Abstract
In this review, we consider the role of cell-cell fusion in cancer development and progression through an evolutionary lens. We begin by summarizing the origins of fusion proteins (fusogens), of which there are many distinct classes that have evolved through convergent evolution. We then use an evolutionary framework to highlight how the persistence of fusion over generations and across different organisms can be attributed to traits that increase fitness secondary to fusion; these traits map well to the expanded hallmarks of cancer. By studying the tumor microenvironment, we can begin to identify the key selective pressures that may favor higher rates of fusion compared to healthy tissues. The paper concludes by discussing the increasing number of research questions surrounding fusion, recommendations for how to answer them, and the need for a greater interest in exploring cell fusion and evolutionary principles in oncology moving forward.
Keywords: Evolution, Cell-cell fusion, Cancer hallmarks, Oncology
1. Introduction
The process of cell fusion is a slowly-growing area of research attention, despite fusion’s frequent occurrence throughout the human body. Cell-cell fusion is a complex and energy-consuming process that requires cytoplasmic and/or nuclear contents to mix, yet is fundamental to the development and survival of many human tissues, both healthy (Brukman et al., 2019; Brukman et al., 2022; Kloc et al., 2021; Kloc et al., 2022; Emans, 1993; Wang, 2020; Hernández and Podbilewicz, 2017; Ahmadzadeh et al., 2022; Kloc, 2022; Elson et al., 2022; Soe, 2011; Petrany and Millay, 2019; Lu and Ikawa, 2022; Deneke and Pauli, 2021) and diseased (Kloc et al., 2021; Rajah et al., 2022; Leroy, 2020; Lazebnik, 2021; Hernández and Podbilewicz, 2017; Ahmadzadeh et al., 2022; Parris, 2013; Boveri, 2008; Hass et al., 2021) (Fig. 1). Oftentimes, the process of cell fusion imitates that of viral entry into cells, which is a direct result of one of its evolutionary origins (Brukman et al., 2019; Brukman et al., 2022; Kloc et al., 2021; Leroy, 2020; Soe, 2011; Hernández and Podbilewicz, 2017; Sapir et al., 2008; Harrison, 2008; Yang and Margam, 2021; Modis, 2013). In fact, in some cases, cell-cell fusion and the resulting syncytia formation occur secondary to viral infection (Leroy, 2020; Lazebnik, 2021; Rajah et al., 2022). While not a traditional “cell” fusion, a closely related example is the fusion of vesicular membranes with cell membranes, such as during synapsis (Emans, 1993; Pérez-Vargas, 2014) and autophagy (Wang, 2016). Regardless of the exact mechanism at play, cell-cell fusion is defined by key characteristics that have been coined the “hallmarks of cell fusion” (Hernández and Podbilewicz, 2017).
Fig. 1.

Key examples of Cell-Cell Fusion in Humans. There are numerous examples of cell-cell fusion that can be found in different healthy tissues throughout the human body (green). These range from gamete-gamete fusion in embryogenesis, to the formation of the placenta which supports its growth until birth. There is growing evidence that identifies cell-cell fusion in cancerous contexts (gold) as well, with a number of potential implications for the future of oncology.
The primary examples of cell-cell fusion in the human body include the formation of multinucleated giant cells (Bühler, 2022; Ahmadzadeh et al., 2022; Leroy, 2020; Kloc, 2022; Skokos et al., 2011; Zhang, 2022), osteoclasts (Elson et al., 2022; Kloc et al., 2022; Soe, 2011), skeletal muscle cells (Schejter, 2016; Gibbs and Pyle, 2023; Lehka and Rędowicz, 2020; Petrany and Millay, 2019), and placentation (Aguilar, 2013; Hernández and Podbilewicz, 2017; Kloc et al., 2021; Liang, 2010), which all produce syncytia (multiple-nucleated cells secondary to cell fusion). Some processes of cell-cell fusion involve nuclear fusion as well; in particular, this is characteristic of gamete-gamete fusion in human fertilization and embryogenesis (Brukman et al., 2022; Deneke and Pauli, 2021; Lu and Ikawa, 2022).
Because fusion can occur in different tissues throughout the body at baseline, it should come as no surprise that fusion can be a characteristic of cancer as well (Parris, 2013). It is well established that all of the classic cancer hallmarks are derived from variations on normal cellular functionality, which in turn promote cancer growth by increasing cellular fitness, and thus yield improved survival of the cancer cells relative to their healthy somatic counterparts (Hanahan and Weinberg, 2011). Despite being first hypothesized as a potential mechanism of carcinogenesis over 120 years ago by Theodore Boveri (Boveri, 2008), it was not until recently that cell-cell fusion in cancer became a more prominent point of discussion in the literature. The past few decades in particular have yielded an increasing number of research articles looking into the mechanism and potential functions of fusion in cancer (Bjerregaard et al., 2006; Demin et al., 2022; Dittmar, 2022; Dittmar et al., 2021; Gast, 2018; Hass et al., 2021),(Hass et al., 2021). There is a steadily growing evidence base that suggests that cell-cell fusion may provide the answers to several key questions in cancer research, including the basis for the organotropic patterns of metastasis (Arena, 2023; Sieler et al., 2021; Tretyakova et al., 2022; Zhang, 2022), immune evasion (Bates, 2023; Tretyakova et al., 2022; Bateman et al., 2000; Shin, 2021; Hass et al., 2021), cancer stemness (Uygur, 2019; Zhang, 2014; Dittmar and Hass, 2023; Hass et al., 2021,Hass et al., 2021; Melzer et al., 2018; Warrier, 2023), genomic instability and the resulting tumor heterogeneity (Wang, 2020; Ogle et al., 2005; Archetti, 2022; Gast, 2018; Hass et al., 2021; Mirzayans and Murray, 2023), and patterns of both chemo- and radio-therapeutic resistance (Casotti, 2023; Druzhkova et al., 2023; Guo, 2023; Hass et al., 2021; Mirzayans and Murray, 2023; Pienta, 2022; Uygur, 2019; Sieler et al., 2021; Yan et al., 2016).
