3–6]. However, for those with recurrent or metastatic disease, the survival rate remains persistently below 25% [3]. Targeted therapy and immunotherapy are actively being investigated as potential treatments for osteosarcoma. Consequently, there is a pressing demand for a thorough investigation into the molecular mechanisms of osteosarcoma, as well as the identification of potent therapeutic targets.
Metabolic reprogramming is a hallmark of malignant progression in tumor cells [7]. Tumor modify their metabolic pathways to adapt to the tumor microenvironment and meet their energy and biosynthetic demands [8]. Recent research suggests that abnormal metabolic activation not only drives malignant differentiation but also shapes the tumor immune microenvironment (TIME) [9–11]. The metabolic interplay between tumor and immune cells influences immune cell recruitment and function, contributing to an immunosuppressive environment [12, 13]. Therefore, understanding metabolic reprogramming is pivotal for making effective tumor treatment strategies.
Solute Carrier (SLC) transporter family is the largest known transmembrane transport superfamily, comprise 65 families and more than 400 members [14, 15]. These transporters are vital for cellular nutrient supply as they facilitate the transport of various molecules, such as carbohydrates, fatty acids, amino acids, nucleotides, inorganic ions, and drugs. Targeting SLCs suppresses the advancement of multiple cancers, including hepatocellular carcinoma, breast cancer, and neuroblastoma [16–18]. Recent studies indicate that SLCs assisting tumor cells in immune evasion by inhibiting the function and infiltration of T cells, NK cells, and other immune cells [19–21]. These results reveal the therapeutic potential of targeting SLCs in tumor treatment.
In our study, we analyzed the Single cell RNA sequencing (scRNA-seq) data from osteosarcoma patients to investigate the expression patterns of SLCs in various cell types. Next, we constructed an SLC-related prognostic signature and identified it as a key prognostic gene. Based on the prognostic signature, patients were stratified into high- or low-risk groups, allowing us to explore the biological characteristics and differences between these groups. Focusing on SLC7A1, scRNA-seq data reveals its correlation with diverse tumor hallmarks and malignant phenotypes, which was confirmed by in vitro assay. Analysis of the immune microenvironment examined the influence of SLC7A1 on tumor cell-macrophage interactions. Co-culture experiments revealed that SLC7A1 expression in tumor cells encourages the differentiation of tumor-associated macrophages (TAMs) into an immunosuppressive phenotype. Through virtual screening of protein structures predicted by AlphaFold2, Cepharanthine (CPE) was identified as a potential inhibitor of SLC7A1. Cellular Thermal Shift Assay (CETSA) demonstrated that CPE could bind to the SLC7A1 protein. CPE effectively inhibited SLC7A1-mediated arginine uptake, impaired cell proliferation, and suppressed osteosarcoma cell-induced M2-like macrophages polarization. This study clarifies the relationship between SLCs and the osteosarcoma microenvironment. Additionally, our findings demonstrate that SLC7A1 has a substantial effect on the tumor malignant progression and its contribution to shaping the immune landscape. Targeting SLC7A1 with CPE holds promise as a potential therapeutic strategy for osteosarcoma patients.
Materials and methods
Dataset source and preprocessing
The Therapeutically Applicable Research to Generate Effective Treatment-Osteosarcoma (TARGET-OS) dataset (n = 85), which includes RNA expression data and clinical information, was obtained from the TARGET database (https://portal.gdc.cancer.gov/). RNA-seq data GSE21257(n = 53) and scRNA-seq datasets GSE152048 (n = 11), GSE162454 (n = 6) of osteosarcoma were obtained from the Gene Expression Omnibus (GEO) databases (https://www.ncbi.nlm.nih.gov/). The 9 pre-metastatic primary osteosarcoma samples from the scRNA-seq dataset GSE152048, along with all samples from GSE162454, were included in the analysis. The solute carrier (SLC) genes were obtained from the SLC tables (slc.bioparadigms.org) and the HGNC database (www.genenames.org/cgi-bin/genefamilies/set/752). The 14 Hallmark gene sets were obtained from the CancerSEA database (http://biocc.hrbmu.edu.cn/CancerSEA/).
Single-cell analysis
Single-cell analysis was conducted within the R statistical environment (version 4.2.1). The “Seurat” package facilitated the processing of raw data and subsequent quality control, which involved the inclusion of genes detected in at least three cells and the exclusion of cells with over 10% mitochondrial reads. Cells exhibiting fewer than 300 or more than 5000 genes were also removed. Harmony was deployed to mitigate batch effects [22]. Cellular annotations were manually assigned based on gene expression profiles. CellChat was used to identify cell–cell communication based on a human database [23]. The AddModuleScore algorithm was used to scoring gene sets within scRNA-seq datasets. The monocle2 algorithm delineated the developmental trajectory of OS cells [24]. The scMetabolism algorithm was employed to calculate the metabolic pathway scores for OS cells and TAMs [25].
Unsupervised clustering of SLCs
We utilized expression data and clinical information from the TARGET osteosarcoma cohort to categorize patients into distinct molecular subtypes through unsupervised clustering techniques. To ascertain the optimal cluster count and their stability, we leveraged the “ConsensusClusterPlus” R package [26]. This determination was based on the consensus matrix, the cumulative distribution function (CDF), and the relative change in the area under the CDF curve. Following the clustering, Kaplan-Meier survival analysis was conducted to evaluate the survival rates associated with each molecular subtype.
Construction and validation of the prognostic signature
We performed Least Absolute Shrinkage and Selection Operator (LASSO) analysis on the SLCs using the “glmnet” package to identify candidate genes [27]. The optimal penalty parameter λ was selected based on the 1-SE (standard error) criterion, which minimized the prediction error. Subsequently, a prognostic model was constructed using the differentially expressed genes with significant prognostic value. Risk scores for each patient were calculated by summing the product of gene expression levels and their respective coefficients. Patients were categorized into low- and high-risk groups based on the median risk score. The prognostic accuracy of the model was evaluated using Kaplan-Meier survival analysis and time-dependent ROC curves. The generalizability of the model was validated by applying it to data from external independent cohorts, thereby confirming the robustness of the findings across various patient populations.
Evaluation of the immunogenomic landscape
Metascape was utilized for Gene Ontology (GO) enrichment analysis and for constructing the enriched pathway network [28]. The ESTIMATE algorithm was employed to determine the immune score, stromal score, ESTIMATE score, and tumor purity for all samples. The differences in the immune-related pathways were calculated and compared using the “IOBR” package [29].
Western blot analysis
Cellular and tissue proteins were extracted with RIPA lysis buffer (Beyotime, P0013B) supplemented with protease and phosphatase inhibitors (Roche, 04693116001, 04906845001). Protein concentrations were measured using the bicinchoninic acid (BCA) assay (ThermoFisher, 23227). SDS-PAGE with a 10% polyacrylamide gel was used to separate total proteins, which were subsequently transferred onto a PVDF membrane (Millipore, IPVH00010). The PVDF membrane was blocked with 5% skim milk for 1 h and then incubated with the primary antibody overnight at 4℃. After being washed three times with TBST buffer, the PVDF membrane was incubated with the corresponding secondary antibody for 2 h. The visualization of target protein was achieved using High-sig ECL. The primary antibodies used in Western blot analysis were: anti-SLC7A1(1:1000, 14195-1-AP, Proteintech), anti-β-Actin (1:1000, 4970 S, CST).
Human samples and cell lines
The OS and paracancerous tissues used in this study were obtained from the Department of Musculoskeletal Oncology, First Affiliated Hospital of Sun Yat-sen University, with the informed consent of all subjects. The clinical information (sex, age, location of tumor, tumor size, and lung metastasis status) of 70 patients were listed in Table S1.
The cell lines used in this study, including 143B, SJSA-1, and THP1, were obtained from the First Affiliated Hospital of Sun Yat-sen University. THP1 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco, A3160801) in an incubator at 33.5 °C with 5% CO2. 143B and SJSA-1 cells were cultured in high-glucose DMEM medium supplemented with 10% fetal bovine serum in an incubator at 33.5 °C with 5% CO2. All cell lines were certified mycoplasma-free.
Plasmid construction and lentiviral transduction
The lentiviruses packaging SLC7A1 shRNAs were purchased from Sigma, and then cloned into the pLKO.1-puro vector (Addgene, #8453) to construct the knockdown plasmids pLKO.1-SLC7A1 vectors. The shRNA sequences are listed in Table S2.
