Abstract
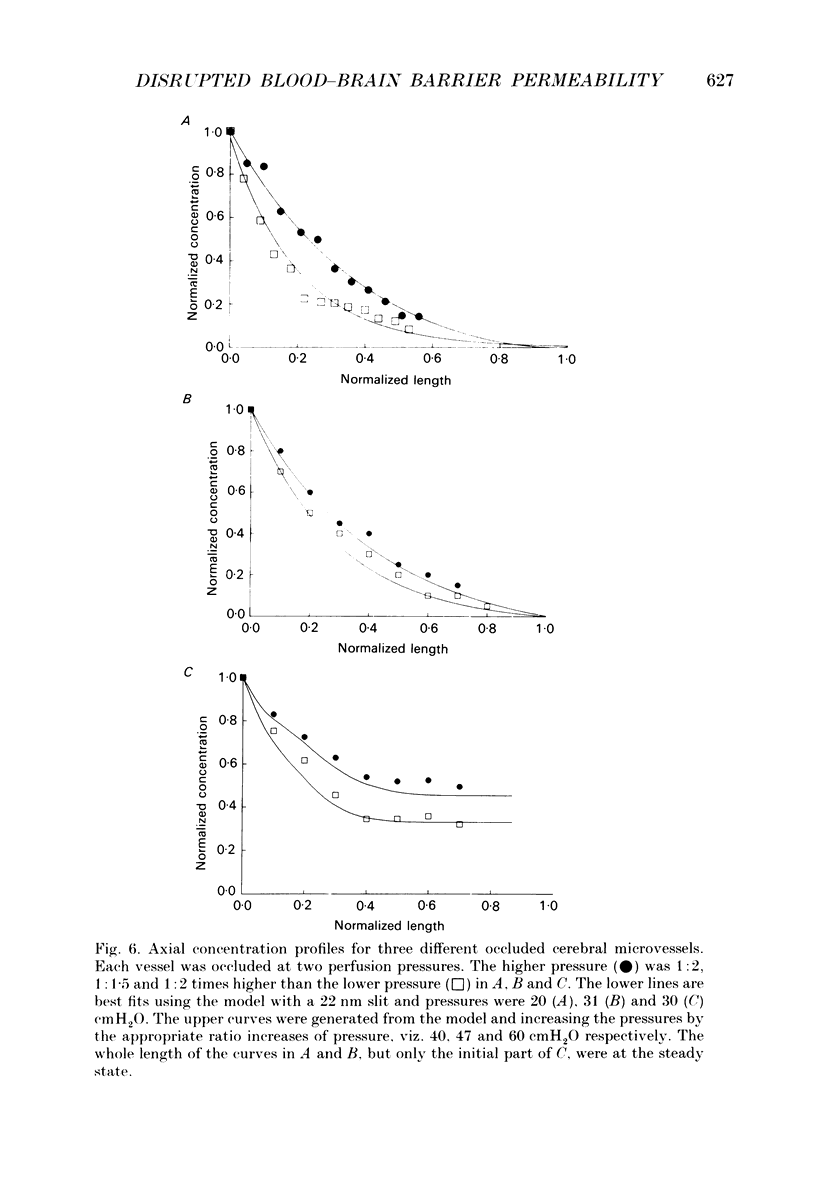
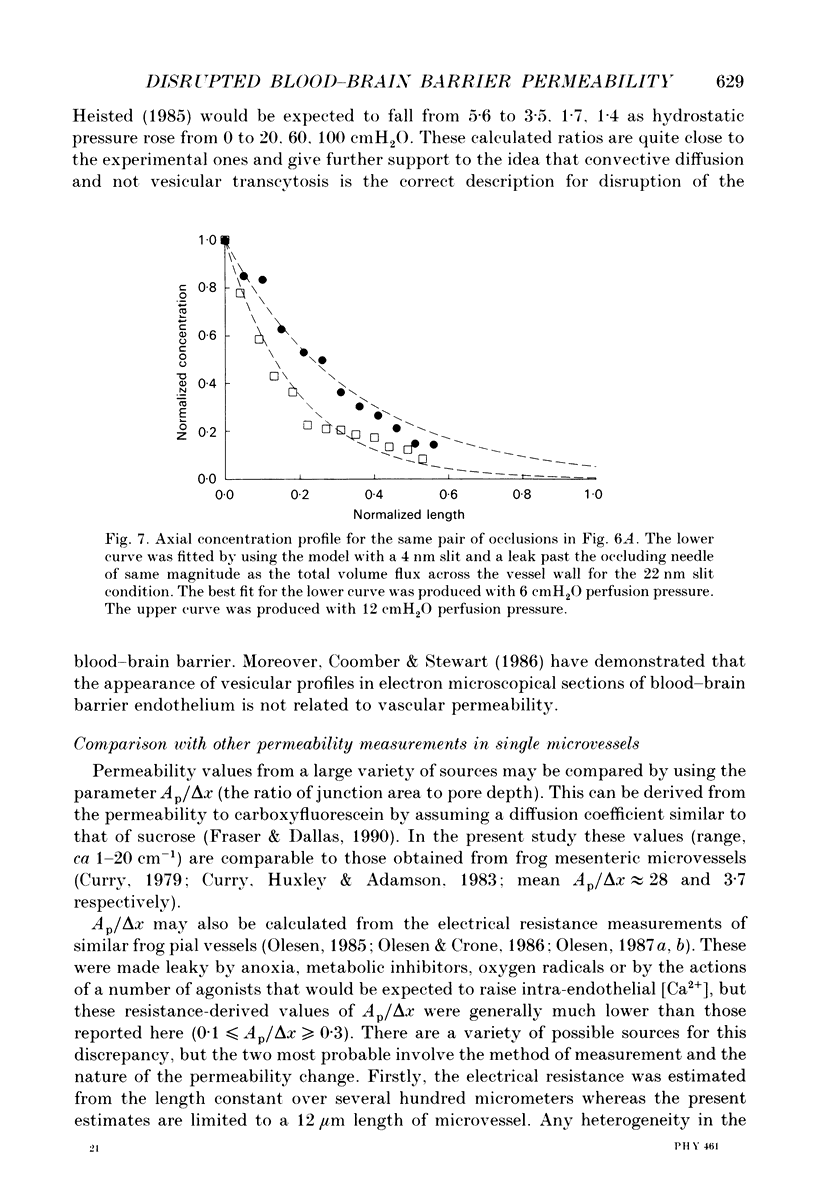
1. This study reports the results of varying the hydrostatic pressure on measurements of permeability coefficient to the low molecular weight impermeant dye carboxyfluorescein (MW = 376) in single leaky cerebral microvessels. A mathematical model, that solved the convective diffusion equations used to analyse the measurements, showed that the measurements were consistent with leakiness being due to 22 nm wide parallel-sided slits between endothelial cells. 2. Microvessels on the surface of the frog's brain were cannulated with a micropipette and perfused with an artificial cerebrospinal fluid containing the dye. Vessels were occluded with a glass microneedle and the rate of change in dye concentration in a 12 microns length section was measured using video-intensified microscopy. 3. It was found that the rate of dye loss at all points along the occluded microvessel segment could be accounted for by a model for convection and diffusion, and that changes in dye concentration at a point remote from the segment entrance can give a good measure of diffusive permeability. 4. When series of measurements were carried out on a single vessel, permeability rose over the course of 20 min. Mean permeability for all measurements was 3.01 x 10(-5) cm sec-1, n = 64 (mode, 2.0; range, 0.48-9.6). The hydrostatic pressure applied during the perfusion had no effect on the measured permeability. 5. The dye concentration along the vessel axis was measured at the steady state and was shown to respond to changes in hydrostatic perfusion pressure in a way predicted by the model. This indicates that hydrostatically driven bulk flow can be important, and thus convection may account for effects previously ascribed to vesicular transcytosis. 6. The possible anatomical basis for the porous pathway is discussed in the light of recent observations on the presence of 0.5 microns perijunctional gaps, the possibility of transendothelial channels, and the unzipping of tight junctions to leave a 22 nm wide slit.
