Abstract
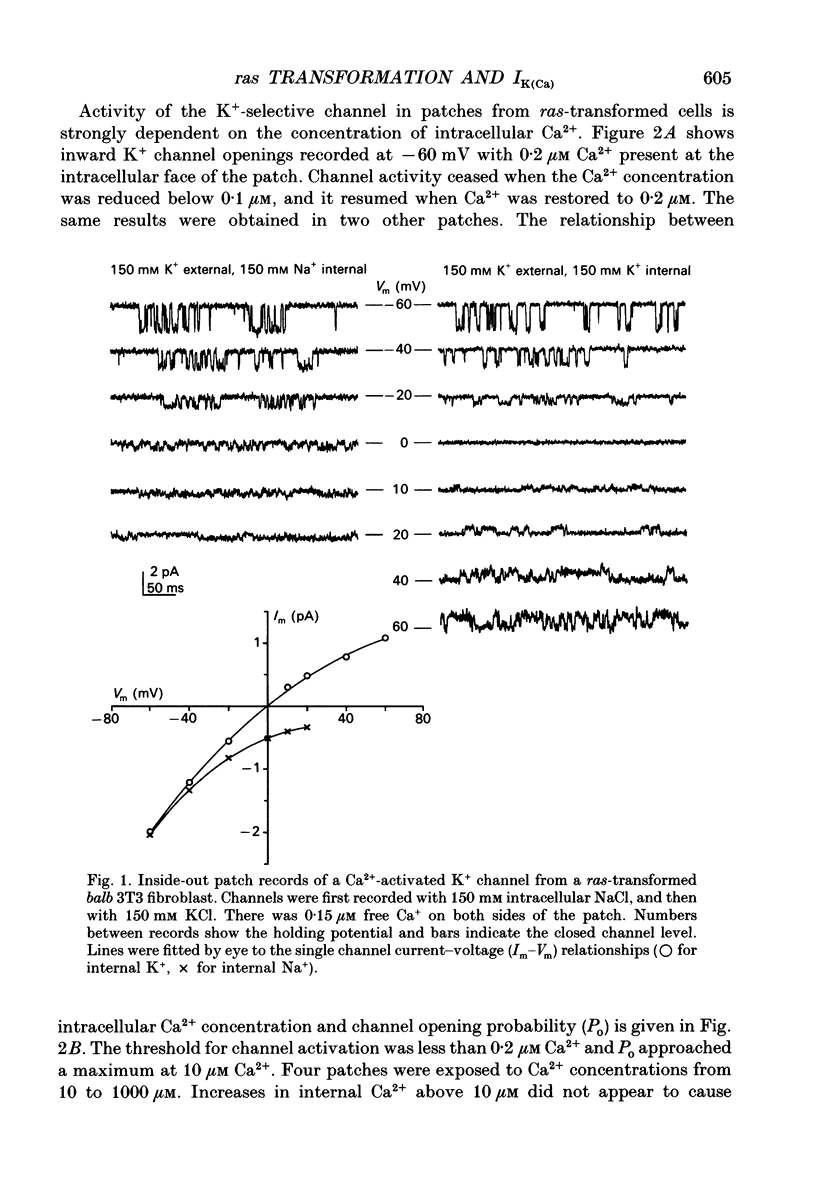
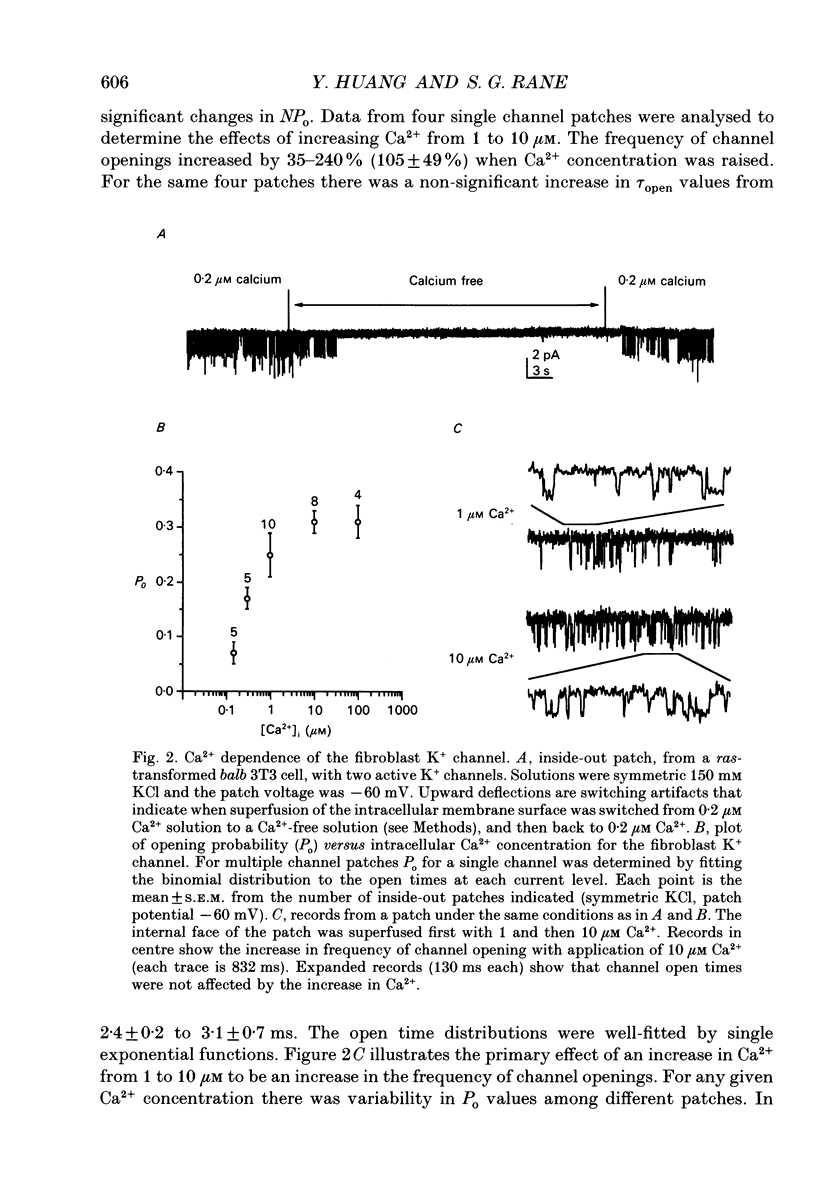
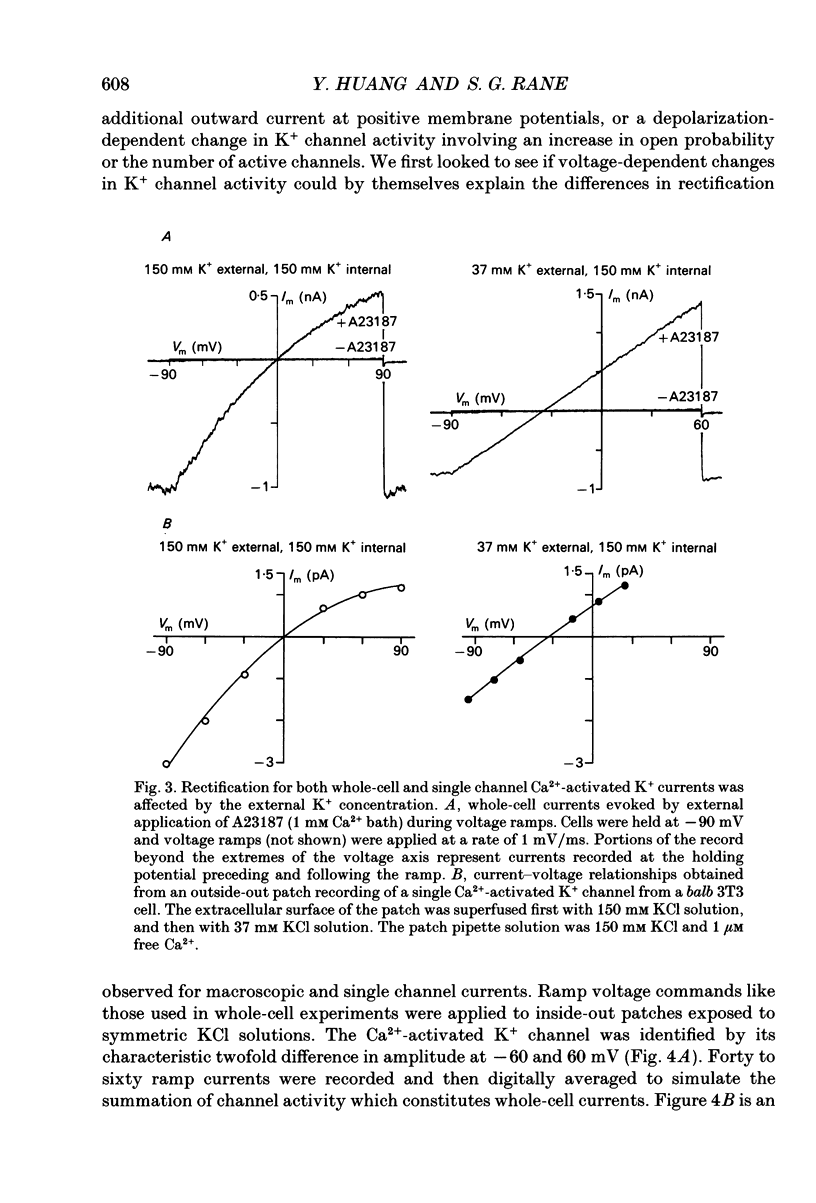
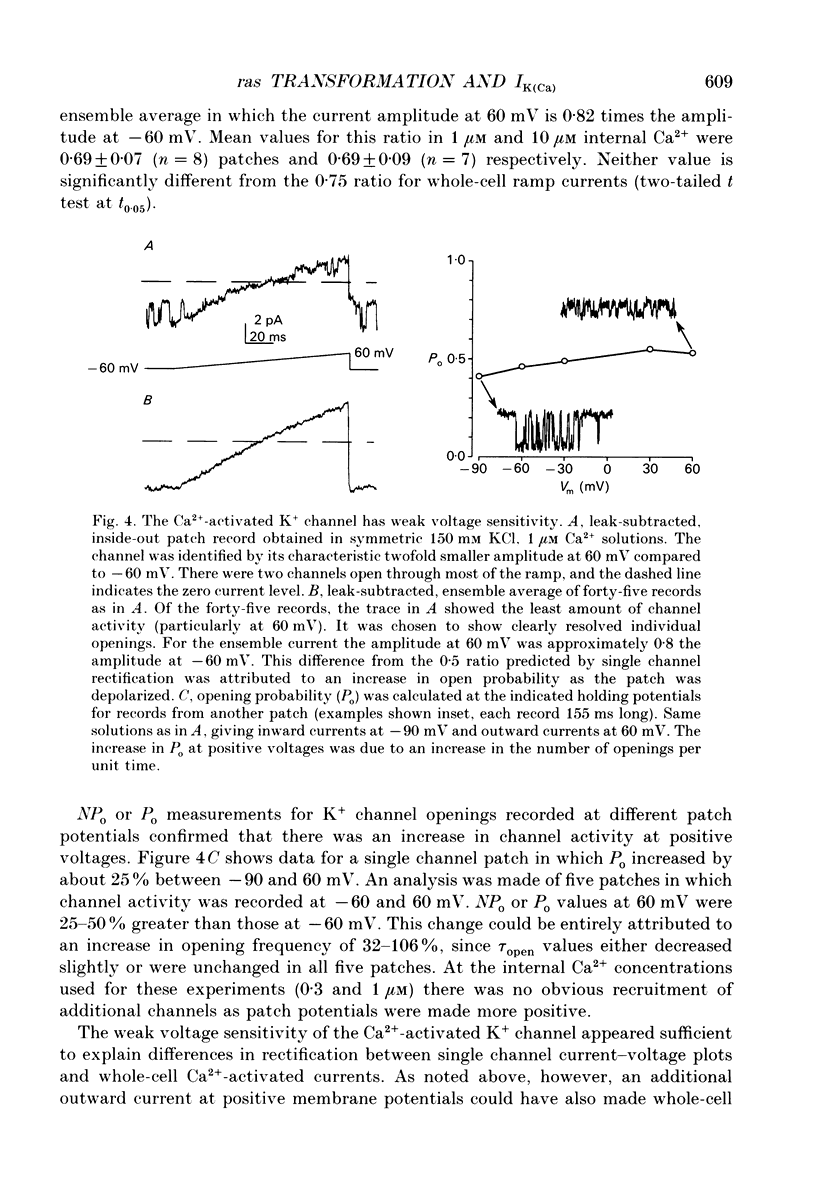
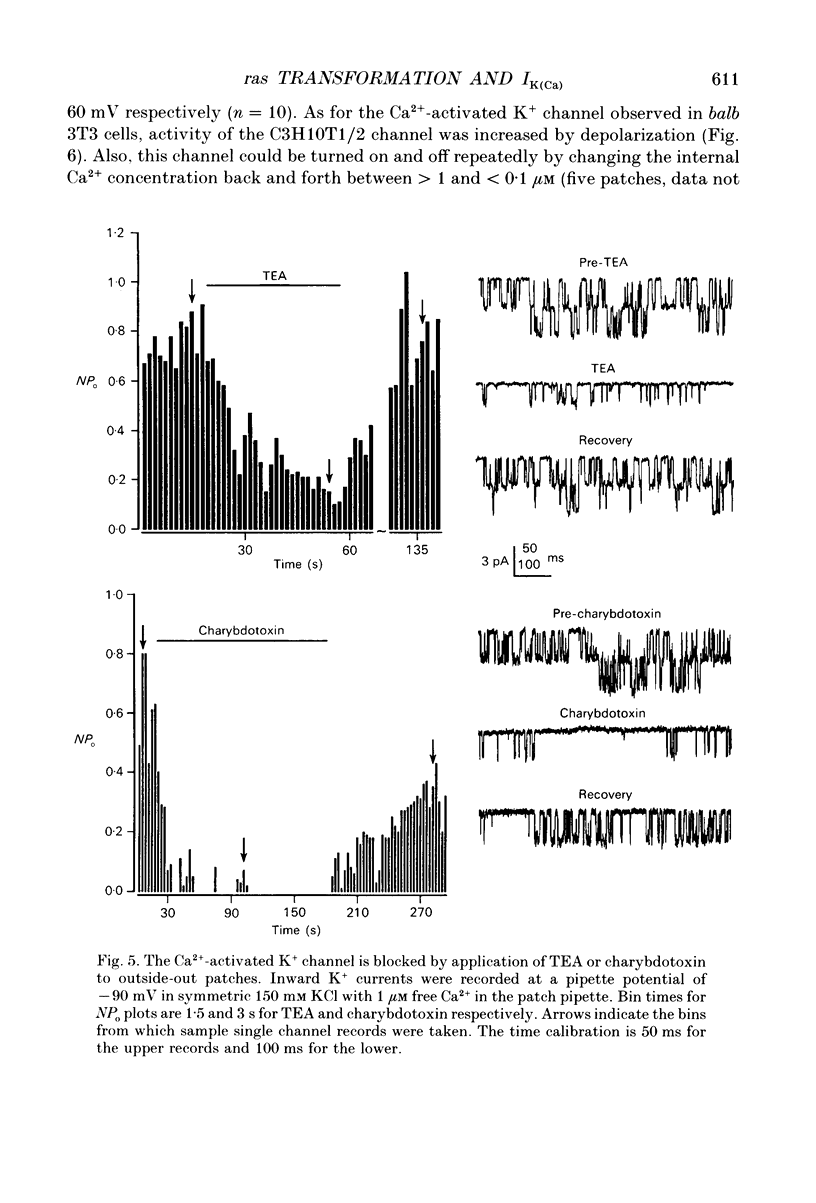
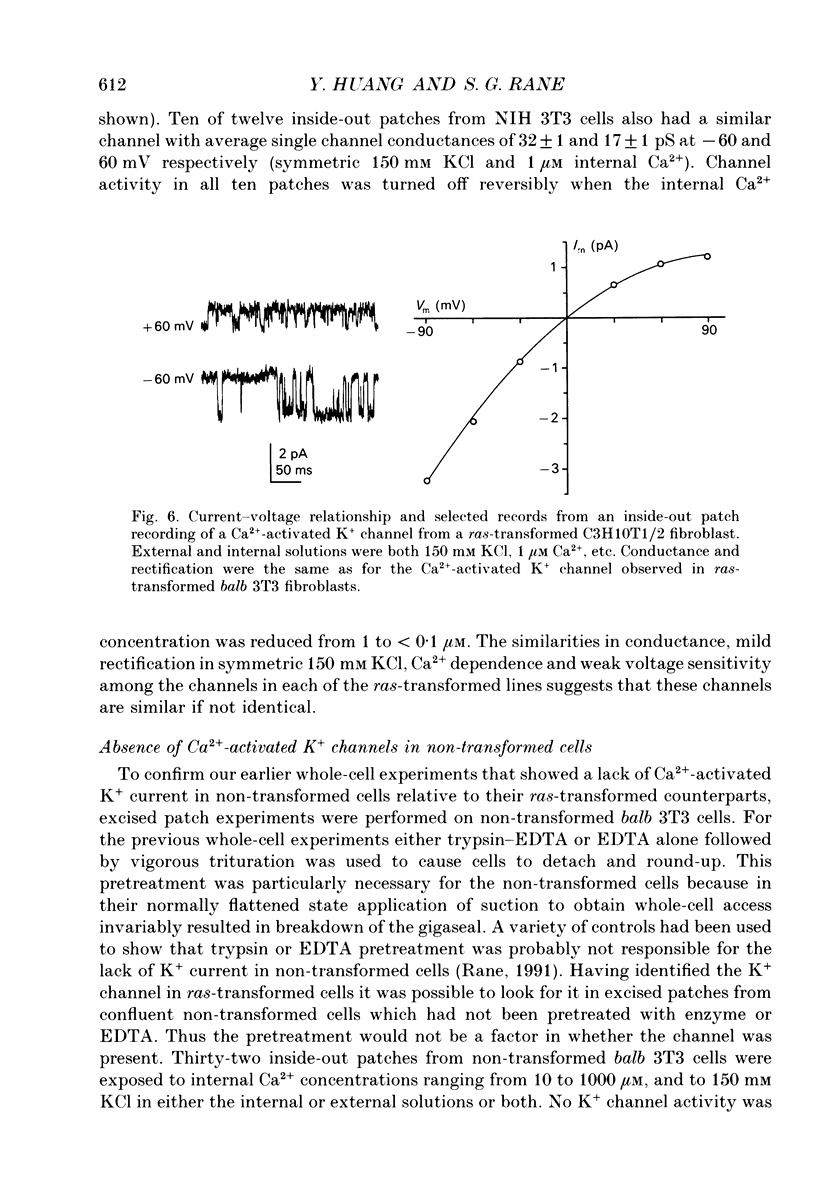
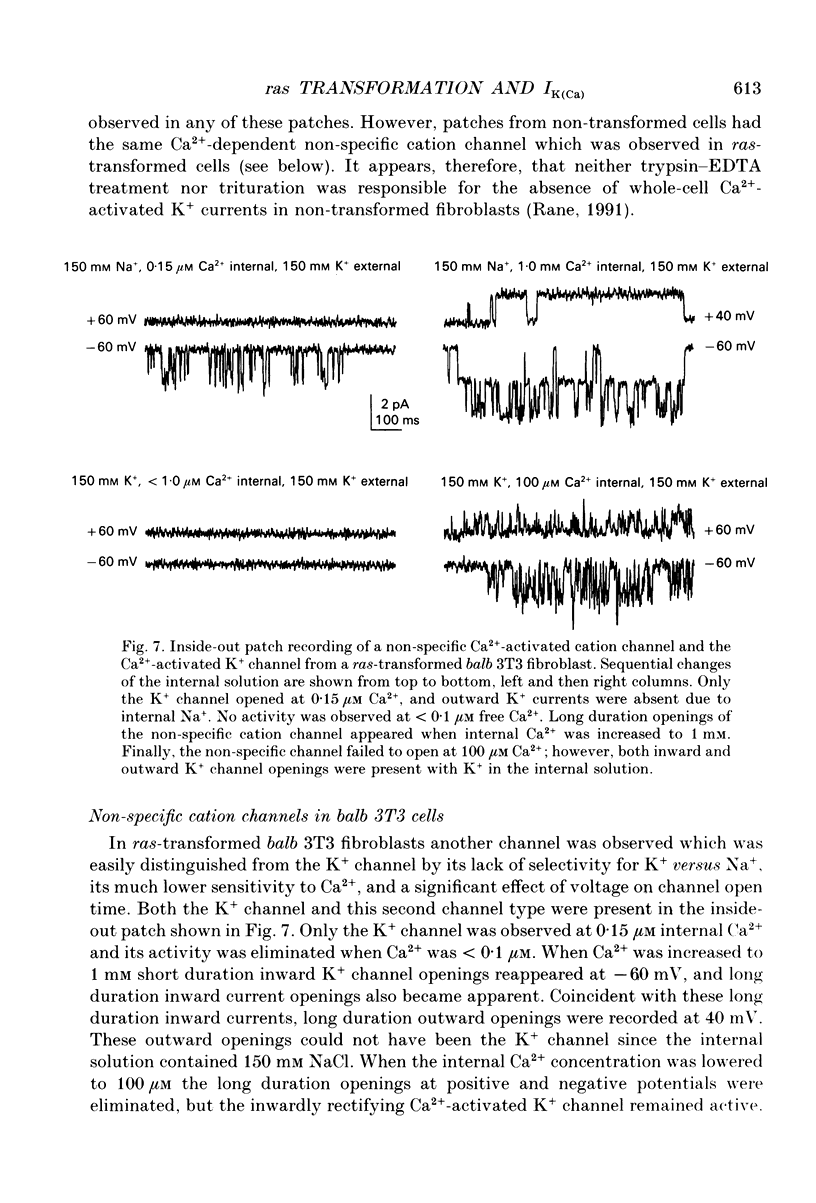
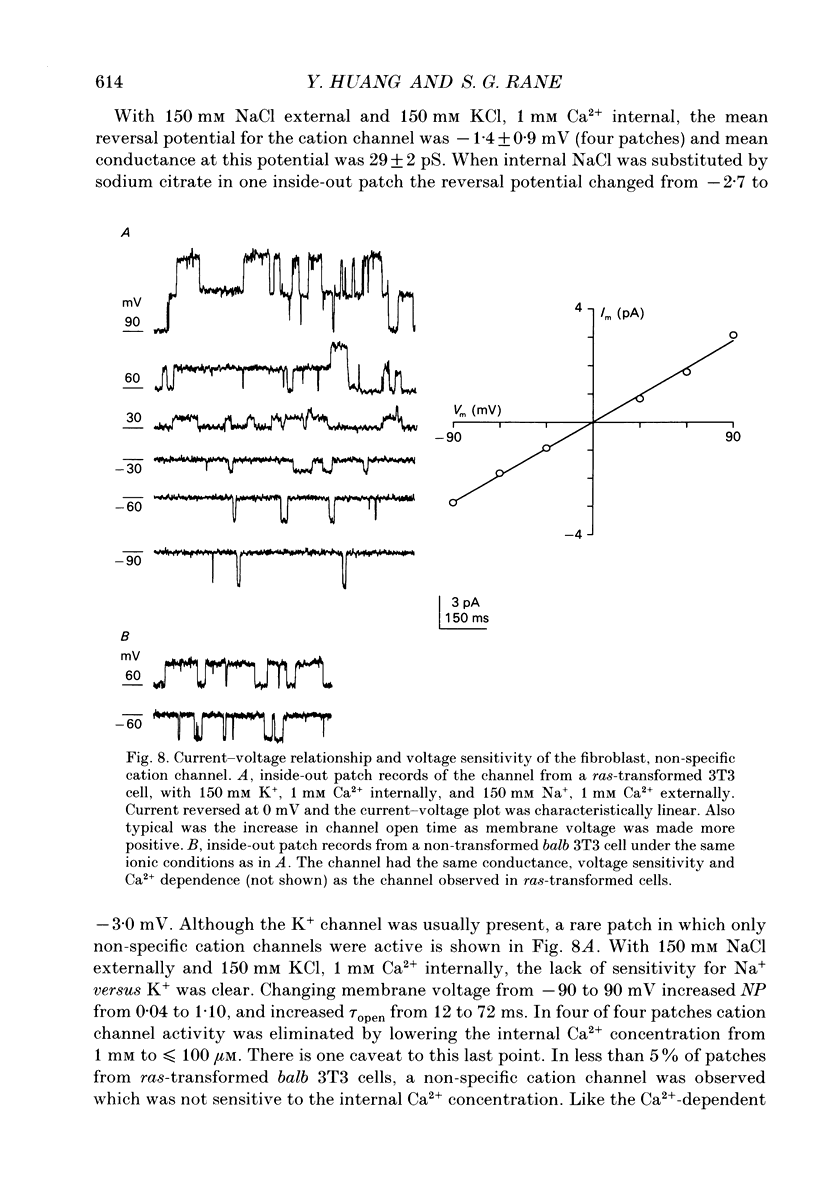
1. Ras-transformed fibroblasts have a whole-cell Ca(2+)-activated K+ current which is either absent or unavailable for activation in their non-transformed counterparts. To better understand the physiological significance of this K+ current the single channel basis for the current was characterized in ras-transformed cells. 2. More than 90% of inside-out patches from ras-transformed balb 3T3 cells had a channel type which was Ca(2+)-activated (threshold < 0.2 microM internal Ca2+), K(+)-selective (permeability ratio PNa:PK < 0.02), and inwardly rectifying in symmetric 