Lentiviral packaging experiments were conducted with polyethylenimine (PEI) (Proteintech, PR40001), as manufacture described. To establish stable cell lines, 2 × 105 OS cells were added to 6-well plate with 1 ml lentiviruses, 1 ml DMEM medium, and 1:1000 polybrene (Biosharp, BL628A). After a 24-hour incubation, cells were cultured with DMEM containing puromycin for 3–5 days.
Cell proliferation and colony formation assay
Osteosarcoma cells in the logarithmic growth phase were seeded into 96-well plates at a density of 1,500 cells per well. Cell viability was assessed daily for 4 consecutive days using the CCK-8 assay (Dojindo, CK04), according to the manufacturer’s instructions. For EUD stain (APExBIO, K1076), osteosarcoma cells were seeded into 24-well plates containing coverslips and incubated for 24 h. Following this, the cells were incubated for 2 h with medium containing 50 µM EdU. The coverslips were then removed, and the cells were fixed with 4% paraformaldehyde. Staining was performed according to the manufacturer’s instructions, and imaging was conducted using an inverted microscope.
For the colony formation assay, a total of 1,000 osteosarcoma cells in the logarithmic growth phase were seeded into 6-well plates, with the culture medium replaced every 3 days. After 2 weeks of incubation, the cells were fixed with paraformaldehyde and stained with crystal violet. Cell colonies with > 50 cells were counted.
Migration and invasion assay
OS cells were seeded into Boyden chambers with 8 μm pore membranes in 24-well Transwell plates (Corning Falcon, 3422). Uncoated chambers were used for the migration assay, while Matrigel-coated were used for the invasion assay. Tumor cells were seeded into the upper chamber with 200 µL of serum-free medium, while 600 µL of medium containing 10% FBS was added to the lower chamber. After incubation for the appropriate duration, the chambers were fixed with 4% paraformaldehyde for 30 min and stained with crystal violet. Cells in the upper chamber were removed, and imaging was conducted using an inverted microscope.
Cell sphere formation
A total of 1000 osteosarcoma cells were seeded into a 6-well ultra-low attachment plate (Corning, 3471) and cultured in serum-free DMEM/F12 medium supplemented with 20 ng/ml epidermal growth factor (R&D Systems, 236-EG), 20 ng/ml basic fibroblast growth factor (R&D Systems, 233-FB), and 20 ng/ml N2 medium (R&D Systems, 2229-N2) for 7 to 14 days. After incubation, the number of spheres with a diameter exceeding 50 μm in each well was counted under a microscope.
Immunohistochemistry (IHC)
For immunohistochemistry (IHC), paraffin-embedded sections of osteosarcoma tissue specimens were first dewaxed and subjected to antigen retrieval using an EDTA antigen retrieval solution (Beyotime, P0085). Subsequently, the sections were incubated with 3% H2O2 for 10 min and then blocked with 5% goat serum for 1 h. The primary antibody (anti-SLC7A1, 1:1000, 14195-1-AP, Proteintech) was incubated overnight at 4 °C. The sections were stained using 3,3′-diaminobenzidine (DAB) (DAKO, K5007) for IHC. The level of IHC staining were evaluated by two independent pathologists according to immunoreactive score (IRS) system. SLC7A1 expression was defined as high or low based on the median of the IHC score as the cutoff value.
Macrophage stimulation and co-culture assay
THP-1 cells (1 × 106) were treated with 320 nmol/L PMA (MedChemExpress, 16561-29-8) for 24 h. The treated THP-1 cells were then seeded into 6-well plates, with 5 × 105 osteosarcoma cells inoculated in the upper chamber(0.4 μm, Falcon, 353090). After 48 h of culture, macrophages were collected for flow cytometry analysis and qRT-PCR.
Quantitative RT-PCR
Total RNA was extracted using the TRIzol cleavage (Beyotime, R0016), followed by reverse transcription into cDNA using the HiScript 1st Strand cDNA Synthesis Kit (Vazyme, R111). Real-time reverse transcription quantitative PCR (RT-qPCR) was performed using the SYBR Green SuperMix (Roche, 4913914001) and the ABI7900HT Fast Real-Time PCR system (Applied Biosystems, CA, USA). ACTB was used as an endogenous control for calculating 2-ΔΔCT values. The primers used are listed in Table S2.
Virtual screening for potential drug candidates
Approved drug structures were retrieved and downloaded from the ZINC database [30], while the SLC7A1 protein structure was predicted using AlphaFold2 [31]. The 3D model of SLC7A1 was evaluated using ProSA-Web (ProSA-web - Protein Structure Analysis (sbg.ac.at)) [32]. Subsequently, a structure-based virtual screening (VS) docking study was conducted using the AutoDock Vina program [33]. Ten compounds were selected based on the lowest binding energy and visualized using PyMOL.
L-arginine uptake
Osteosarcoma cells (5 × 105) in the logarithmic growth phase were seeded into a six-well plate and cultured in arginine-free DMEM supplemented with 0.2 mM arginine and 10% FBS for 24 h. Subsequently, the culture medium was collected, and the arginine concentration was measured using L-Arginine Colorimetric Assay Kit (Elabscience, E-BC-K850-M) according to the manufacturer’s instructions. The arginine uptake ratio was calculated based on the difference in arginine concentration in the culture medium. Drugs including Paritaprevir (HY-12594), Cepharanthine (HY-N6972), Midostaurin (HY-10230), Ledipasvir (HY-15602), Alcuronium (HY-106707), Venetoclax (HY-15531), Eltrombopag (HY-15306), Simeprevir (HY-10241), Vaprisol (HY-18347 A) and Glecaprevir (HY-17634) were purchased from MedChemExpress.
Cellular Thermal Shift Assay (CETSA)
Osteosarcoma cells in the logarithmic growth phase were seeded into a 15 cm dish. The cells were pretreated with or without 40 µM CPE for 12 h prior to the cellular thermal shift assays. Following incubation, the cells were trypsinized, collected, and resuspended in PBS supplemented with a protease inhibitor cocktail. The cell suspension was then aliquoted into six PCR tubes for subsequent thermal treatment using a Veriti 96-well thermal cycler at the indicated temperature points for 3 min, followed by a 3-minute incubation at room temperature. The cells were then lysed through three freeze-thaw cycles using liquid nitrogen and a thermal cycler. The tubes were centrifuged at 13,000 rpm for 10 min at 4 °C, and the supernatant containing the cell lysate was collected for subsequent Western Blot analysis.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0, SPSS 25.0. or R 4.2.1. Each experiment was repeated three times independently. Data were presented as mean ± SD unless otherwise specified. Quantitative data were compared by the Student’s t-test or one-way ANOVA, and Overall survival and lung metastasis-free survival analysis were conducted using the Kaplan–Meier method with the log-rank test. A p-value of less than 0.05 was considered statistically significant. In all cases, the p values are represented as follows: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Results
Landscape of SLCs in the scRNA-seq dataset for osteosarcoma
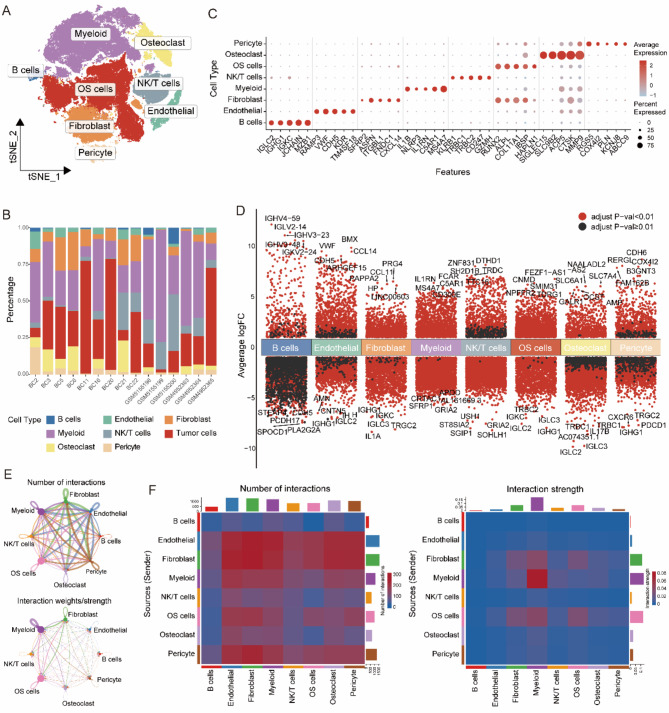
To investigate the landscape of solute carrier (SLC) family genes in osteosarcoma, we analyzed single-cell RNA sequencing (scRNA-seq) datasets from GSE152048 and GSE162454. After quality control, a total of 110,364 cells were retained for further examination. Dimensionality reduction and clustering revealed eight major cell lineages: B cells, endothelial cells, fibroblasts, myeloid cells, NK/T cells, osteoclasts, osteosarcoma (OS) cells, and pericytes (Fig. 1A). The proportion of these cell types varied across samples (Fig. 1B). Bubble plots displayed scaled expression levels and the proportion of specific markers for each cell type (Fig. 1C), while volcano plots illustrated significantly differentially expressed genes among the lineages (Fig. 1D). Utilizing CellChat analysis, we identified diverse interactions between different cell types (Fig. 1E, F).