Recent studies highlight cell-cell fusion in human cancers, which can range in effect from enabling endothelial-mesothelial transition (EMT), the first step in local invasion and metastasis, via cancer-endothelium fusion (Hass et al., 2020; Hass et al., 2021; Parris, 2013; Dittmar et al., 2021; Melzer et al., 2019) to the formation of cancer stem cells (CSCs) and immune evasion following cancer-macrophage fusion (Li et al., 2023; Dittmar, 2022; Hass et al., 2021; Zhang, 2022; Dittmar et al., 2021). The examples of cell-cell fusion in healthy tissue and cancer have been summarized in Fig. 1.
Thus, there is an increasing evidence base that cell-cell fusion is extremely important to consider in cancer. While one of the first papers to do so was written almost 20 years ago (Ogle et al., 2005), the past 5 years have had a surge in the number of review papers that connect cell-cell fusion to the expanded hallmarks of cancer described by Hanahan and Weinberg, (Hanahan and Weinberg, 2011). Several recent reviews highlight how cell-cell fusion may promote tumorigenesis, propagate treatment resistance, and worsen patient prognosis (Hass et al., 2021; Dittmar, 2022; Dittmar et al., 2023,Dittmar et al., 2021; Hass et al., 2021; Wang, 2021; Dietz, 2021; Gast, 2018; Sieler et al., 2021).
While evidence of fusion has been repeatedly observed in a variety of tissue types in vitro and in vivo (Melzer et al., 2019; Yan et al., 2016; Dietz, 2021; Hass et al., 2021; Ruano, 2022), the frequency at which fusion occurs in vivo and the impact of these fusion events are unclear. This has been complicated by difficulties in observing fusion events between cells of shared cell lineages. In clinical studies, cell-cell fusion events between distinct cellular lineages, have been identified using several experimental techniques. For example, using genotyping, Pawelek et al. demonstrated fusion between cells from genetically distinct bone marrow donors (Pawelek, 2014), while other studies have utilized highly multiplexed immuno-histochemical analyses to reveal co-expression of proteins associated with distinct epithelial and lymphocytic or monocytic lineages (Melzer et al., 2019; Ruano, 2022). It is only in experimental in vitro and in vivo settings, where the use of fluorescent proteins or barcoding has allowed researchers to detect similar fusion events occurring between cancerous epithelial cells of more closely related lineages (Miroshnychenko, 2021; Su, 2015; Bjerregaard et al., 2006; Wang, 2021). Unlike the studies of cell fusion between independent cell lineages, these epithelial tumor populations did not have unique surface markers to distinguish the fused cells from their parental lineages. Due to these challenges, the research of cell-cell fusion, particularly within tumor cell populations, is still in its earliest stages.
Additionally, the molecular biology of the cell-cell fusion pathway is generally well-studied only in certain biological contexts (e.g. vesicular fusion in synapsis Emans, 1993; Pérez-Vargas, 2014), gamete-gamete fusion (Brukman et al., 2022; Deneke and Pauli, 2021; Lu and Ikawa, 2022), and viral-host fusion (Leroy, 2020; Brukman et al., 2022; Sapir et al., 2008; Harrison, 2008; Rajah et al., 2022; Lazebnik, 2021). Further study in other contexts, particularly in settings of pathology and increased biological variation (e.g. across cancer), is necessary. A deeper mechanistic understanding of cell fusion in cancer may unlock new avenues of cancer treatment that limit the fundamental ability of cancer (i.e. pre-malignant lesions) to evolve.
In this review, we utilize the framework of evolutionary oncology (Niculescu, 2023; Gatenby and Brown, 2020; Pepper et al., 2009) to demonstrate our current understanding of how fusion happens, what fitness costs are associated with fusion, how rare fusion events can impact tumor evolution and patient outcomes, and highlight key unanswered questions in this burgeoning field of cancer research. We further exploit this evolutionary framework to emphasize how interclonal cell-cell fusion events in cancer may contribute to oncogenesis, metastasis, and the evolution of therapeutic resistance.