150 mM KCl solutions (conductances at -60 and 60 mV of 33 +/- 1 and 17 +/- 1 pS respectively). Channel opening probability increased 25-50% between -60 and 60 mV due to an increase in the frequency of opening. Single K+ channels in outside-out patches were blocked by externally applied 10 mM TEA or 100 nM charybdotoxin, as were whole-cell Ca(2+)-activated K+ currents. The properties of this class of K+ channel are sufficient to account for the whole-cell Ca(2+)-activated current in ras-transformed cells. 3. Inside-out patches from C3H10T1/2 and NIH 3T3 fibroblasts transformed by the H-ras oncogene had Ca(2+)-activated K+ channels identical to those observed in K-ras-transformed balb 3T3 cells. 4. As predicted from whole-cell experiments Ca(2+)-activated K+ channels were not observed in inside-out patches from non-transformed balb 3T3 cells. The purpose of the excised patch recordings was, instead, to rule out potential technical complications with the whole-cell experiments. For instance A23187, which evoked whole-cell K+ currents in transformed cells, may not have elevated Ca2+ sufficiently to allow K+ channel activation in non-transformed cells. Another possibility was that trypsin pretreatment used to round-up cells for whole-cell recording may have preferentially disabled channels in non-transformed cells. The first problem was addressed by exposing patches from non-transformed cells to 100-1000 microM Ca2+. Excised patches were also taken from non-transformed cells which had not been exposed to trypsin. K+ channel activity was not observed under either condition. 5. Patches from both ras-transformed and non-transformed cells had a type of non-specific cation channel which was activated at internal Ca2+ concentrations > or = 100 microM. This channel was sensitive to membrane voltage, mean open time increasing from 12 to 72 ms between -90 and 90 mV.