Fig. 1.
Single-cell landscape in osteosarcoma. (A) t-SNE plot of single cells profiled in osteosarcoma. (B) Cell proportions in each samples. (C) Bubble plot of the average and percent expression of different markers in each cell types. (D) Differential gene expression analysis reveals up and down-regulated genes across various cell types. An adjusted p value < 0.01 is indicated in red, while an adjusted p value ≥ 0.01 is indicated in black. (E) Network of cell-cell communication; Number of interactions (top); Strength of interactions (bottom). (F) Heatmap of cell-cell communication; Number of interactions (left); Strength of interactions (right)
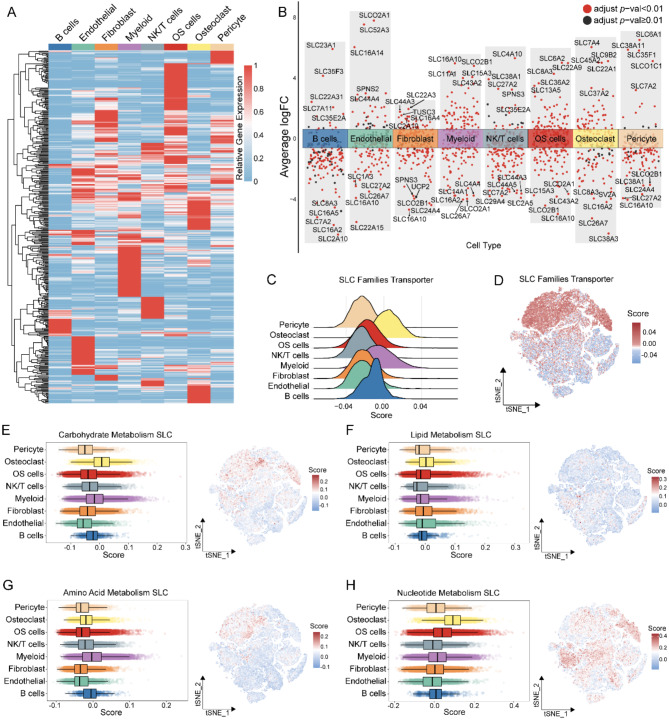
The single-cell sequencing data revealed distinct expression patterns of solute carrier (SLC) superfamily genes across different cell types (Fig. 2A, B). Using the AddModuleScore algorithm, we calculated SLC scores for each cell type, incorporating all 449 SLC genes as a gene set. Unexpectedly, osteoclasts had the highest SLC scores, followed by myeloid cells and B cells, then OS cells, while other cell types exhibited lower scores (Fig. 2C). Based on the metabolic pathways of the substrates they transport, we categorized the SLCs into four groups: Carbohydrate Metabolism SLC (CM-SLC), Lipid Metabolism SLC (LM-SLC), Amino Acid Metabolism SLC (AAM-SLC), and Nucleotide Metabolism SLC (NM-SLC). Further analysis revealed that osteoclasts had relatively higher CM-SLC and NM-SLC scores, myeloid cells showed elevated AAM-SLC scores, and tumor cells had NM-SLC scores second only to osteoclasts (Fig,1 A, Fig. 2D- E). In contrast, LM-SLC scores were consistently low across different cell types (Fig. 2F).
Fig. 2.
Landscape of SLCs in the scRNA-seq dataset for osteosarcoma. (A) Heatmap showing the expression of SLCs in each cell type. (B) Differential expression analysis of SLCs across each cell types. An adjusted p value < 0.01 is indicated in red, while an adjusted p value ≥ 0.01 is indicated in black. (C) Ridge plot of SLC score in each cell type. (D) t-SNE plot of SLC score in osteosarcoma dataset. (E) Enrichment score of Carbohydrates Metabolism SLC; CM-SLC Score in each cell type (left), t-SNE of CM-SLC Score(right). (F) Enrichment score of Lipid Metabolism SLC; LM-SLC Score in each cell type (left), t-SNE of LM-SLC Score(right). (G) Enrichment score of Amino Acid Metabolism SLC; AAM-SLC Score in each cell type (left), t-SNE of AAM-SLC Score(right). (H) Enrichment score of Nucleotide Metabolism SLC; NM-SLC Score in each cell type (left), t-SNE of NM-SLC Score(right)
Construction of a prognostic risk scoring model for osteosarcoma based on SLCs and survival analysis
We conducted a consensus clustering analysis based on 449 solute carrier (SLC) genes within osteosarcoma cases from the TARGET database(n = 85). This analysis identified two distinct clusters (K = 2, Cluster A = 41, Cluster B = 44) as determined by the consensus matrix, area under the curve, and cumulative distribution function (CDF) (Fig S1A- D). Survival analysis indicated that patients in Cluster B had a worse prognosis than those in Cluster A (p = 0.037) (Fig S1E). A clustering heatmap illustrated the correlation of SLC family gene expression with clinical features such as gender, age, ethnicity, and tumor site, revealing no significant inter-cluster differences in these characteristics (Fig S1F, Supplementary Table 3).
To further investigate the role of the SLC family in osteosarcoma progression and clinical prognosis, we randomly divided the TARGET osteosarcoma cohort into a training group (n = 50) and a testing group (n = 35) in a 6:4 ratio. Subsequently, we employed LASSO penalized Cox regression analysis to identify SLC family genes associated with prognosis, selecting the penalty parameter based on the minimum criterion (Fig. 3A, B). This analysis identified nine prognostic genes, among which SLC7A1 had the highest risk ratio of 2.19 (p = 0.011) (Fig. 3C). Based on the median risk score, we classified the training set into high-risk and low-risk groups. Survival analysis demonstrated that the high-risk group had significantly shorter overall survival compared to the low-risk group. The prognostic model exhibited high sensitivity and specificity, with area under the receiver operating characteristic (ROC) curve values of 0.856 for 1-year survival prediction, 0.961 for 3-year survival prediction, and 0.964 for 5-year survival prediction (Fig. 3D, E). Similar results were observed in the testing set and the external validation cohort GSE21257 (Fig. 3F- I). Further survival analysis of the genes identified through LASSO regression revealed that NIPA2 and SLC45A4 expression levels were positively correlated with prognosis, indicating that high-expression groups had better outcomes; In contrast, expressions of SLC13A5, SLC38A5, and SLC7A1 were negatively correlated with prognosis (Fig S2A- I).
Fig. 3.
Construction of an SLCs-related prognostic signature in osteosarcoma. (A) Coefficient profiles of SLCs. (B) Identification of the best parameter(lambda) in LASSO. (C) Cox analysis of identified 9 genes to construct the risk model. (D) Kaplan-Meier analysis of overall survival in training group. (E) Receiver operating characteristic (ROC) curve analysis for 1-,3-, and 5-year survival for risk model in training group. (F) Kaplan-Meier analysis of overall survival in testing group. (G) ROC curve analysis for 1-,3-, and 5-year survival for risk model in testing group. (H) Kaplan-Meier analysis of overall survival in GSE21257 cohort. (I) ROC curve analysis for 1-,3-, and 5-year survival for risk model in GSE21257 cohort. (J) Nomogram for predicting 1-, 3-, and 5-year survival rate in osteosarcoma. (K) Calibration curves for internal validation of the nomogram. (L) ROC curve analysis of the nomogram in predicting the overall survival
Subsequently, we constructed a nomogram that incorporated clinical parameters alongside the SLC risk model to predict overall survival in the TARGET-OS cohort (Fig. 3J). The internal validation calibration plot of the nomogram demonstrated a strong correlation between predicted probabilities and actual observed outcomes (Fig. 3K). The combined nomogram achieved an AUC value of 0.848, indicating good accuracy in predicting overall survival rates (Fig. 3L).
