Abstract
1. Male frogs (Xenopus laevis) were castrated then given either empty or testosterone-filled implants to produce animals with low or high levels of circulating testosterone. Eight weeks later the contractile properties of an androgen-sensitive forelimb flexor, the flexor carpi radialis muscle (FCR), were measured in vitro. Another forelimb flexor muscle, the coracoradialis, and a hindlimb muscle, the iliofibularis, were analysed similarly. 2. Plasma testosterone levels were 0.9 +/- 0.3 ng/ml (+/- S.E.M.) in castrated frogs with blank implants (C) and 61.3 +/- 4.7 ng/ml in castrates with testosterone implants (CT). Unoperated males, sampled at various times of the year, ranged between 10.8 and 51.0 ng/ml. 3. With direct electrical stimulation of the FCR, contraction time of the isometric twitch was not affected by testosterone levels. Relaxation times were affected, however. Half- and 90% relaxation times were 27 and 42% longer, respectively, for CT compared to C muscles. 4. Testosterone also had no effect on the contraction time of twitches elicited by stimulation of the FCR nerve. Half- and 90% relaxation times were 51 and 76% longer, respectively, for CT compared to C muscles. 5. Tetanus tension, elicited by direct stimulation of the FCR at 50 Hz, was 86% greater in CT compared to C muscles. The average cross-sectional area of FCR muscle fibres was 84% greater in CT muscles. These results implied that testosterone treatment had no effect on specific muscle tension. 6. Stimulation of the FCR nerve at 50 Hz resulted in 53% less tension than the same stimulus applied directly to CT muscles. In C muscles the difference was only 14%. This suggested that testosterone treatment reduced synaptic efficacy. 