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag G., McCormick F. Regulators and effectors of ras proteins. Annu Rev Cell Biol. 1991;7:601–632. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- Chen C. F., Corbley M. J., Roberts T. M., Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science. 1988 Feb 26;239(4843):1024–1026. doi: 10.1126/science.2449730. [DOI] [PubMed] [Google Scholar]
- Collin C., Papageorge A. G., Lowy D. R., Alkon D. L. Early enhancement of calcium currents by H-ras oncoproteins injected into Hermissenda neurons. Science. 1990 Dec 21;250(4988):1743–1745. doi: 10.1126/science.2176747. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Decoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 1987 Mar;89(3):405–420. doi: 10.1085/jgp.89.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Krause D., Lee S. C. Voltage-gated potassium conductance in human T lymphocytes stimulated with phorbol ester. J Physiol. 1986 Mar;372:405–423. doi: 10.1113/jphysiol.1986.sp016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto K., Furuya K., Maeno T., Edwards C., Oka T. Oscillating activity of a calcium-activated K+ channel in normal and cancerous mammary cells in culture. J Membr Biol. 1991 Jan;119(2):133–139. doi: 10.1007/BF01871412. [DOI] [PubMed] [Google Scholar]
- Estacion M. Inhibition of voltage-dependent Na+ current in cell-fusion hybrids containing activated c-Ha-ras. J Membr Biol. 1990 Feb;113(2):169–175. doi: 10.1007/BF01872890. [DOI] [PubMed] [Google Scholar]
- Flamm R. E., Birnberg N. C., Kaczmarek L. K. Transfection of activated ras into an excitable cell line (AtT-20) alters tetrodotoxin sensitivity of voltage-dependent sodium current. Pflugers Arch. 1990 Apr;416(1-2):120–125. doi: 10.1007/BF00370232. [DOI] [PubMed] [Google Scholar]