Immune landscape of SLC-related prognostic signature in osteosarcoma patients
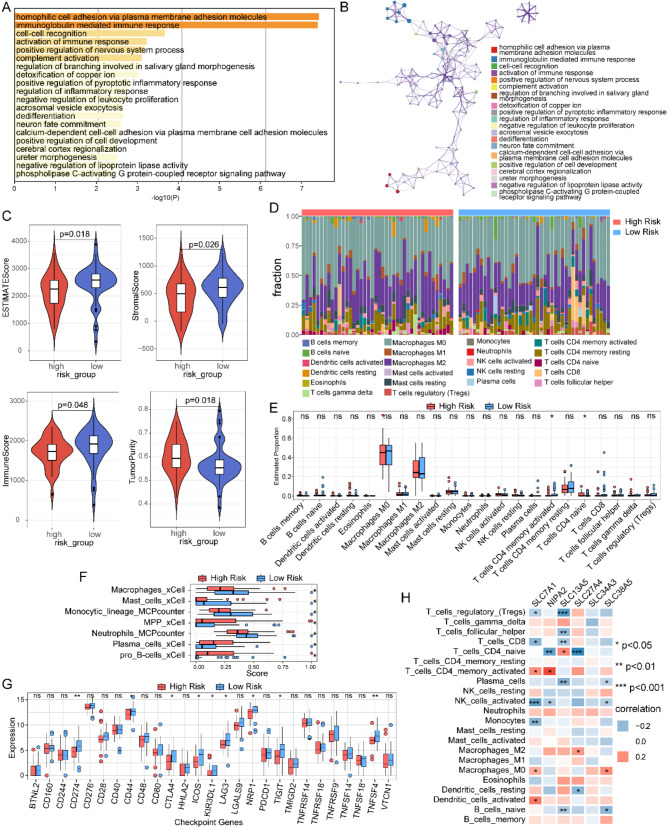
We conducted a differential analysis of RNA sequencing data across different risk groups in the TARGET-OS cohort. Using the online tool Metascape, we performed Gene Ontology (GO) enrichment analysis on differential genes (|logFC| > 1, p < 0.05) and constructed an enrichment network. The results revealed significant enrichment of differential genes in pathways related to homophilic cell adhesion through plasma membrane adhesion molecules, immunoglobulin-mediated immune response, and activation of immune responses (Fig. 4A, B).
Fig. 4.
Immune landscape of SLC-related prognostic signature in osteosarcoma patients. (A) GO annotation of differential genes between high and low-risk groups. (B) Network of enriched terms. (C) Comparison of immune score, stromal score, estimate score, and tumor purity between low- and high-risk groups. (D) Immune cell fraction analysis by CIBERSORT. (E) Immune cell infiltration proportions between low- and high-risk groups by CIBERSORT. (F) Immune cell infiltration proportions between low- and high-risk groups by XCELL and MCPcounter. (G) Expression of immune checkpoint genes between low- and high-risk groups
The ESTIMATE algorithm assessed the infiltration of stromal and immune cells in the TARGET-OS cohort, indicating that the high-risk group exhibited lower immune scores, stromal scores, and ESTIMATE scores, alongside a higher tumor purity (Fig. 4C). Further analysis using the CIBERSORT algorithm evaluated the composition of immune cells in each sample (Fig. 4D), revealing differences in the infiltration of naive CD4 T cells and memory-activated CD4 + T cells between high- and low-risk groups (Fig. 4E). Additionally, the XCELL and MCPcounter algorithms indicated significant differences in the infiltration levels of macrophages, mast cells, and monocytes between the two groups (Fig. 4F).
Differential expression analysis of immune checkpoint genes revealed that the expression levels of CD274, CD44, CTLA4, ICOS, KIR3DL1, LAG3, NPR1, TIGIT, and TNFSF4 were significantly higher in the low-risk group compared to the high-risk group (Fig. 4G). We also examined the correlation between six SLC family genes identified as significantly associated with prognosis in the risk model and immune cell infiltration. Notably, these genes correlated significantly with the infiltration of multiple immune cell types; specifically, SLC7A1 showed a significant negative correlation with activated NK cells and monocytes, while demonstrating a positive correlation with activated dendritic cells (DCs) (Fig. 4H). These findings suggest that the expression of SLC family genes is associated with immunomodulation in osteosarcoma, highlighting their potential regulatory role in the immune microenvironment.
Single-cell analysis of SLC7A1 in osteosarcoma cells
Given that SLC7A1 demonstrated the highest risk ratio in the TARGET-OS cohort and is negatively correlated with prognosis, we further analyzed it using osteosarcoma single-cell data. The bubble plot revealed elevated expression levels of SLC7A1 and a higher proportion of expressing cells among the OS cells (Fig. 5A). Further clustering analysis categorized OS cells into five distinct groups: OS1, OS2, OS3, OS4, and OS5, each with unique gene expression profiles (Fig. 5B, C). Notably, SLC7A1-positive cells were predominantly found in the OS4 group (Fig. 5D- F).
Fig. 5.
Single-cell analysis of SLC7A1 in osteosarcoma cells. (A) Average and percent expression of SLC7A1 in each cell types. (B) t-SNE plot showing the clustering of OS cells. (C) Heatmap showing the differential genes in each OS Clusters. (D) Average and percent expression of SLC7A1 in each OS Clusters. (E) t-SNE plot of SLC7A1 expression in OS cells. (F) t-SNE plot showing density of SLC7A1 in OS cells. (G) Pseudotemporal ordering trajectory map of OS cells. (H) Pseudotemporal ordering of OS Clusters. (I) Trajectory plot showing the expression of SLC7A1. (J) SLC7A1 pseudotemporal expression map. (K) scMetabolism analysis of OS Clusters. (L) The tumor Hallmark scores of SLC7A1-positive and SLC7A1-negative cells in OS cells
Using the Monocle algorithm to infer the differentiation trajectory of OS cells (Fig. 5G), we determined that the OS4 group cells occupy the terminal end of this trajectory (Fig. 5H), with SLC7A1 expression differing significantly along it (p<0.001) (Fig. 5I, J). We assessed the metabolic pathway activity across tumor groups using the scMetabolism algorithm and found that, consistent with high SLC7A1 expression, OS4 cells exhibited enhanced activity in pathways such as arginine biosynthesis, arginine and proline metabolism, and glycolysis/gluconeogenesis (Fig. 5K). Hanahan et al. identified 14 hallmarks of cancer, including “limitless replicative potential,” “tissue invasion and metastasis,” and “tumor-promoting inflammation,” which reflect the potential for malignant progression [34]. Based on these hallmarks, we retrieved the corresponding gene sets to evaluate the malignancy of the tumor cells [35]. Scoring of 14 tumor characteristic gene sets revealed that SLC7A1-positive cells possess greater proliferative and metastatic capabilities, as well as enhanced stemness (Fig. 5L).
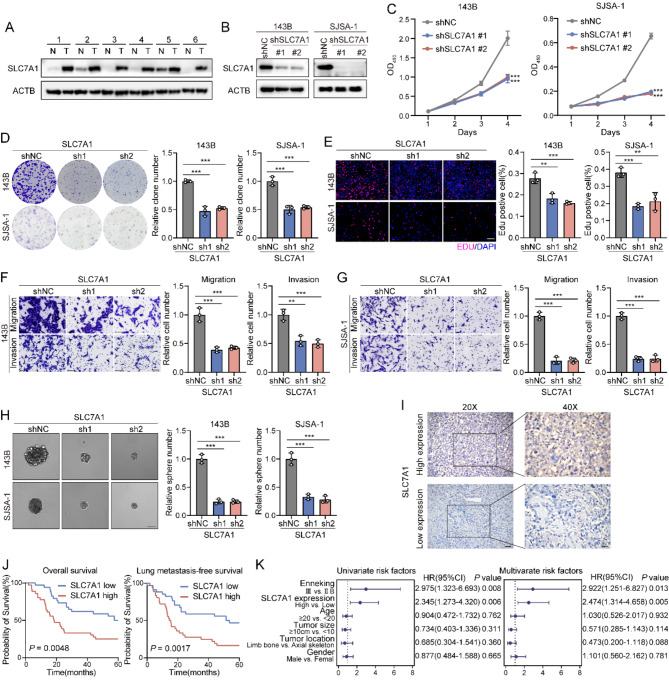
SLC7A1 promotes the proliferation and metastasis of osteosarcoma in vitro and correlates with poor prognosis
Immunoblotting revealed that SLC7A1 is upregulated in tumor tissues (Fig. 6A). Subsequently, we selected the 143B and SJSA-1 osteosarcoma cell lines and performed SLC7A1 knockdown in vitro (Fig. 6B). This knockdown resulted in reduced proliferative capacity (Fig. 6C), which was also indicated by a lower proportion of positive EdU cells and fewer colony formations (Fig. 6D, E). Furthermore, SLC7A1 knockdown significantly inhibited cell invasion, migration, and sphere-forming abilities in both cell lines (Fig. 6F- H). To validate the prognostic impact of SLC7A1, we analyzed its expression in 70 osteosarcoma specimens using immunohistochemistry (IHC) (Fig. 6I). Results indicated that 53% of patients exhibited strong or moderate SLC7A1 staining, while 47% showed weak or negative staining. Kaplan-Meier analysis revealed that high SLC7A1 expression correlated with poorer overall survival (OS) (p = 0.0048) and lung metastasis-free survival (LMFS) (p = 0.0017) (Fig. 6J). Both univariate and multivariate regression analyses identified SLC7A1 levels as an independent prognostic factor (Fig. 6K). These findings suggest that SLC7A1 may be a valuable therapeutic target and prognostic marker in osteosarcoma.