2. Evolutionary oncology: understanding cancer as somatic evolution
Nearly 50 years ago, cancer was first proposed to be the result of somatic evolution (Nowell, 1976). While we as a field now understand cancer to often be the result of many different factors, this idea of somatic evolution still offers us important insights. Using this framework, carcinogenesis can be understood as the expansion and ongoing mutation of cancerous clones into a population of cells with different genetic and physical characteristics and behaviors (Dasari et al., 2021; Frank, 2010; Gatenby and Brown, 2020; Gerstung, 2020; Huang, 2021; Maley et al., 2011; Merlo et al., 2006; Miura, 2022; Nowak et al., 2003; Nowell, 1976; Olafsson and Anderson, 2021; Rozhok and DeGregori, 2020). The combination of genetic instability and environmental stress (e.g. inflammation, hypoxia, etc.) is thought to select for traits that we now know define the cancer hallmarks, and thus lead to further cancer progression via invasion, metastasis, and cancer stemness, among others (Hanahan and Weinberg, 2011). Furthermore, as clones evolve, ecological interactions start to occur between the clones, which in turn drive the further evolution of tumors via selection for the fittest clones within each ecological niche in the tumor microenvironment (Dasari et al., 2021; Frank, 2010; Gatenby and Brown, 2020; Gerstung, 2020; Huang, 2021; Maley et al., 2011; Merlo et al., 2006; Miura, 2022; Nowak et al., 2003; Nowell, 1976; Olafsson and Anderson, 2021; Rozhok and DeGregori, 2020). The key terminology used in this framework is summarized in Box 1.
Box 1. An Evolutionary Framework: Terms and Definitions.
Evolutionary oncology: field of oncology based on the idea that cancer is derived from somatic mutation and selective pressures and is thus inherently governed by evolutionary principles (Cairns, 1975)
Evolutionary time: time measured in generations; for cancer this is measured in cell generations (Gingerich, 2019)
Relative fitness: an ecological measure of an individual’s fitness, usually quantified as the reproductive success of an individual compared to competitors (Steven and Kirkwood, 2015)
Fitness cost: decreased relative fitness; many adaptations result in a trade-off where the new trait or mutation is adaptive in some contexts but not others e.g. sickle-cell trait vs malaria (Yubero and Poyatos, 2021; Dobkin et al., 2023)
Selection: also referred to as selective pressure, the environmental factors that favor certain traits over others, often resulting in a population shift in favor of the fitter (favored) trait (Komarova et al., 2008; Scott and Marusyk, 2017)
Convergent evolution: the process by which similar phenotypic traits arise from genotypically distinct lineages (Stern, 2013)
One of the most important takeaways of this approach is the idea that rare events such as cell-cell fusion can have drastic impacts in biology. Cancer itself is fundamentally a rare event. Despite its rarity, the incidence of cancers keeps increasing across the globe. The biggest factor at play seems to be that people are just living longer; evolutionary oncology allows us to understand that fundamentally this creates longer timescales from which rare events such as initiating cancer mutations can arise (Dasari et al., 2021; Frank, 2010; Gatenby and Brown, 2020; Gerstung, 2020; Huang, 2021; Maley et al., 2011; Merlo et al., 2006; Miura, 2022; Nowak et al., 2003; Nowell, 1976; Olafsson and Anderson, 2021; Rozhok and DeGregori, 2020).
In this review, we argue that these same principles that define cancer incidence and progression are at play in the process of cell-cell fusion. More specifically, we claim that while cell-cell fusion is fundamentally a rare event, it offers adaptive benefits that the tumor microenvironment selects for. In this way, another type of rare event can have impacts of unknown significance on cancer initiation and progression, and patient outcomes as a result. Keeping this perspective in mind, let us now consider the mechanisms by which cell-cell fusion occurs.
3. How fusion happens: key molecular pathways
The process of cell fusion follows a distinct series of steps that are mediated by a special class of membrane-bound glycoproteins known as fusogens or fusion proteins (Brukman et al., 2019; Yang and Margam, 2021). This process has been studied extensively due to its role in placentation and fertilization, so the basic mechanics on a cellular level are well agreed upon and have been reviewed extensively (Pérez-Vargas, 2014; Yang and Margam, 2021; Brukman et al., 2019).
Fundamentally, the process begins with the recognition and approach of two cells capable of fusing (Brukman et al., 2019). The steps leading up to the cell-cell recognition and approach are not well understood, although there is evidence that suggests that cells must be “primed” to a pro-fusogenic state prior to the initiation of cell fusion (Dittmar and Hass, 2023). Following docking and partial fusion (of external membranes only), the more energetically costly steps of pore formation and resultant fusion of the inner membranes eventually yield a multinucleated cell known as a syncytium (Ogle et al., 2005). This process is summarized in Fig. 2. These steps are described more extensively in several review papers (Pérez-Vargas, 2014; Yang and Margam, 2021; Brukman et al., 2019).
Fig. 2.

The key steps of cell fusion. Cell fusion occurs in a contact-dependent manner. Membrane bound receptors initiate (1) recognition and approach as well as (2) initial cell contact and docking. Upon close contact, the outer membranes can fuse in a process called (3)hemifusion, leaving the intracellular contents in separate compartments. The initial mixing of cellular contents occurs upon (4) pore formation. The pore expands and eventually the inner membranes merge as well resulting in the (5) syncytium formation, where there is complete membrane fusion but no fusion of nuclear contents.
Following their formation, these syncytia can undergo a number of additional steps. In some cases, the syncytium exits the cell cycle and enters a quiescent state. Alternatively, these cells can undergo additional fusion events with other cells; this is the process that underlies the formation of multinucleated giant cells. The syncytium can undergo nuclear fusion instead resulting in a polyploid (e.g. 4 N) mononucleated cell. This polyploid cell can either enter a form of mitosis to yield two novel non-identical daughter cells, undergo ploidy reduction (via ejection of extra genetic material) to return to a standard ploidy state, or can again enter a quiescent cell state (Miroshnychenko, 2021; Hass et al., 2021). These potential outcomes are summarized in Fig. 3.