- Furuya K., Enomoto K., Furuya S., Yamagishi S., Edwards C., Oka T. Single calcium-activated potassium channel in cultured mammary epithelial cells. Pflugers Arch. 1989 Jun;414(2):118–124. doi: 10.1007/BF00580952. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jurnak F. The three-dimensional structure of c-H-ras p21: implications for oncogene and G protein studies. Trends Biochem Sci. 1988 Jun;13(6):195–198. doi: 10.1016/0968-0004(88)90080-1. [DOI] [PubMed] [Google Scholar]
- Kojima I., Matsunaga H., Kurokawa K., Ogata E., Nishimoto I. Calcium influx: an intracellular message of the mitogenic action of insulin-like growth factor-I. J Biol Chem. 1988 Nov 15;263(32):16561–16567. [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Schlichter L. C. Ca2(+)-activated K+ channels in human B lymphocytes and rat thymocytes. J Physiol. 1989 Aug;415:69–83. doi: 10.1113/jphysiol.1989.sp017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., de Laat S. W., van der Saag P. T. Serum triggers a sequence of rapid ionic conductance changes in quiescent neuroblastoma cells. Nature. 1979 Jun 21;279(5715):721–723. doi: 10.1038/279721a0. [DOI] [PubMed] [Google Scholar]
- Price M., Lee S. C., Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G. A Ca2(+)-activated K+ current in ras-transformed fibroblasts is absent from nontransformed cells. Am J Physiol. 1991 Jan;260(1 Pt 1):C104–C112. doi: 10.1152/ajpcell.1991.260.1.C104. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sauvé R., Simoneau C., Monette R., Roy G. Single-channel analysis of the potassium permeability in HeLa cancer cells: evidence for a calcium-activated potassium channel of small unitary conductance. J Membr Biol. 1986;92(3):269–282. doi: 10.1007/BF01869395. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Tsai M. H., Yu C. L., Smith J. K. Critical role of cellular ras proteins in proliferative signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):871–881. doi: 10.1101/sqb.1988.053.01.100. [DOI] [PubMed] [Google Scholar]
- Taparowsky E. J., Heaney M. L., Parsons J. T. Oncogene-mediated multistep transformation of C3H10T1/2 cells. Cancer Res. 1987 Aug 1;47(15):4125–4129. [PubMed] [Google Scholar]
- Villereal M. L., Cook J. S. Regulation of active amino acid transport by growth-related changes in membrane potential in a human fibroblast. J Biol Chem. 1978 Nov 25;253(22):8257–8262. [PubMed] [Google Scholar]
- Yatani A., Okabe K., Polakis P., Halenbeck R., McCormick F., Brown A. M. ras p21 and GAP inhibit coupling of muscarinic receptors to atrial K+ channels. Cell. 1990 Jun 1;61(5):769–776. doi: 10.1016/0092-8674(90)90187-j. [DOI] [PubMed] [Google Scholar]