Fig. 6.
SLC7A1 promotes the proliferation and metastasis of osteosarcoma in vitro and is correlates with poor prognosis. (A) SLC7A1 protein levels measured in 6 matched pairs of osteosarcoma and adjacent normal tissues by immunoblotting. (B) Knockdown of SLC7A1 in 143B and SJSJ1 cell lines. (C) Proliferation assay of SLC7A1-KD 143B (left) and SJSA-1 (right) cells (n = 6). (D) Colony formation of SLC7A1-KD 143B and SJSA-1 cells (left). Quantification of colony formation (right) (n = 3). (E) EdU incorporation assay of SLC7A1-KD 143B and SJSA-1 cells (left). Quantification of EdU incorporation assay (right). Scale bar: 100 μm (n = 3). (F) Migration and invasion of SLC7A1-KD 143B cells (left). Quantification of migration and invasion (right). Scale bar: 100 μm (n = 3). (G) Migration and invasion of SLC7A1-KD SJSA-1 cells (left). Quantification of migration and invasion (right). Scale bar: 100 μm (n = 3). (H) Sphere formation assay of SLC7A1-KD 143B and SJSA-1 cells (left). Quantification of Sphere formation assay (right) (n = 3). (I) Representative IHC images showing high or low expression of SLC7A1 in patient tumor specimens. Scale bars: 50 μm (left), 25 μm (right). (J) Overall survival (left) and LMFS (right) of patients according to the expression levels of SLC7A1, by log-rank test. (K) Univariate and multivariate analyses of prognostic factors for overall survival and LMFS among patients with osteosarcoma, by Wald test. Data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, by two-way ANOVA with Dunnett’s multiple comparisons test (C) and one-way ANOVA with Dunnett’s multiple comparisons test (D, E, F, G, and H)
Relationship between SLC7A1 expression and tumor immune microenvironment
To investigate the relationship between SLC7A1 expression in tumor cells and the tumor immune microenvironment, we categorized 15 single-cell samples into high and low SLC7A1 expression groups based on the levels of SLC7A1 in the tumor cells. The analysis revealed distinct cell type compositions, with a notable decrease in myeloid cells in the high-expression group (Fig. 7A). Further dimensionality reduction and clustering of isolated myeloid cells identified four distinct types: tumor-associated macrophages (TAMs), dendritic cells, monocytes, and proliferative myeloid cells, each with unique expression profiles (Fig. 7B- D). A re-analysis of cellular composition confirmed a significant reduction in TAMs in the high-expression group (Fig. 7E).
Fig. 7.
Analysis of immune cell proportions and cell communication in SLC7A1-associated groups (A) Cell proportion in different SLC7A1 expression groups. (B) Clustering of myeloid cells. (C) Feature plots of marker genes in each clusters of myeloid cells. (D) Bubble plot of the average and percent expression of different markers in each clusters of myeloid cells. (E) Cell proportion of TAMs in different SLC7A1 expression groups. (F) scMetabolism analysis of TAMs in different SLC7A1 expression groups. (G) Heatmap of cell-cell communication between OS cells and immune cells. Number of interactions (left); Strength of interactions (right). (H) Network of cell-cell communication between OS cells and immune cells. Number of interactions (left); Strength of interactions (right). (I) Heatmap showing differential interactions between OS cells and immune cells. (J) Differential cell-cell communication signaling pathways between OS cells and TAMs in different SLC7A1 expression groups. (K) Bubble heatmap showing upregulated receptor-ligand interactions between OS cells and TAMs in High-SLC7A1 group. (L) Chord diagram showing upregulated receptor-ligand interactions between OS cells and TAMs in High-SLC7A1 group
Utilizing the scmetabolism package, we conducted metabolic analyses on TAMs and found that the high SLC7A1 expression group exhibited enhanced arginine biosynthesis, while arginine and proline metabolism was diminished, suggesting a potential arginine deficiency in this microenvironment (Fig. 7F). Additionally, using CellChat, we explored ligand-receptor interactions between tumor cells and various immune cells, finding a higher frequency and intensity of interactions between tumor cells and macrophages (Fig. 7G). TAMs received more signals from osteosarcoma cells compared to other immune cells (Fig. 7H).
Further comparisons of intercellular communication between the high and low SLC7A1 expression groups indicated that osteosarcoma cells in the high SLC7A1 expression group sent more signals to TAMs, albeit with slightly reduced signal intensity (Fig. 7I). Differential signal pathway analysis revealed enhanced signaling in the high SLC7A1 expression group for pathways such as ANGPTL, THBS, and IFN-I, whereas the low SLC7A1 expression group exhibited stronger signaling in MHC-I, CD86, and CD80 (Fig. 7J). Notably, pathways including THBS1-CD47, SPP1-CD44, PTN-SDC3, and IL6-(IL6R + IL6ST) were significantly upregulated in the high SLC7A1 expression group (Fig. 7K).
SLC7A1 influenced M2 macrophage polarization
To further clarify the regulatory role of SLC7A1 on macrophages, we examined the effect of SLC7A1 expression in osteosarcoma cells on the differentiation of tumor-associated macrophages (TAMs) using a co-culture model (Fig. 8A). THP1 cells were treated with PMA to induce macrophage differentiation and then co-cultured with SLC7A1-knockdown osteosarcoma cells (143B). Following this, THP1 cells were collected for analysis. Flow cytometry revealed that the SLC7A1-knockdown group exhibited a significant decrease in CD206 expression and a slight increase in CD86 expression compared to control cells (Fig. 8B, C). Additionally, qPCR analysis demonstrated significant upregulation of M2 macrophage markers, including CD206, ARG1, IL-10, TGFB1, and CD163 (Fig. 8D). These findings indicate that SLC7A1 expression in osteosarcoma cells facilitates the polarization of M2-like macrophages within the microenvironment.
Fig. 8.
SLC7A1 influenced M2 macrophage polarization. (A) Schematic diagram of the co-culture model workflow between osteosarcoma cells and macrophages derived from induced THP1 cells. (B) CD206 expression in TAMs co-cultured with control or SLC7A1-KD 143B cells, detected by flow cytometry (left). Quantification of CD206 + TAMs (right) (n = 3). (C) CD86 expression in TAMs co-cultured with control or SLC7A1-KD 143B cells, detected by flow cytometry (left). Quantification of CD86 + TAMs (right) (n = 3). (D) The expression of CD206, ARG1, IL10, TGFB1, and CD163 mRNA in TAMs co-cultured with control or SLC7A1-KD 143B cells, detected by qRT-PCR analyses (n = 3). Data are presented as the mean ± SD, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, by Students’ t-test (B, C, and D)
Structure-based virtual screen identified Cepharanthine as a potential inhibitor of SLC7A1
Structural-based virtual screening was conducted to identify approved drugs that may inhibit SLC7A1 function (Fig. 9A). We utilized the ZINC20 database, which contains nearly two billion downloadable compound structures, to retrieve and download the structural files of 5,903 approved drugs, converting them into 3D models. The SLC7A1 protein structure was predicted using AlphaFold2 (Fig. 9B), with a predicted alignment error plot indicating high confidence in most segments (Fig. 9C). Model quality assessment via the ProSA server yielded a ProSA Z-score of -6.78, indicating that the model’s quality is comparable to those derived from NMR and X-ray crystallography (Fig. 9D). Virtual screening was performed using AUTODOCK VINA, designating SLC7A1 as the receptor and the 5,903 approved drugs as ligands, from which the top 10 drugs with the lowest docking scores were selected (Table 1). The molecular docking visualization of these drugs is depicted, showing the SLC7A1 protein model in white, the drug molecules in blue sticks, hydrogen bonds as yellow dashed lines, and labeled amino acid residues involved in interactions (Fig. 9E- N).
Fig. 9.