7. In CT muscles, direct or nerve stimulation of fibres in the shoulder region of the FCR elicited twitches that contracted and relaxed more slowly than fibres in the elbow region. In C muscles there was no difference in contraction or relaxation time between fibres in the shoulder and elbow regions. 8. Testosterone treatment had little effect on contraction and relaxation times or tension levels of coracoradialis or iliofibularis muscles.
Full text
PDF


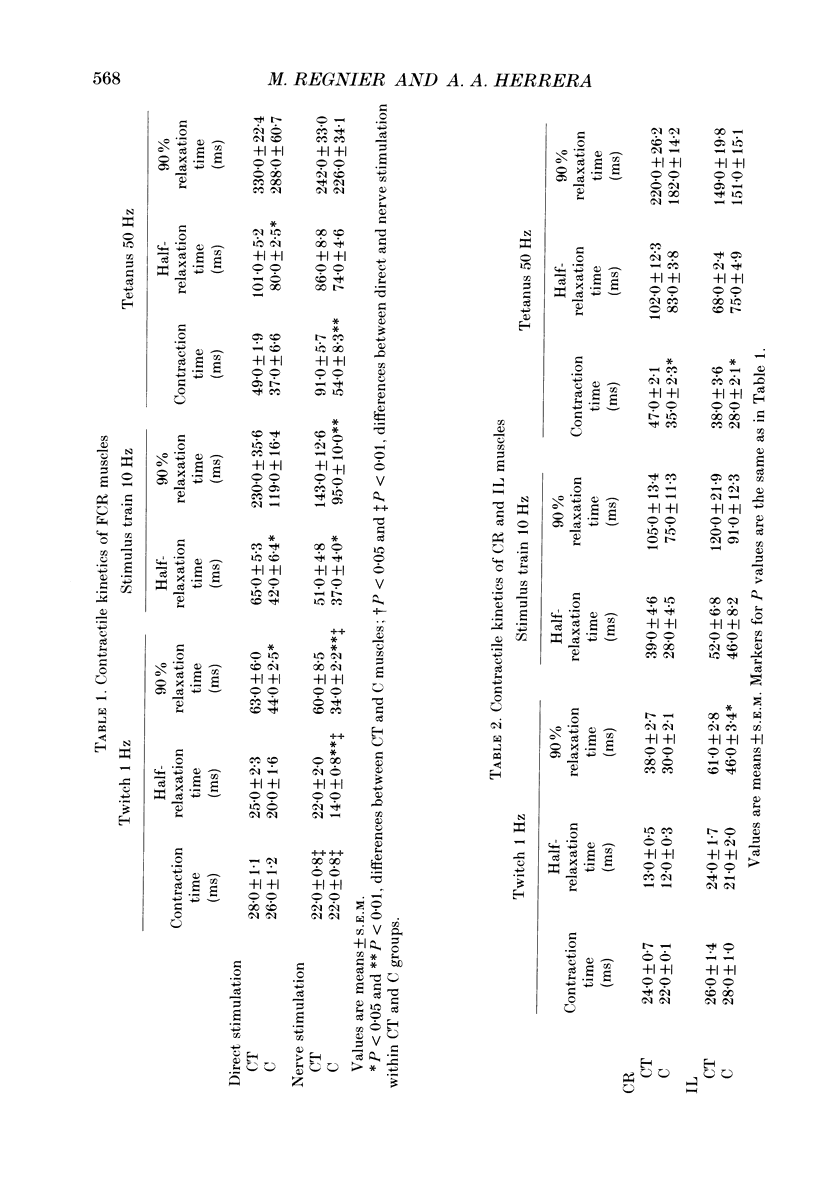
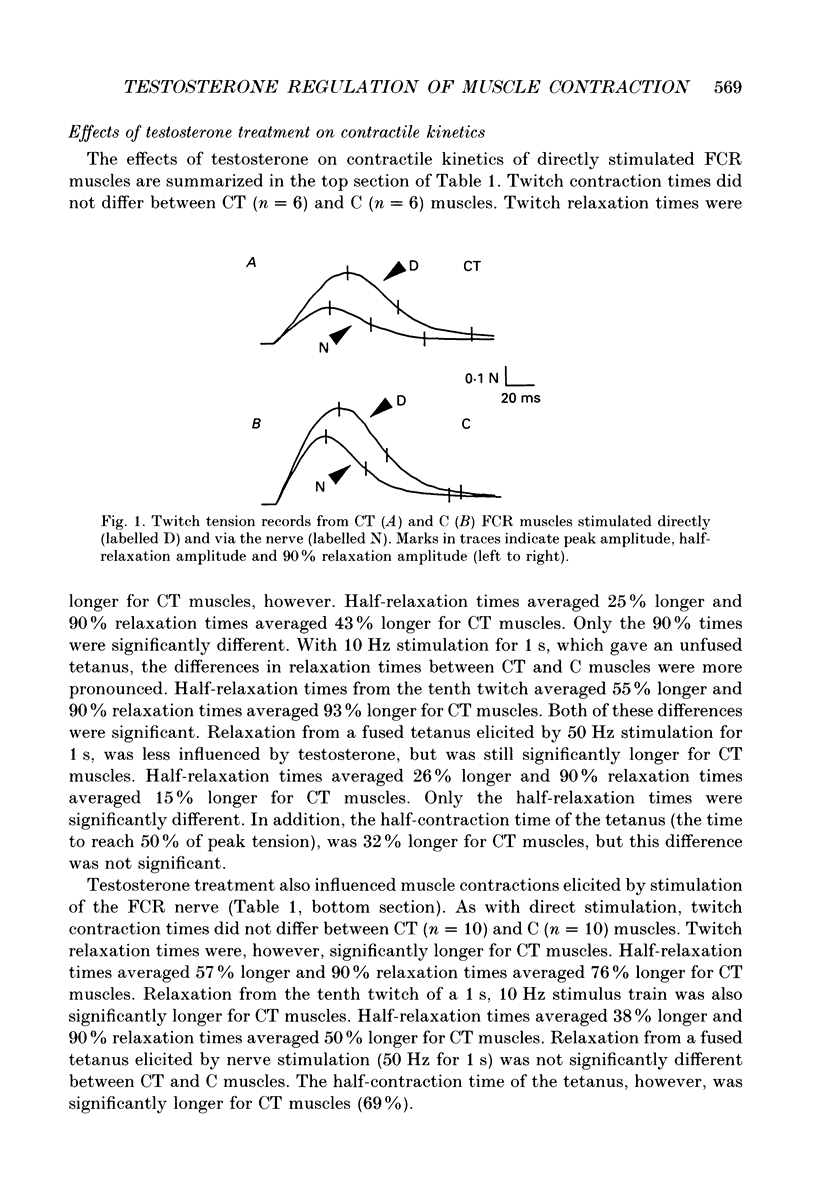










Selected References
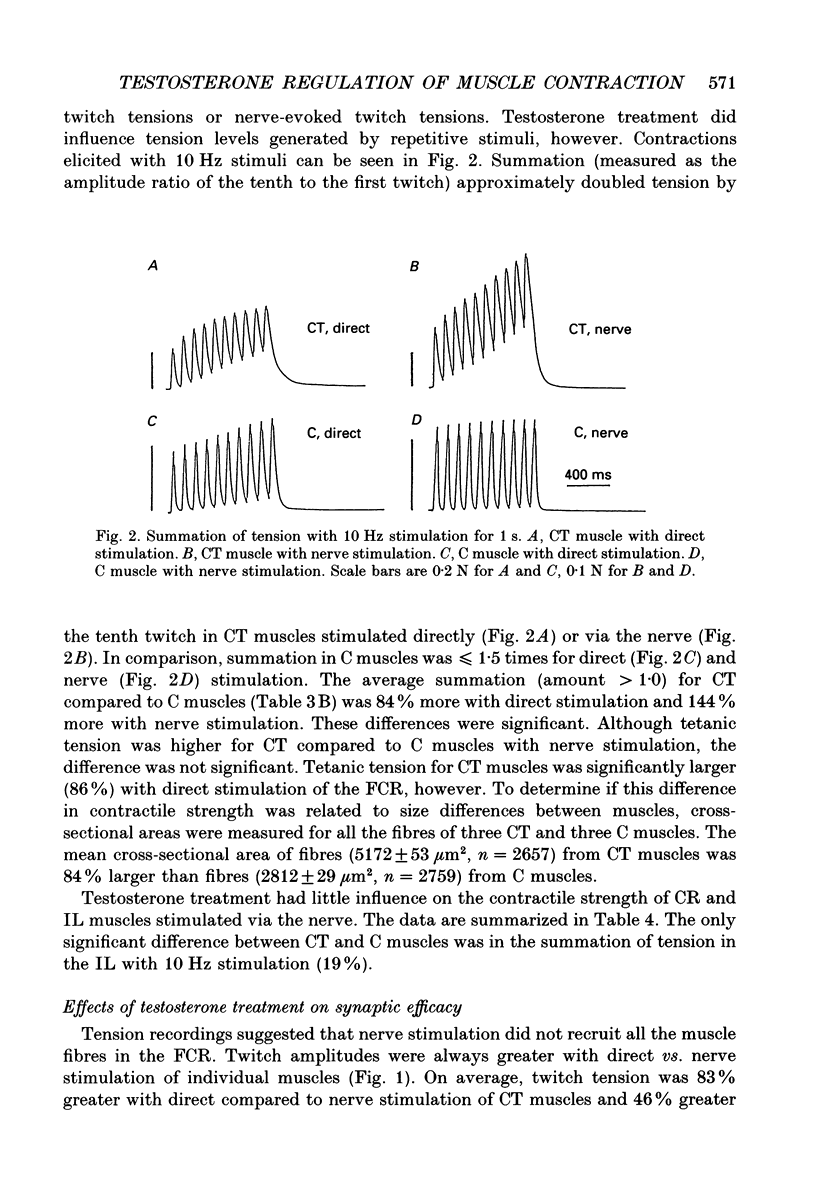
These references are in PubMed. This may not be the complete list of references from this article.