Full text
PDF

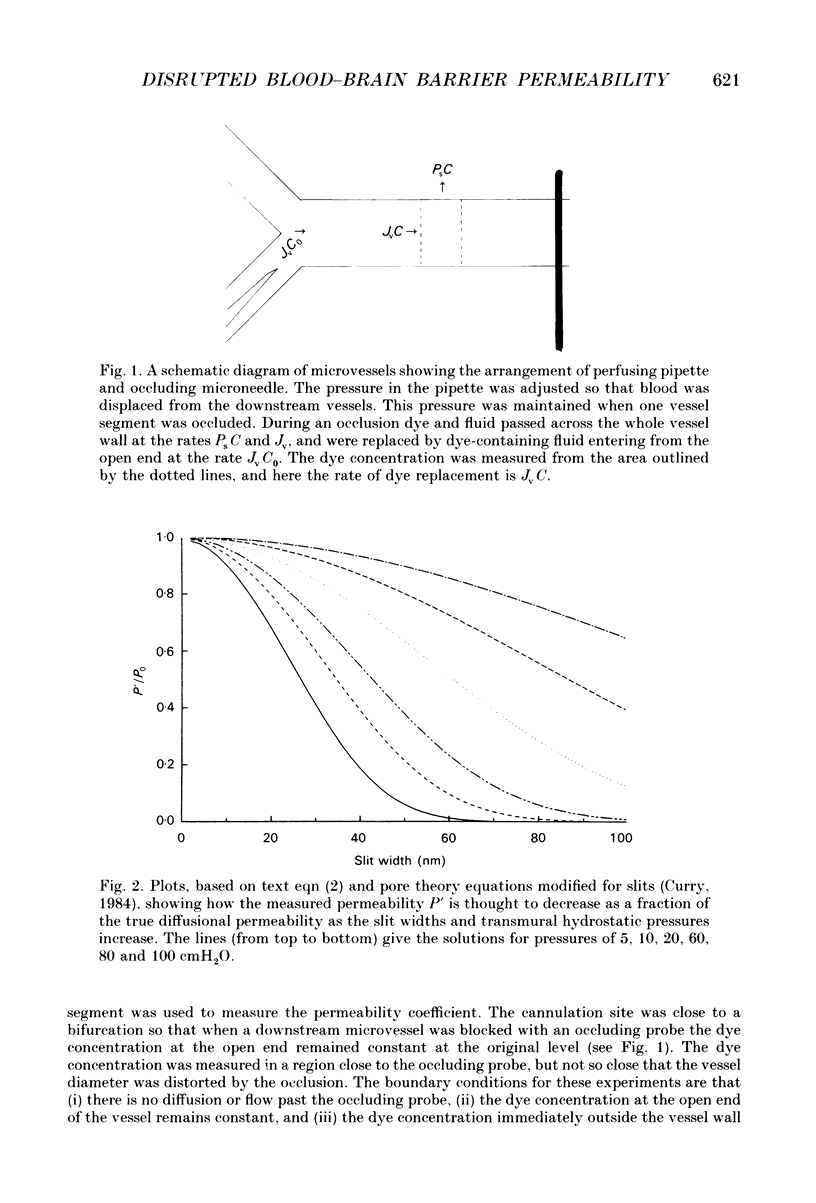


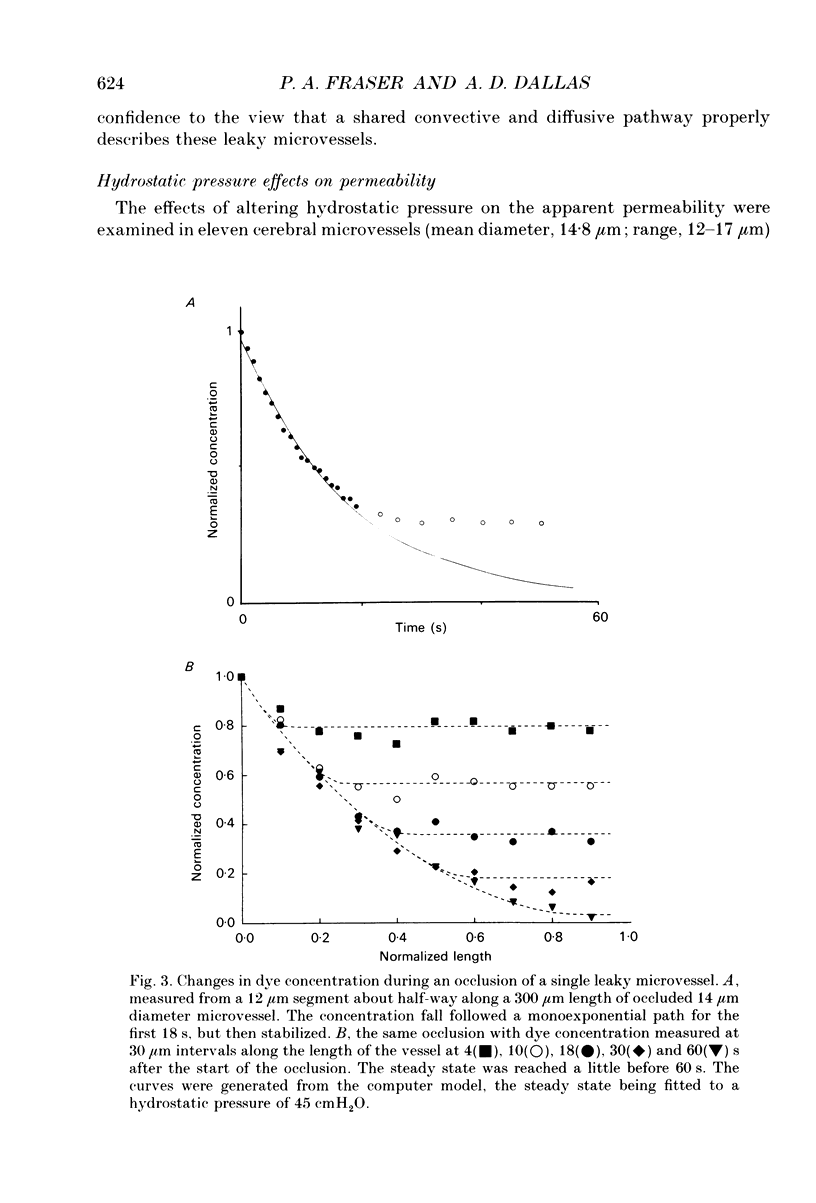
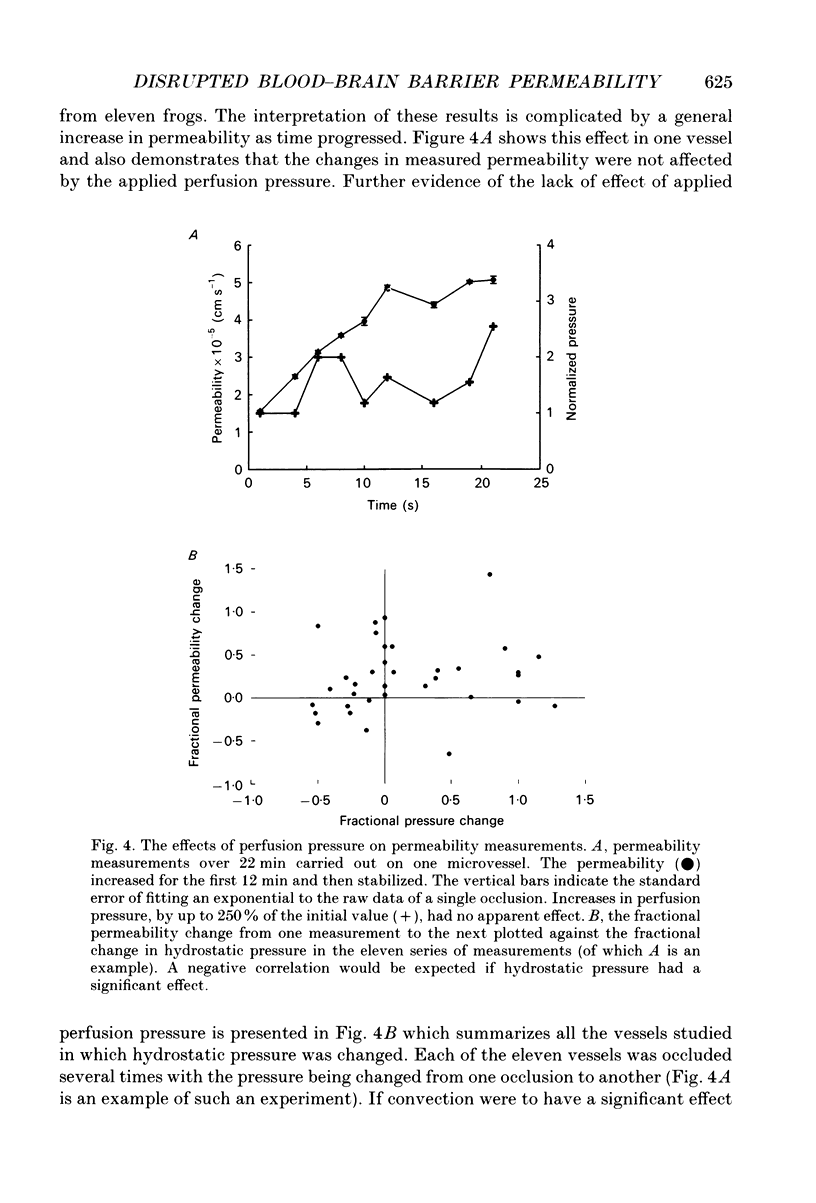
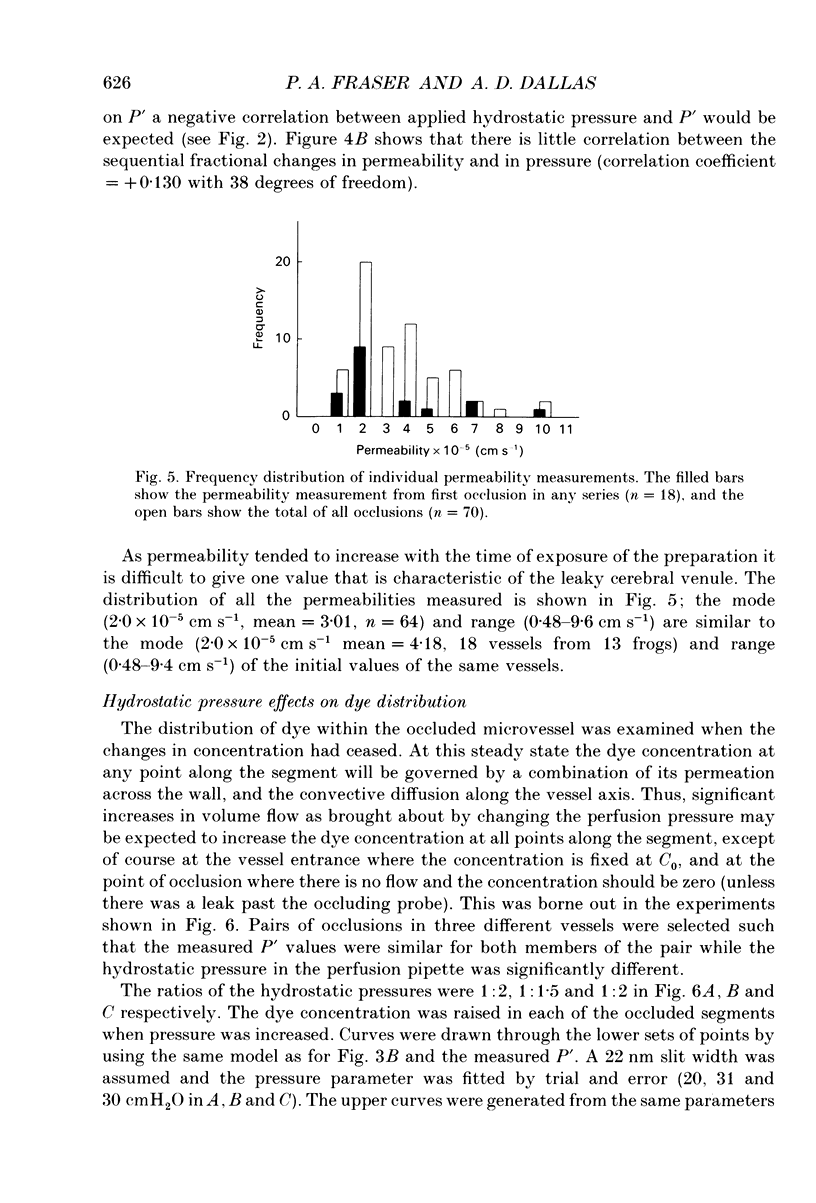




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundgaard M. Ultrastructure of frog cerebral and pial microvessels and their impermeability to lanthanum ions. Brain Res. 1982 Jun 3;241(1):57–65. doi: 10.1016/0006-8993(82)91228-8. [DOI] [PubMed] [Google Scholar]
- Clough G., Michel C. C., Phillips M. E. Inflammatory changes in permeability and ultrastructure of single vessels in the frog mesenteric microcirculation. J Physiol. 1988 Jan;395:99–114. doi: 10.1113/jphysiol.1988.sp016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough G., Michel C. C. Quantitative comparisons of hydraulic permeability and endothelial intercellular cleft dimensions in single frog capillaries. J Physiol. 1988 Nov;405:563–576. doi: 10.1113/jphysiol.1988.sp017348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B. L., Stewart P. A. Three-dimensional reconstruction of vesicles in endothelium of blood-brain barrier versus highly permeable microvessels. Anat Rec. 1986 Jul;215(3):256–261. doi: 10.1002/ar.1092150308. [DOI] [PubMed] [Google Scholar]