Structure-based virtual screen of SLC7A1 (A) Schematic diagram of the workflow for structure-based virtual screening. (B) The predicted 3D structure of SLC7A1 by AlphaFold2. (C) Predicted aligned error of the SLC7A1 3D model. (D) Overall model quality of the SLC7A1 3D analyzed by ProSA-Web. (E) The calculated binding mode between Paritaprevie and SLC7A1. (F) The calculated binding mode between Cepharanthine and SLC7A1. (G) The calculated binding mode between Midostaurin and SLC7A1. (H) The calculated binding mode between Ledipasvir and SLC7A1. (I) The calculated binding mode between Alcuronium and SLC7A1. (J) The calculated binding mode between Venetoclax and SLC7A1. (K) The calculated binding mode between Eltrombopag and SLC7A1. (L) The calculated binding mode between Simeprevir and SLC7A1. (M) The calculated binding mode between Vaprisol and SLC7A1. (N) The calculated binding mode between Glecaprevir and SLC7A1
Table 1.
10 drugs with the lowest docking scores
| Rank | Score | Chemical Formula | Drug |
|---|---|---|---|
| 1 | -9.5 | C40H43N7O7S | Paritaprevir |
| 2 | -9.3 | C37H38N2O6 | Cepharanthine |
| 3 | -9.2 | C35H30N4O4 | Midostaurin |
| 4 | -9.2 | C49H54F2N8O6 | Ledipasvir |
| 5 | -9.1 | C44H50N4O2 | Alcuronium |
| 6 | -9.1 | C45H50ClN7O7S | Venetoclax |
| 7 | -9 | C25H22N4O4 | Eltrombopag |
| 8 | -9 | C38H47N5O7S2 | Simeprevir |
| 9 | -9 | C32H26N4O2 | vaprisol |
| 10 | -8.9 | C38H46F4N6O9S | Glecaprevir |
To further screen these 10 drugs, we first assessed their impact on arginine uptake in osteosarcoma cells. The results revealed that at a concentration of 10 µM, only Paritaprevir, Cepharanthine, Eltrombopag, and Simeprevir significantly inhibited arginine uptake (Fig. 10A). IC50 analysis indicated that Cepharanthine (CPE) (5.13 µM) had the lowest half-inhibitory concentration against osteosarcoma cells (Fig. 10B). Cellular Thermal Shift Assay (CETSA) demonstrated that CPE inhibited heat-induced degradation of SLC7A1 protein (Fig. 10C), suggesting a potential interaction between CPE and SLC7A1. Treatment of osteosarcoma cells with CPE at concentrations of 0, 2, and 5 µM revealed that, with increasing concentration, CPE enhanced its inhibitory effects on both arginine uptake and cell proliferation (Fig. 10D, E). To evaluate the effect of CPE pre-treatment on osteosarcoma cell-induced macrophage polarization, we collected conditioned media from osteosarcoma cells pretreated with or without CPE for 24 h and used it to culture THP1-derived macrophages (Fig. 10F). After 48 h of incubation, flow cytometry analysis revealed that CPE pretreatment significantly downregulated CD206 expression and upregulated CD86 expression, with these effects intensifying as CPE concentration increased (Fig. 10G, H). These findings indicate that Cepharanthine exerts anti-tumor effects by binding to SLC7A1 and inhibiting its activity.
Fig. 10.
Cepharanthine is a potential targeted inhibitor of SLC7A1. (A) Inhibition of arginine uptake in 143B cells by 10 drugs. (B) IC50 analysis of Paritaprevir, Cepharanthine, Eltrombopag, and Simeprevir. (C) CETSA showing the binding of CPE to SLC7A1 protein. (D) Arginine uptake in 143B cells treated with 0, 2, and 5 µM CPE. (E) CCK8 assay evaluating the proliferation of 143B cells treated with 0, 2, and 5 µM CPE. (F) Schematic of conditioned medium production from CPE -pretreated 143B cells. Cells were treated with 0, 2, or 5 µM CPE, followed by medium replacement after 24 h. After an additional 24-hour incubation, the conditioned medium was collected. This medium was used to culture THP1-induced macrophages for 48 h before flow cytometry analysis. (G) Flow cytometry analysis of CD206 expression in macrophages. (H) Flow cytometry analysis of CD86 expression in macrophages
Discussion
Recent studies suggest that the SLCs are instrumental in shaping the metabolic landscape of tumors [36–38]. By regulating the transport of metabolites, SLCs mediates the homeostasis of metabolism [39, 40]. The overexpression of SLCs in malignant cells sustains the rapid activity of cellular metabolic networks and promotes cell proliferation and invasion. According to recent studies, SLC25A51 exhibits increased expression in multiple tumors, including adenocarcinoma, gastric cancer, and liver cancer [41]. This enhanced expression is implicated in promoting oncogenic progression through the maintenance of mitochondrial protein acetylation and the facilitation of proline synthesis. In liver cancer, the activation of β-catenin upregulates SLC13A3, thereby modulating intracellular levels of glutathione and leucine; silencing of SLC13A3 induces autophagic ferroptosis [42]. In this study, we analyzed the expression patterns of SLCs across various cell types within the osteosarcoma microenvironment. The results reflects the distinct metabolic substrate uptake preferences of these cell types. In addition, we have delineated a novel SLC-related prognostic signature, which enables the assessment of patient prognosis. According to the prognostic signature, SLCs such as NIPA2, SLC13A5, SLC38A5, SLC45A4, and SLC7A1 are associated with prognosis in osteosarcoma.
The tumor immune microenvironment is characterized by its complex composition [43]. There is an intricate regulatory network involving tumor cells, immune cells, and stromal cells [9, 44]. SLCs allowed malignant cells to modulate the levels of various metabolites in the tumor microenvironment, thereby influencing immune responses [45–47]. Guo et al. demonstrated that tumor cells and conventional dendritic cells (cDC1s) compete for glutamine via SLC38A2, which regulates the antitumor activity of CD8 + T cells [48]. Research has indicated that KRAS-mutated colorectal cancer stimulates an expansion of MDSCs via SLC25A22, consequently establishing an immunosuppressive tumor microenvironment [49]. These studies indicate that SLCs modulate the levels of key metabolites within the tumor microenvironment, thereby affecting the function and activity of immune cells and promoting the establishment of an immunosuppressive microenvironment. Our findings indicate that the expression of SLC family genes correlates with several immune-related pathways, including homophilic cell adhesion and activation of the immune response. Compared to the low-risk SLC group, the high-risk group exhibited reduced infiltration of immune and stromal cells, while the tumor purity score was higher. Additionally, the high-risk group showed a lower proportion of activated memory CD4 + T cells, naive CD4 + T cells, macrophages, mast cells, monocytic lineage cells, and neutrophils, whereas the proportion of pro-B cells, MPPs, and plasma cells was higher. This is consistent with the poorer prognostic outcomes observed in the high-risk group. Furthermore, the expression of various immune checkpoint genes was associated with SLC expression levels. Our results suggest that SLCs may mediate the formation of an immunosuppressive microenvironment in osteosarcoma by modulating the metabolic microenvironment, inhibiting the proliferation and activation of immune cells, or inducing a pro-tumor phenotype in immune cells.
SLC7A1 (Cationic Amino Acid Transporter 1, CAT1) belongs to the cationic amino acid transporter family and exhibits a high affinity for arginine, lysine, and ornithine [50]. SLC7A1 is highly expressed in various tumors and is associated with tumor progression [51, 52]. In hepatocellular carcinoma, arginine uptake is uniquely dependent on SLC7A1. Inhibition of SLC7A1 significantly suppresses the growth of hepatocellular carcinoma in vitro and in vivo [53]. Breast cancer and chronic lymphocytic leukemia cells also exhibit a pronounced reliance on SLC7A1, with its downregulation markedly impeding cellular proliferation and triggering apoptosis [54, 55]. In our study, SLC7A1 exhibited elevated expression in OS cells and was positively correlated with tumor differentiation processes. SLC7A1-positive osteosarcoma cells exhibited higher tumor hallmark scores, indicating increased malignancy. In vitro experiments further confirmed that silencing SLC7A1 significantly inhibit malignant phenotypes. Additionally, IHC and survival analyses in osteosarcoma cohorts revealed an association between SLC7A1 and adverse clinical outcomes.