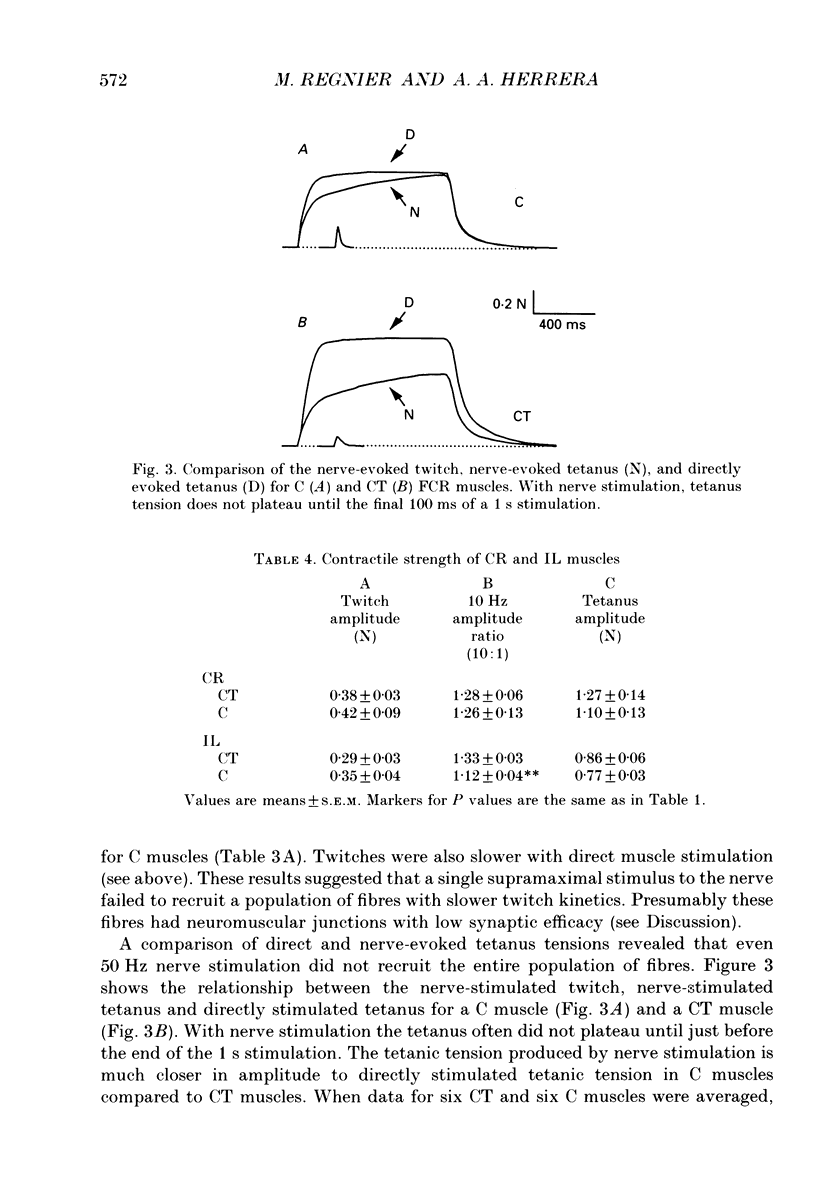
- Blanco C. E., Sieck G. C., Edgerton V. R. Quantitative histochemical determination of succinic dehydrogenase activity in skeletal muscle fibres. Histochem J. 1988 Apr;20(4):230–243. doi: 10.1007/BF01747468. [DOI] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Kelley D. B., Jurman M. E., Zemlan F. P., Schneider G. T., Krieger N. R. Modulation of the neural control of the clasp reflex in male Xenopus laevis by androgens: a multidisciplinary study. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5876–5880. doi: 10.1073/pnas.78.9.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Wetzel D. M. 5 alpha-dihydrotestosterone has nonspecific effects on membrane channels and possible genomic effects on ACh-activated channels. J Neurophysiol. 1989 May;61(5):1036–1052. doi: 10.1152/jn.1989.61.5.1036. [DOI] [PubMed] [Google Scholar]
- Heglund N. C., Cavagna G. A. Mechanical work, oxygen consumption, and efficiency in isolated frog and rat muscle. Am J Physiol. 1987 Jul;253(1 Pt 1):C22–C29. doi: 10.1152/ajpcell.1987.253.1.C22. [DOI] [PubMed] [Google Scholar]
- Heilman C., Müller W., Pette D. Correlation between ultrastructural and functional changes in sarcoplasmic reticulum during chronic stimulation of fast muscle. J Membr Biol. 1981 Apr 15;59(2):143–149. doi: 10.1007/BF01875712. [DOI] [PubMed] [Google Scholar]
- Iaizzo P. A., Poppele R. E. Twitch relaxation of the cat soleus muscle at different lengths and temperatures. Muscle Nerve. 1990 Dec;13(12):1105–1112. doi: 10.1002/mus.880131204. [DOI] [PubMed] [Google Scholar]
- Kelley D. B., Pfaff D. W. Hormone effects on male sex behavior in adult South African clawed frogs, Xenopus laevis. Horm Behav. 1976 Jun;7(2):159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Kirby A. C. Physiology of the sternoradialis muscle: sexual dimorphism and role in amplexus in the Leopard frog (Rana pipiens). Comp Biochem Physiol A Comp Physiol. 1983;74(3):705–709. doi: 10.1016/0300-9629(83)90572-8. [DOI] [PubMed] [Google Scholar]
- Klug G., Wiehrer W., Reichmann H., Leberer E., Pette D. Relationships between early alterations in parvalbumins, sarcoplasmic reticulum and metabolic enzymes in chronically stimulated fast twitch muscle. Pflugers Arch. 1983 Dec;399(4):280–284. doi: 10.1007/BF00652753. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Proske U. Properties of motor units of the frog sartorius muscle. J Physiol. 1976 Jul;258(3):673–685. doi: 10.1113/jphysiol.1976.sp011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G. E., Kelly A. M., Rubinstein N. A. Testosterone-induced changes in contractile protein isoforms in the sexually dimorphic temporalis muscle of the guinea pig. J Biol Chem. 1986 Oct 5;261(28):13278–13284. [PubMed] [Google Scholar]