- Crone C. Lack of selectivity to small ions in paracellular pathways in cerebral and muscle capillaries of the frog. J Physiol. 1984 Aug;353:317–337. doi: 10.1113/jphysiol.1984.sp015338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E. A hydrodynamic description of the osmotic reflection coefficient with application to the pore theory of transcapillary exchange. Microvasc Res. 1974 Sep;8(2):236–252. doi: 10.1016/0026-2862(74)90097-1. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Huxley V. H., Adamson R. H. Permeability of single capillaries to intermediate-sized colored solutes. Am J Physiol. 1983 Sep;245(3):H495–H505. doi: 10.1152/ajpheart.1983.245.3.H495. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Joyner W. L., Rutledge J. C. Graded modulation of frog microvessel permeability to albumin using ionophore A23187. Am J Physiol. 1990 Feb;258(2 Pt 2):H587–H598. doi: 10.1152/ajpheart.1990.258.2.H587. [DOI] [PubMed] [Google Scholar]
- Curry F. E. Permeability coefficients of the capillary wall to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery. Microvasc Res. 1979 May;17(3 Pt 1):290–308. doi: 10.1016/s0026-2862(79)80005-9. [DOI] [PubMed] [Google Scholar]
- Fraser P. A., Dallas A. D., Davies S. Measurement of filtration coefficient in single cerebral microvessels of the frog. J Physiol. 1990 Apr;423:343–361. doi: 10.1113/jphysiol.1990.sp018026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley V. H., Curry F. E., Adamson R. H. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol. 1987 Jan;252(1 Pt 2):H188–H197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- Lossinsky A. S., Pluta R., Song M. J., Badmajew V., Moretz R. C., Wisniewski H. M. Mechanisms of inflammatory cell attachment in chronic relapsing experimental allergic encephalomyelitis: a scanning and high-voltage electron microscopic study of the injured mouse blood-brain barrier. Microvasc Res. 1991 May;41(3):299–310. doi: 10.1016/0026-2862(91)90030-f. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Heistad D. D. Permeability of blood-brain barrier to various sized molecules. Am J Physiol. 1985 May;248(5 Pt 2):H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. A calcium-dependent reversible permeability increase in microvessels in frog brain, induced by serotonin. J Physiol. 1985 Apr;361:103–113. doi: 10.1113/jphysiol.1985.sp015635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Crone C. Substances that rapidly augment ionic conductance of endothelium in cerebral venules. Acta Physiol Scand. 1986 Jun;127(2):233–241. doi: 10.1111/j.1748-1716.1986.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. Free oxygen radicals decrease electrical resistance of microvascular endothelium in brain. Acta Physiol Scand. 