Macrophages are key components of the tumor immune microenvironment [56]. Current research suggests that macrophages can differentiate into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes under the stimulation of the tumor microenvironment [57, 58]. In hepatocellular carcinoma (HCC), SRSF10 enhances the expression of glycolytic enzymes, resulting in increased lactic acid levels. Lactic acid accumulation induces H3K18 acetylation in macrophages, promoting their polarization toward M2 phenotype [59]. Nian et al. demonstrated that IL-34 secreted by p53-mutated tumor cells stimulates macrophages to upregulate CD36, enhancing fatty acid uptake and driving M2-like polarization, which suppresses CD8 + T cell-mediated anti-tumor immunity [60]. These studies indicate that tumor cells can regulate macrophage activation through multiple mechanisms, driving their polarization toward an M2-like pro-tumor phenotype. In our study, the high SLC7A1 expression group exhibited a significant reduction in myeloid cell infiltration, particularly in TAMs. We observed extensive interactions between osteosarcoma cells and TAMs, with notable differences in signaling pathways, such as THBS1 and SPP1, between osteosarcoma cells and TAMs across different SLC7A1 groups. Furthermore, in the low SLC7A1 expression group, TAMs exhibited enhanced arginine metabolism, while the high expression group showed increased arginine biosynthesis. Co-culture assays demonstrated that suppression of SLC7A1 in osteosarcoma cells significantly inhibited the polarization of M2-like macrophages and induced an M1 phenotype in macrophages, as evidenced by the downregulation of CD206 and upregulation of CD86 expression. Our results suggest that the expression of SLC7A1 in osteosarcoma cells may regulate the infiltration and function of TAMs through arginine uptake and OS-TAMs cell communication.
Structure-based virtual screening is an important technique in drug development, significantly expediting the drug discovery process and minimizing associated costs [61]. The advent of AlphaFold2 has further accelerated this process by providing accurate protein structure predictions [62, 63]. Xu et al. discovered that AO-022 significantly inhibits breast cancer progression by targeting TALDO1 via virtual screening [64]. Ding et al. reported, after high-throughput virtual screening and in vitro drug effect evaluation, ZYZ384 was identified as an SMYD3 inhibitor with anti-hepatocellular carcinoma activity [65]. Given the pro-oncogenic role of SLC7A1 in osteosarcoma, investigating inhibitors that target SLC7A1 holds substantial clinical relevance. In our study, we utilized AlphaFold2 to predict the structure of the SLC7A1 protein and evaluated the model’s reliability. This was followed by virtual screening of approved drugs from the ZINC20 database. We demonstrate that Cepharanthine binds to the SLC7A1 protein, effectively inhibiting arginine uptake and cell proliferation in osteosarcoma cells, and suppresses osteosarcoma cell-induced M2-like polarization of macrophages. Our result paves the way for further drug development of SLC7A1.
While this study explores the expression patterns and functions of SLC family proteins in osteosarcoma, specifically SLC7A1, and identifies potential SLC7A1 inhibitors through virtual screening, it has certain limitations. The study utilized publicly available databases to develop and validate SLC-related risk model. Significant differences in macrophage and lymphoid cell infiltration were observed between different risk groups and SLC7A1 expression groups. Further analysis and validation of other immune cell types, beyond macrophages, would provide a more comprehensive understanding of SLC7A1’s impact on the tumor immune microenvironment. While in vitro functional experiments confirmed the role of SLC7A1 in osteosarcoma, additional in vivo experiments are required to validate these findings. Furthermore, the molecular mechanisms by which SLC7A1 promotes proliferation and induces M1/M2 macrophage polarization need further exploration. Furthermore, a more thorough validation and evaluation of the anti-osteosarcoma effects and clinical efficacy of SLC7A1 inhibitors are essential.
Conclusions
Collectively, we found that SLCs, and the representative molecule SLC7A1 in particular, were associated with osteosarcoma prognosis. The study revealed that solute carrier family (SLCs) significantly influence immune cell infiltration in osteosarcoma, and the constructed SLC-related risk prognostic signature demonstrated robust predictive efficacy for overall survival. Single-cell analysis and in vitro experiments demonstrated that SLC7A1 promotes the malignant progression of osteosarcoma. SLC7A1 in tumor cells modulates the communication between the tumor and macrophages, facilitating the polarization towards M2-like macrophages. Moreover, we identified Cepharanthine as a potential SLC7A1 inhibitor. These findings provide novel insights into the importance of SLCs, particularly SLC7A1, in the tumor microenvironment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All contributors to this study are included in the list of authors. We gratefully acknowledge the GEO and TARGET database and the uploader of the datasets.
Abbreviations
- OS
Osteosarcoma
- TIME
Tumor Immune Microenvironment
- SLC
Solute Carrier
- scRNA-seq
Single cell RNA sequencing
- TAMs
Tumor-associated macrophages
- GEO
Gene Expression Omnibus
- LASSO
Least Absolute Shrinkage and Selection Operator
- TARGET
Therapeutically Applicable Research to Generate Effective Treatment
- IHC
Immunohistochemistry
- GO
Gene Ontology
- CM-SLC
Carbohydrate Metabolism SLC
- LM-SLC
Lipid Metabolism SLC
- AAM-SLC
Amino Acid Metabolism SLC
- NM-SLC
Nucleotide Metabolism SLC
- CETSA
Cellular Thermal Shift Assay
Author contributions
XX, LW and YZ designed the study. YL conducted the bioinformatic analysis and analyzed the data. YL and JC performed the experiments and analyzed the data. YL and HY drafted the manuscript. TZ, JT, WC and ZG made a significant contribution to the acquisition and integration of the data. TZ and ZG reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) Project (Grants 82273357).
Data availability
Publicly available datasets were analyzed in this study. These data can be found in: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgiacc=GSE21257,GSE152048,GSE162454 and https://ocg.cancer.gov/programs/target.
Declarations
Ethics approval and consent to participate
Human sample collection for this study was conducted in accordance with the Declaration of Helsinki, and was approved by the Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University, with the reference number [2021]755.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Liao, Junkai Chen and Hao Yao contributed equally to this work.
Contributor Information
Yutong Zou, Email: zouyt23@mail2.sysu.edu.cn.
Lili Wen, Email: wenll@sysucc.org.cn.
Xianbiao Xie, Email: xiexbiao@mail.sysu.edu.cn.
References
- 1.Whelan JS, Davis LE. Osteosarcoma, Chondrosarcoma, and Chordoma. J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36(2):188–93. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Reviews Clin Oncol. 2021;18(10):609–24. [DOI] [PubMed] [Google Scholar]
- 4.Ritter J, Bielack SS, Osteosarcoma. Annals of Oncology. Official J Eur Soc Med Oncol. 2010;21(Suppl 7):vii320–5. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer PS, Helman LJ. New Horizons in the Treatment of Osteosarcoma. N Engl J Med. 2021;385(22):2066–76. [DOI] [PubMed] [Google Scholar]
- 6.Crompton JG, Ogura K, Bernthal NM, Kawai A, Eilber FC. Local Control of Soft Tissue and Bone Sarcomas. J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36(2):111–7. [DOI] [PubMed] [Google Scholar]
- 7.Tufail M, Jiang C-H, Li N. Altered metabolism in cancer: insights into energy pathways and therapeutic targets. Mol Cancer. 2024;23(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Y, Yu T-J, Xu Y, Ding R, Wang Y-P, Jiang Y-Z, et al. Emerging therapies in cancer metabolism. Cell Metabol. 2023;35(8):1283–303. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Ma Y, Li D, Wei J, Chen K, Zhang E, et al. Cancer associated fibroblasts and metabolic reprogramming: unraveling the intricate crosstalk in tumor evolution. J Hematol Oncol. 2024;17(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Guo J, Yu S, Zheng Y, Duan M, Zhao L et al. Cellular senescence and metabolic reprogramming: Unraveling the intricate crosstalk in the immunosuppressive tumor microenvironment. Cancer Communications (London, England). 2024. [DOI] [PMC free article] [PubMed]
- 11.Dang Q, Li B, Jin B, Ye Z, Lou X, Wang T, et al. Cancer immunometabolism: advent, challenges, and perspective. Mol Cancer. 2024;23(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Pei T, Liu C, Cao M, Hu X, Yuan J et al. Glutamine metabolic competition drives immunosuppressive reprogramming of intratumour GPR109A + myeloid cells to promote liver cancer progression. Gut. 2024. [DOI] [PubMed]
- 13.Vilbois S, Xu Y, Ho P-C. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer. 2024;10(3):242–55. [DOI] [PubMed] [Google Scholar]
- 14.Hediger MA, Romero MF, Peng J-B, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflug Arch: Eur J Physiol. 2004;447(5):465–8. [DOI] [PubMed] [Google Scholar]