Fig. 3.
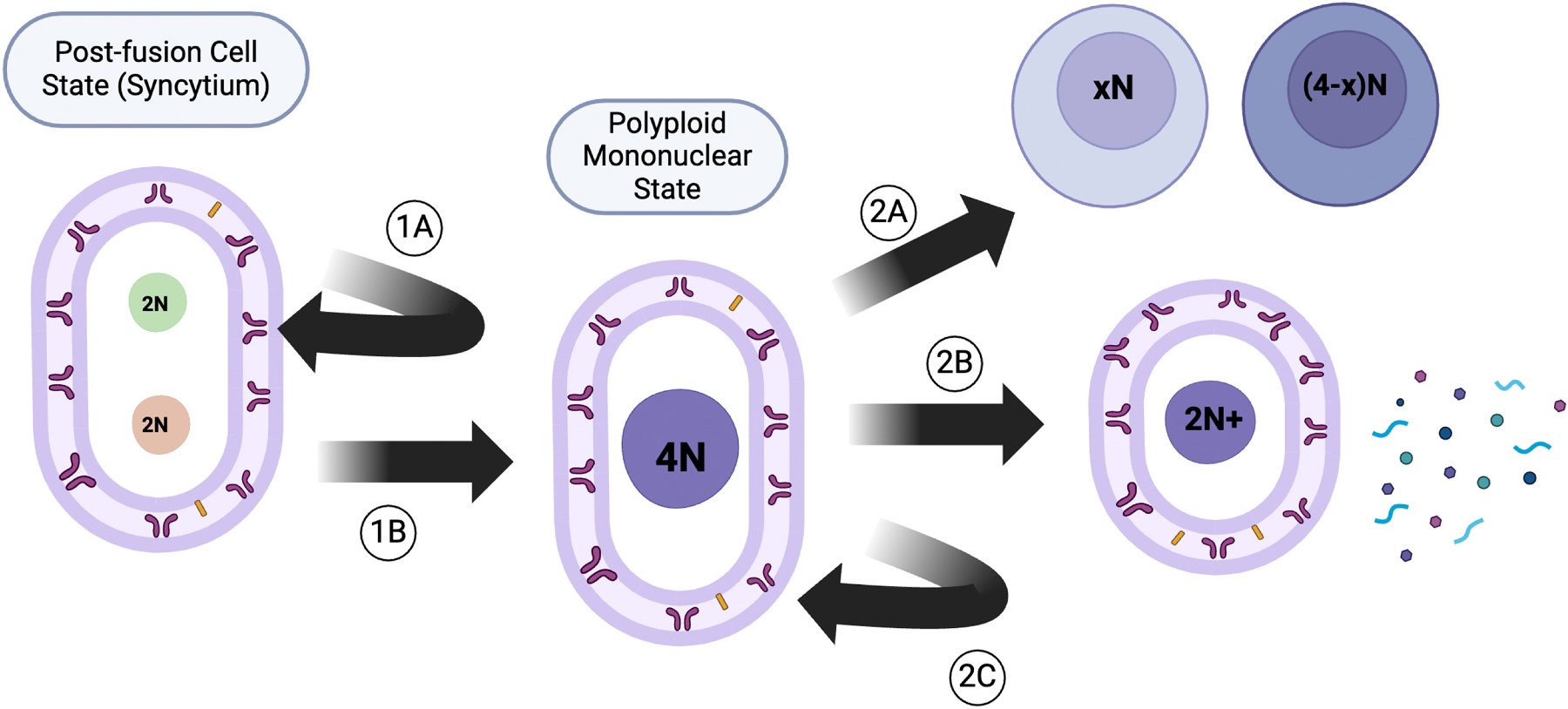
Potential outcomes of cell fusion. Following syncytial formation, there are a number of additional steps that can be taken, each leading to a different final cell state. Every step of the way, the cell may exit the cell cycle to enter a quiescent state (1A or 2C). There may be additional fusions with other cells (not shown) or nuclear fusion (1B). If the cell undergoes nuclear fusion, one additional possibility includes the formation of non-identical daughter cells following a mitosis event that may split the DNA content unequally (2A). The cell may normalize its DNA content via an alternative method known as polyploidy reduction, depicted by (2B).
There are several potential outcomes, and what influences or directs each of these outcomes is still unknown. In this way, the process of cell fusion is dynamic. The rate at which each of these outcomes is reached following a fusion event is also unknown. Recent work has investigated potential selective pressures that may drive the steady state of this system in different biological settings, if a steady state exists. Hass summarized this concept - that of selecting the final state of the cell following fusion - as the Post-Hybrid Selection Process (PHSP) (Hass et al., 2021).