1987 Feb;129(2):181–187. doi: 10.1111/j.1748-1716.1987.tb08057.x. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. Regulation of ion permeability in frog brain venules. Significance of calcium, cyclic nucleotides and protein kinase C. J Physiol. 1987 Jun;387:59–68. doi: 10.1113/jphysiol.1987.sp016562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak C. S., Goldstein D. A., Hoffman J. F. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963 Nov;5(3):426–442. doi: 10.1016/0022-5193(63)90088-2. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Size selectivity of blood-brain barrier permeability at various times after osmotic opening. Am J Physiol. 1987 Sep;253(3 Pt 2):R459–R466. doi: 10.1152/ajpregu.1987.253.3.R459. [DOI] [PubMed] [Google Scholar]
- Schmidley J. W., Wissig S. L. Anionic sites on the luminal surface of fenestrated and continuous capillaries of the CNS. Brain Res. 1986 Jan 22;363(2):265–271. doi: 10.1016/0006-8993(86)91011-5. [DOI] [PubMed] [Google Scholar]
- van Deurs B. Structural aspects of brain barriers, with special reference to the permeability of the cerebral endothelium and choroidal epithelium. Int Rev Cytol. 1980;65:117–191. doi: 10.1016/s0074-7696(08)61960-9. [DOI] [PubMed] [Google Scholar]