- 15.Schlessinger A, Yee SW, Sali A, Giacomini KM. SLC classification: an update. Clin Pharmacol Ther. 2013;94(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai W-Y, Chuang T-P, Borenäs M, Lind DE, Hallberg B, Palmer RH. Anaplastic Lymphoma Kinase signaling stabilizes SLC3A2 expression via MARCH11 to promote neuroblastoma cell growth. Cell Death Differ. 2024. [DOI] [PMC free article] [PubMed]
- 17.Zhang Q, Wei T, Jin W, Yan L, Shi L, Zhu S, et al. Deficiency in SLC25A15, a hypoxia-responsive gene, promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J Hepatol. 2024;80(2):293–308. [DOI] [PubMed] [Google Scholar]
- 18.Tan Z, Boyapati K, Tressler CM, Jenkinson NM, Glunde K. Glutamine transporter SLC38A3 promotes breast cancer metastasis via Gsk3β/β-catenin/EMT pathway. Cancer Lett. 2024;586:216653. [DOI] [PubMed] [Google Scholar]
- 19.Cao T, Zhang W, Wang Q, Wang C, Ma W, Zhang C et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8 + T cells. Cell. 2024;187(9). [DOI] [PubMed]
- 20.Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappellesso F, Orban M-P, Shirgaonkar N, Berardi E, Serneels J, Neveu M-A, et al. Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat Cancer. 2022;3(12):1464–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16(12):1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan C-H, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12(1):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14(10):979–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at single-cell level. Cancer Discov. 2022;12(1):134–53. [DOI] [PubMed] [Google Scholar]
- 26.Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinf (Oxford England). 2010;26(12):1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized Linear models via Coordinate Descent. J Stat Softw. 2010;33(1). [PMC free article] [PubMed]
- 28.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng D, Ye Z, Shen R, Yu G, Wu J, Xiong Y, et al. IOBR: Multi-omics Immuno-Oncology Biological Research to Decode Tumor Microenvironment and signatures. Front Immunol. 2021;12:687975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin JJ, Tang KG, Young J, Dandarchuluun C, Wong BR, Khurelbaatar M, et al. ZINC20-A free Ultralarge-Scale Chemical database for ligand Discovery. J Chem Inf Model. 2020;60(12):6065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varadi M, Bertoni D, Magana P, Paramval U, Pidruchna I, Radhakrishnan M, et al. AlphaFold protein structure database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024;52(D1):D368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35(Web Server issue):W407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and Python Bindings. J Chem Inf Model. 2021;61(8):3891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D. Hallmarks of Cancer: New dimensions. Cancer Discov. 2022;12(1):31–46. [DOI] [PubMed] [Google Scholar]
- 35.Yuan H, Yan M, Zhang G, Liu W, Deng C, Liao G, et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019;47(D1):D900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Y, Huang Y-H, Skopelitis DS, Iyer SV, Costa ASH, Yang Z, et al. SLC5A3-Dependent Myo-Inositol Auxotrophy in Acute myeloid leukemia. Cancer Discov. 2022;12(2):450–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J, Long G, Hu K, Xiao D, Liu S, Xiao L, et al. Targeting USP8 inhibits O-GlcNAcylation of SLC7A11 to promote Ferroptosis of Hepatocellular Carcinoma via stabilization of OGT. Adv Sci (Weinheim Baden-Wurttemberg Germany). 2023;10(33):e2302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Zong H, Liu W, Lin W, Sun A, Ding Z, et al. Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11. J Experimental Clin Cancer Research: CR. 2024;43(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlessinger A, Zatorski N, Hutchinson K, Colas C. Targeting SLC transporters: small molecules as modulators and therapeutic opportunities. Trends Biochem Sci. 2023;48(9):801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (London England). 2018;38(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Bie J, Zhao L, Song C, Zhang T, Li M, et al. SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis. Cell Death Differ. 2023;30(8):1916–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao W, Wang X, Han L, Zhang C, Wang C, Kong D, et al. SLC13A3 is a major effector downstream of activated β-catenin in liver cancer pathogenesis. Nat Commun. 2024;15(1):7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khosravi G-R, Mostafavi S, Bastan S, Ebrahimi N, Gharibvand RS, Eskandari N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun (London England). 2024;44(5):521–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haynes NM, Chadwick TB, Parker BS. The complexity of immune evasion mechanisms throughout the metastatic cascade. Nat Immunol. 2024;25(10):1793–808. [DOI] [PubMed] [Google Scholar]
- 45.Chen R, Chen L. Solute carrier transporters: emerging central players in tumour immunotherapy. Trends Cell Biol. 2022;32(3):186–201. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Zou W. Amino acids and their transporters in T cell immunity and Cancer therapy. Mol Cell. 2020;80(3):384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng M, Ren J, Jing Y, Jiang X, Xiao Q, Huang J, et al. Tumour-derived small extracellular vesicles suppress CD8 + T cell immune function by inhibiting SLC6A8-mediated creatine import in NPM1-mutated acute myeloid leukaemia. J Extracell Vesicles. 2021;10(13):e12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo C, You Z, Shi H, Sun Y, Du X, Palacios G, et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature. 2023;620(7972):200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Q, Peng Y, Ji F, Chen H, Kang W, Chan L-S, et al. Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat Commun. 2023;14(1):4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino acid transporters (CATs). J Membr Biol. 2006;213(2):67–77. [DOI] [PubMed] [Google Scholar]
- 51.You S, Han X, Xu Y, Yao Q. Research progress on the role of cationic amino acid transporter (CAT) family members in malignant tumors and immune microenvironment. Amino Acids. 2023;55(10):1213–22. [DOI] [PubMed] [Google Scholar]
- 52.He W, Zhang J, Liu B, Liu X, Liu G, Xie L, et al. S119N mutation of the E3 ubiquitin ligase SPOP suppresses SLC7A1 degradation to regulate Hepatoblastoma Progression. Mol Ther Oncolytics. 2020;19:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Missiaen R, Anderson NM, Kim LC, Nance B, Burrows M, Skuli N et al. GCN2 inhibition sensitizes arginine-deprived hepatocellular carcinoma cells to senolytic treatment. Cell Metabol. 2022;34(8). [DOI] [PMC free article] [PubMed]
- 54.Abdelmagid SA, Rickard JA, McDonald WJ, Thomas LN, Too CKL. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J Cell Biochem. 2011;112(4):1084–92. [DOI] [PubMed] [Google Scholar]
- 55.Werner A, Pieh D, Echchannaoui H, Rupp J, Rajalingam K, Theobald M, et al. Cationic amino acid transporter-1-Mediated Arginine Uptake is essential for chronic lymphocytic leukemia cell proliferation and viability. Front Oncol. 2019;9:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taranto D, Kloosterman DJ, Akkari L. Macrophages and T cells in metabolic disorder-associated cancers. Nat Rev Cancer. 2024. [DOI] [PubMed]
- 57.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [DOI] [PubMed] [Google Scholar]
- 58.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40. [DOI] [PubMed] [Google Scholar]
- 59.Cai J, Song L, Zhang F, Wu S, Zhu G, Zhang P et al. Targeting SRSF10 might inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy in hepatocellular carcinoma. Cancer Communications (London, England). 2024. [DOI] [PMC free article] [PubMed]
- 60.Nian Z, Dou Y, Shen Y, Liu J, Du X, Jiang Y et al. Interleukin-34-orchestrated tumor-associated macrophage reprogramming is required for tumor immune escape driven by p53 inactivation. Immunity. 2024;57(10). [DOI] [PubMed]
- 61.Zhou G, Rusnac D-V, Park H, Canzani D, Nguyen HM, Stewart L, et al. An artificial intelligence accelerated virtual screening platform for drug discovery. Nat Commun. 2024;15(1):7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo S-B, Meng Y, Lin L, Zhou Z-Z, Li H-L, Tian X-P, et al. Artificial intelligence alphafold model for molecular biology and drug discovery: a machine-learning-driven informatics investigation. Mol Cancer. 2024;23(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z, Zeng X, Zhao Y, Chen R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct Target Therapy. 2023;8(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu G, Huang R, Wumaier R, Lyu J, Huang M, Zhang Y, et al. Proteomic profiling of serum extracellular vesicles identifies diagnostic signatures and therapeutic targets in breast Cancer. Cancer Res. 2024;84(19):3267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding Q, Cai J, Jin L, Hu W, Song W, Rose P, et al. A novel small molecule ZYZ384 targeting SMYD3 for hepatocellular carcinoma via reducing H3K4 trimethylation of the Rac1 promoter. MedComm. 2024;5(10):e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found in: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgiacc=GSE21257,GSE152048,GSE162454 and https://ocg.cancer.gov/programs/target.