Despite our mechanistic understanding of the basics of cell-cell fusion in humans, which has been established through comprehensive study over the past few decades, there are still numerous questions that remain. For one, it is unclear which internal cell signaling pathways are triggered by fusogenic signaling, or if these pathways can vary depending on fusogen-independent factors in the cellular microenvironments. While extracellular events necessary for fusion have been studied and summarized by Dittmar and Hass (Dittmar and Hass, 2022), the interplay between the intra- and extracellular pathways is still not well understood in a fusion-specific context. Furthermore, it is unclear to what extent tumor microenvironments take advantage of or manipulate these extrinsic pathways to their benefit. Preliminary evidence suggests that the tumor microenvironment encourages fusion (Yan, 2017; Melzer et al., 2018,Melzer et al., 2019; Sieler et al., 2021; Dittmar et al., 2023).
The identified triggers of cell fusion - hypoxia (Dittmar and Hass, 2022; Knerr, 2005) and inflammation (Skokos et al., 2011; Strick, 2007; Weiler et al., 2018; Dittmar and Hass, 2022) - have been studied more comprehensively. However, it remains unclear just how many pathways can trigger fusogen production, or what the signaling hierarchy is between these pathways (i.e. which are necessary, which can be triggered secondary to other fusogenic pathways, etc.). Interestingly, many of the pathways implicated thus far have major roles in regulating the cell cycle and therefore are often associated with oncogenesis. For example, syncytin-1 and syncytin-2, the most commonly expressed human fusogens, are regulated in association with the cAMP/PKA pathways via GCM1 and AP1 transcription factors respectively (Dittmar, 2022; Knerr, 2005; Liang, 2010; Toufaily et al., 2015; Fei, 2019). In addition, Myomaker and Myomerger, the fusogens associated with human muscle formation and maturation (Petrany and Millay, 2019; Lehka and Rędowicz, 2020; Gibbs and Pyle, 2023; Schejter, 2016), have been similarly associated with Wnt/β-catenin signaling pathways in mice (Lehka and Rędowicz, 2020; Dittmar, 2022). Additional evidence associated myomaker and myomerger with MAPK/ERK signaling (Schejter, 2016). Finally, a recent study out of Dalhousie University in Nova Scotia provided data implicating the PI3K/AKT/mTORC1 pathway (MacKenzie, 2023).
Inflammation can also trigger and/or upregulate fusion events, via several different mechanisms. The pro-inflammatory signaling of TNF-α upgregulates Matrix Metalloproteinase (MMP) expression, which helps reduce ECM volume and allows for improved cell-cell contact in the initial stages of cell fusion (Skokos et al., 2011; Weiler et al., 2018). TNF-α expression has also been correlated with increased fusogen (syncytin) levels (Dittmar and Hass, 2022; Dittmar, 2022; Skokos et al., 2011; Yan, 2017; Weiler et al., 2018), particularly in certain cancerous contexts including squamous cell carcinoma (SCC) (Yan, 2017). The anti-inflammatory signaling molecule TGF-β was demonstrated to reduce fusion in endometrial carcinoma (Strick, 2007). This study noted the importance of hormone signaling as a driver of fusion in cancerous contexts (Strick, 2007). If this connection is strengthened, it would suggest that hormonally-driven cancers such as carcinomas of the breast, endometrium, and prostrate could be upregulating cell-cell fusion (Strick, 2007).
All of these potential triggers are limited to the cell-cell fusion process before the initiating cell-cell contact occurs, and may define ways in which cells could be “primed” to favor fusion (Dittmar et al., 2023). However, it is important to note that there is likely great additional complexity involved in cell-cell fusion, as the presence of fusogenic content is seemingly insufficient to trigger fusion (Dittmar et al., 2023; Brukman et al., 2019). For example, there are clearly many potential pathways that could be contributing to the steps before the fusogens are even transcribed. To what extent other pathways or mechanisms may be involved in the steps following is unknown, as are the implications of this transcriptional upregulation. So far, there is no clear evidence regarding whether the fusogens play key roles strictly inside the induced fusogenic cell, or if exported fusogens can propagate fusogenic potential to cellular neighbors. These key pathways in fusogen transcription, and the unknown impacts following its production, are summarized in Fig. 4. More recent work has started to investigate the molecular changes necessary for fusogen signaling to result in fusion, including the roles of fusogen-receptor binding, but this work is in its early stages and so far includes the study of similar proteins known as fusexins in plants (e.g. A. thaliana) and the model organism C. elegans (Brukman et al., 2022).
Fig. 4.

Main biochemical pathways identified to be associated with fusogenic signaling There are many key chemical pathways that have been implicated in fusion via pro-fusogenic signaling, many of which are well-studied in cancerous contexts. These pathways include the Ras/Raf (light blue), PIP3/AKT/mTOR(light green), cAMP/PKA, and Wnt (dark blue)pathways. There is additional evidence that inflammatory markers such as TNF-α, which are known to be at higher concentrations in the tumor microenvironment (TME), may be involved as well. To what extent the fusogen signals trigger intrinsic or extrinsic cellular changes following their induction is unclear (red question mark).
4. The cost of fusion
The biggest cost known to be associated with cell fusion is its energetic cost. As one might expect, the process of pore formation, membrane fusion and cytoplasmic/nuclear mixing described previously is energetically costly, especially for cancer cells. Cancer cells have extensive energetic needs which far exceed those of healthy cells physiologically. (Liberti and Locasale, 2016; Vander Heiden et al., 2009; Purcell et al., 2016; Johnson, 2008; Argilés and Azcón-Bieto, 1988; Gouirand et al., 2018) (refer to Box 1).