- Lännergren J., Lindblom P., Johansson B. Contractile properties of two varieties of twitch muscle fibres in Xenopus laevis. Acta Physiol Scand. 1982 Apr;114(4):523–535. doi: 10.1111/j.1748-1716.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Melichna J., Gutmann E., Herbrychová A., Stichová J. Sexual dimorphism in contraction properties and fibre pattern of the flexor carpi radialis muscle of the frog (Rana temporaria L.). Aust Vet J. 1972 Jan;48(1):89–91. [PubMed] [Google Scholar]
- Muller E. R., Galavazi G., Szirmai J. A. Effect of castration and testosterone treatment on fiber width of the flexor carpi radialis muscle in the male frog (Rana temporaria L.). Gen Comp Endocrinol. 1969 Oct;13(2):275–284. doi: 10.1016/0016-6480(69)90249-4. [DOI] [PubMed] [Google Scholar]
- Pette D. J.B. Wolffe memorial lecture. Activity-induced fast to slow transitions in mammalian muscle. Med Sci Sports Exerc. 1984 Dec;16(6):517–528. [PubMed] [Google Scholar]
- Saborido A., Vila J., Molano F., Megías A. Effect of anabolic steroids on mitochondria and sarcotubular system of skeletal muscle. J Appl Physiol (1985) 1991 Mar;70(3):1038–1043. doi: 10.1152/jappl.1991.70.3.1038. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Sassoon D. A., Gray G. E., Kelley D. B. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci. 1987 Oct;7(10):3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segil N., Silverman L., Kelley D. B. Androgen-binding levels in a sexually dimorphic muscle of Xenopus laevis. Gen Comp Endocrinol. 1987 Apr;66(1):95–101. doi: 10.1016/0016-6480(87)90354-6. [DOI] [PubMed] [Google Scholar]
- Thibert P. Androgen sensitivity of skeletal muscle: nondependence on the motor nerve in the frog forearm. Exp Neurol. 1986 Mar;91(3):559–570. doi: 10.1016/0014-4886(86)90052-x. [DOI] [PubMed] [Google Scholar]
- Thibert P., Nicolet M. Tonic properties of the Flexor carpi radialis muscle of the male frog (Rana temporaria). Pflugers Arch. 1975;356(3):253–265. doi: 10.1007/BF00583837. [DOI] [PubMed] [Google Scholar]
- Tobias M. L., Kelley D. B. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis. J Neurosci. 1988 Jul;8(7):2422–2429. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Gutmann E. Electrophysiological and contractile properties of the levator ani muscle after castration and testosterone administration. Pflugers Arch. 1977 Mar 11;368(1-2):105–109. doi: 10.1007/BF01063461. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Gutmann E. Spontaneous transmitter release from motor nerve endings in muscle fibres of castrated and old animals. Experientia. 1969 Sep 15;25(9):945–946. doi: 10.1007/BF01898080. [DOI] [PubMed] [Google Scholar]