Measuring the cost of cell fusion is an area of ongoing research, but the fields of physics and computational modeling have offered some insight (Akimov et al., 2020). A 2020 review by Akimov et al (Akimov et al., 2020). summarizes the extensive work that has gone into energetic estimations of membranous cell and vesicle fusion. They highlight how the estimations have changed as both the potential complexity of the fusion process and the number of methods available to study fusion have increased over time (Akimov et al., 2020). In some studies, this energetic cost of fusion has been inferred in vitro as a decrease in cellular growth or division rates relative to the hybrid’s cellular precursors (Miroshnychenko, 2021). This is representative of a classic evolutionary trade-off (refer to Box 1).
Despite the high energetic needs of cancer cells and the resulting metabolically challenged state, it has been increasingly observed that cancer cells fuse in numerous contexts. They have been repeatedly observed fusing with adjacent somatic cells (Bjerregaard et al., 2006; Delespaul, 2020; Druzhkova et al., 2023; Gast, 2018; Li et al., 2023; Melzer et al., 2019; Pawelek, 2020; Shin, 2021; Su, 2015; Uygur, 2019) and increasingly with other cancer cells (Bühler, 2022; Druzhkova et al., 2023; Hass et al., 2021,Hass et al., 2021; Mirzayans and Murray, 2023; Strick, 2007; Wakeling et al., 1994; Yan et al., 2016) as well. Importantly, published examples of cell-cell fusion have been associated with poor clinical outcomes through increased tumor growth, progression, and metastasis relative to their non-fused counterparts (Bühler, 2022; Druzhkova et al., 2023; Hass et al., 2021,Hass et al., 2021; Mirzayans and Murray, 2023; Strick, 2007; Wakeling et al., 1994; Yan et al., 2016). Even so, this suggests that there must be some significant adaptive benefit(s) of fusion to the proliferation and survival of cancer, benefits that favor cell-cell fusion despite its associated energetic burden. To better understand how cell-cell fusion can improve the relative fitness of cancer cells (and thus can persist due to selective pressures of the TME), we will now examine different reported incidences of cell fusion in cancer.
5. Cell-cell fusion: roles in cancer development and progression
5.1. Cell-cell fusion and tetraploidization in cancer
In the context of diploid organisms – such as humans – tetraploidization refers to the process by which genomes are doubled (Ben-David and Amon, 2020; Davoli and de Lange, 2012; Galofré, 2020; Jemaa, 2021; Jemaá, 2023; Lee et al., 2009; Otto, 2007; Potapova et al., 2013; Shu et al., 2018; Storchova and Kuffer, 2008; Tanaka, 2018; Vitale, 2011; Zack, 2013). In humans, this most often occurs via mitotic failure (from mitotic slippage or cytokinetic failure) (Davoli and de Lange, 2012; Galofré, 2020; Jemaa, 2021; Jemaá, 2023), endoreplication (Lee et al., 2009; Shu et al., 2018), or in some cases, via cell-cell fusion (Storchova and Kuffer, 2008; Tanaka, 2018; Zack, 2013). Each of these processes differ greatly in their mechanisms and triggers, but tetraploid cells as a class have several key shared characteristics that are important to consider. For more information on mitotic slippage, endoreplication, and cytokinetic failure – their triggers and underlying mechanism – please refer to the work of Tanaka et al., and others (Ben-David and Amon, 2020; Davoli and de Lange, 2012; Galofré, 2020; Jemaa, 2021; Jemaá, 2023; Lee et al., 2009; Otto, 2007; Potapova et al., 2013; Shu et al., 2018; Storchova and Kuffer, 2008; Tanaka, 2018; Vitale, 2011; Zack, 2013).
The most important consideration when studying tetraploidy is the occurrence of aneulpoidy, or non-2 N chromosome numbers as a result of chromosomal loss or gain, that often results from chromosomal instability (CIN) following a tetraploidy state (Ben-David and Amon, 2020; Otto, 2007; Potapova et al., 2013). According to the National Cancer Institute (NCI), aneuploidy is one of the most common cellular features of cancer at an average 90% occurrence, even in early stage cancers (Aneuploidy May Help Tumors Become Resistant to Treatment - NCI (2021). Archive Location: nciglobal,ncienterprise.). Aneuploidy, in turn, has been associated with several key beneficial phenotypes including chemo- and radio-therapeutic resistance profiles, cancer stemness, and genomic instability (Ben-David and Amon, 2020; Otto, 2007; Potapova et al., 2013). It is important to note that in healthy cells, tetraploidy and the aneuploidy that results often leads to apoptosis or senescence (an exit from the cell cycle), but in cancer cells that have a dysregulated cell cycle, tetraploidy can act as a stepping stone to enable many of the hallmarks of cancer (Ben-David and Amon, 2020; Otto, 2007; Potapova et al., 2013). In this way, it is logical to conclude that cell-cell fusion, as another pathway to tetraploidization, can similarly act as a hallmark-enabling mechanism.
5.2. Cell-cell fusion and the hallmarks of cancer
Let us now consider the roles of cell-cell fusion in potentiating the hallmarks of cancer as defined by Hanahan and Weinberg, (2011). For each cancer hallmark that cell-cell fusion is associated with, we can interpret the hallmark as an adaptive benefit or feature of increased relative fitness following cell-cell fusion. Furthermore, each hallmark produced secondary to a fusion event - despite its relative rarity of incidence - can have a prolonged impact on the overall evolution of the tumor and the resultant tumor burden perceived by a patient and their clinical team (refer to section two: Understanding Cancer as Somatic Evolution) (Hass et al., 2021; Wang, 2021).
Consider the hallmark of increased genetic instability and mutations, which cell-cell fusion is known to contribute to following nuclear fusion (Parris, 2013; Hass et al., 2021; Searles et al., 2018; Su, 2015). Genetic instability, particularly following a polyploid state, has many known key adaptive benefits for cancer; in many cases it allows the tumor to evolve treatment resistance and thus out-compete the relatively treatment-sensitive tumor cells neighboring it (Vittoria et al., 2023; Mandelia, 2021; Pienta, 2022). Similarly, the cancer hallmark of increasing invasive and metastatic potential, which too has been connected to a post-fusion state (Jang, 2022; Arena, 2023), allows cancerous cells to seed distant sites. It is not yet well understood at what rate each of these cancer hallmarks arises from cell-cell fusion, but it is likely that several can co-occur following a single fusion event as many of these hallmarks are fundamentally interrelated (Hanahan and Weinberg, 2011). Therefore, the net effect of a single cell-cell fusion event as a hallmark-enabling mechanism can have drastic consequences for cancer’s overall survival, especially when considered within the selective pressures of the human body.
Perhaps even more fascinating is the idea that fusion can act as a form of parasexual recombination in tumors, especially when fusion occurs between two cancer cells (Miroshnychenko, 2021). Parasexual recombination between cancerous cells could result in the amassing of multiple adaptive benefits and mutations – whether chemoresistant or pro-proliferating or pro-invasive etc. – into one single hybrid cell, which can then undergo more division and/or more fusion to propagate those adaptations further Miroshnychenko, (2021). In the computational model of tumor evolution described by Miroshnychenko et al. in 2021, it was demonstrated that even in the absence of any chemotherapeutic selective pressure, tumor evolution could diverge from a tumor with no fusion with just one fusion event per 1000 mitoses (Miroshnychenko, 2021). This is too frequent to be convincing on its own, but it is strong evidence that the ubiquitous selective pressures within tumors likely select for fused cells, even if their occurrence is rare. The question of just how rare fusion events can be while causing a divergence in tumor evolution remains and will continue to persist until we devise better ways of measuring rates of cell-cell fusion. Selective pressures do affect tumors in vivo (e.g. chemotherapy, blood supply, etc.), and the relationship between these selective pressures and fusion rates is yet undefined (Miroshnychenko, 2021).
Another key evolutionary benefit of cell-cell fusion is the potential formation of cancer-initiating stem cells, often just referred to as cancer stem cells or CSCs (Dittmar, 2022), and/or polyploid/giant cancer cells (PCCs/GCCs) (Zhang, 2014; Casotti, 2023; Bühler, 2022; Ben-Shoshan, 2014). At this point, nearly all cancers have been associated with CSCs and PCCs, but their formation is not usually connected to cell-cell fusion. For reference, cancer stem cells are usually thought to be due to a sum of initiating mutations that result in an initial cancer clone, whereas polyploid cancer cells are usually thought to be the result of a series of mitotic failures like those that are often associated with tetraploidization. (Kloc, 2022; Dittmar, 2022; Ben-David and Amon, 2020; Davoli and de Lange, 2012; Galofré, 2020; Jemaa, 2021; Jemaá, 2023; Lee et al., 2009; Otto, 2007; Potapova et al., 2013; Shu et al., 2018; Storchova and Kuffer, 2008; Tanaka, 2018; Vitale, 2011; Zack, 2013) This is in spite of the establishment of a lab protocol that allows for the induction of PCCs/GCCs via cell fusion in vitro (Zhang, 2014). Furthermore, recent evidence suggests that cell-cell fusion can and often does lead to the formation of CSCs and/or PCCs in vivo (Melzer et al., 2019), with further evidence that each cell type can yield distinct adaptive benefits (Mirzayans and Murray, 2023). The difficulty in proving the significance of cell-cell fusion in these cases inherently lies in the practical realities surrounding the identification of the origins of these cells. While these cells are nearly ubiquitously observed in histological studies of biopsied tumor samples (Mirzayans and Murray, 2023), it is seemingly impossible to separate cells based on their origins (refer to Fusion and Tetraploidization in Cancer for more details). Therefore, while these cell types greatly improve the survivability of cancer cells, the extent to which the presence of CSCs and PCCs can be attributed to fusion events is unclear.
A summary of these key adaptive benefits as defined by the cancer hallmarks (Hanahan and Weinberg, 2011), some of their established cancerous affiliations, and whether or not fusion has been directly identified in these contexts is summarized in Table 2. Other evolutionary benefits of fusion can be identified by examining the established triggers of fusion, which we discuss next.
Table 2.
Summary of key evidence supporting the importance of fusion in cancer based on associated cancer hallmarks i.e. adaptive benefits, the cancers associated with each, and whether fusion was observed directly.
| Adaptive Benefit / Cancer Hallmark | Cancers Associated | Observations | Fusion Observed (all/ some/none) | References |
|---|---|---|---|---|
|
| ||||
| Treatment resistance; resilience to environmental stress | Potentially all; gastrointestinal tumors | • Polyploidization (polyploid giant cell formation) treatment resistance and resilience to environmental stress • Cell-in-cell formation leading to increased treatment resistance, worse prognosis, and resilience to stress |
Some | (Mirzayans and Murray, 2023; Casotti, 2023; Archetti, 2022; Buhler, 2022; Pienta, 2022; Vittoria et al., 2023; Druzhkova et al., 2023; Anatskaya and Vinogradov, 2022; Ben-Shoshan, 2014; Mandelia, 2021; Kaufmann, 2022) |
| Sternness/ replicative immortality | Bladder cancer (Urothelial cell carcinoma) | • Extended 3’LTR → increased c-Myb interaction → upregulated syncytin levels associated with increased stemness of cancer cells | None | (Yu, 2014) |
| Migration; Invasion/ Metastasis | Colon adenocarcinoma + melanoma, sarcomas, prostate cancer, ovarian cancer, breast cancer | • Macrophage-neoplastic cell fusion to form circulating hybrid/tumor cells (CHCs/CTCs) • Increased TNF-α levels leading to increased MMP9 expression and decreased ECM → increased fusion between breast epithelium and and breast cancer cells |
All | (Weiler et al., 2018; Vittoria et al., 2023; Druzhkova et al., 2023; Ruano, 2022; Gast, 2018) |
| Immune evasion | Colon adenocarcinoma | • Colon cancer cell-lymphocyte fusion resulting in cancer acquisition of immune regulatory surface proteins including CTLA4 | All | (Shin, 2021) |
| Enabling replicative immortality | Sarcomas, prostate cancer, endometrial carcinoma | • Immortal myoblast-transformed fibroblast fusion leading to tumor initiation insarcomas • Prostate cancer cells fused with nearby muscle cells → increased sternness/immortality and drug resistance • Increased fusion of endometrial cells with increased syncytin-1 expression and down-regulation of TGF-α signaling |
All | (Weiler et al., 2018; Druzhkova et al., 2023; Wang, 2020) |
| Genetic heterogeneity/genetic instability | Prostate cancer, breast cancer | • Cancer-stromal cell fusion leading to hybrid formation (but with reduced growth rates) • Somatic cell-cell fusion leading to cancer initiation; a potential marker for differentiation of basal vs luminal breast cancer |
All | (Wang, 2020; Su, 2015) |
5.3. Triggers of cell-cell fusion in cancer
The established triggers of cell fusion - and more specifically the known triggers of fusogen upregulation - are hypoxia and inflammation (Knerr, 2005; Yan, 2017; Strick, 2007). To understand how these triggers offer insights into the shifts in tumor evolutionary trajectory that can arise following fusion events, one only needs to consider the process of tumorigenesis and the formation of the associated tumor microenvironment (TME) (Jang, 2022; Gouirand et al., 2018; Nunes, 2020; Kutova et al., 2021; Hass et al., 2020).
The TME is well-established as being highly inflammatory, and current evidence suggests that this inflammation is tumor-promoting for many reasons. Some of the effects of inflammation on tumor progression include increased angiogenesis and improved immune evasion secondary to lactic acid production (Jang, 2022; Gouirand et al., 2018; Nunes, 2020; Kutova et al., 2021; Hass et al., 2020). Tumorigenesis also consistently results in areas of hypoxia. The angiogenesis induced by the TME and general hyper-inflammatory state is insufficient in comparison to the rapid growth that is characteristic of many tumors. Oftentimes the cancerous cells rapidly outgrow the availability of oxygen, usually resulting in necrotic cores throughout the tumors once they grow above a certain volume and/or mass (Scott et al., 2016; Jang, 2022; Gouirand et al., 2018; Nunes, 2020; Kutova et al., 2021; Hass et al., 2020). Interestingly, independent studies have observed that increasing density and mechanical stress, as are associated with these hypoxic cores of solid tumors, are directly correlated with higher rates of cancer cell-cell fusion in vitro (Bühler, 2022).
Thus the two key triggers of fusion - hypoxia and inflammation- are known to be nearly universally present in the TME. This, of course, has some important implications when considering the yet unexplored impacts of cell fusion in cancer. Several effects could explain why fusion events are promoted when cells are in these tumorous microenvironments, and these benefits (Knerr, 2005; Yan, 2017; Strick, 2007) are summarized briefly here.
In hypoxic states, cell fusion can allow for a consolidation of oxygen, energetic reserves, and metabolic precursors to prolong hybrid cell survival until oxygenation improves (Knerr, 2005; Yan, 2017; Strick, 2007). Simultaneously, secondary to the TME inflammatory state, the increased rates of cell fusion following profusogenic priming and the resulting higher fusogen production (Knerr, 2005; Yan, 2017; Strick, 2007) can promote immune evasion and cancer stem cell formation (adaptive effects discussed previously). These effects can compound with the more immediate effects of the inflammatory state, including increased angiogenesis and higher rates of immune surveillance, to have even more drastic but yet unmeasured effects on the tumor’s evolution over time (Nunes, 2020; Hass et al., 2020).
Now consider an additional phenotype sometimes derived from cell-fusion: a higher migratory ability of cancer hybrid cells following macrophage-cancer cell fusion. In the context of the inflammatory TME (and its angiogenesis-promoting state), we can see a perfect storm of environmental factors that, in combination with hybrid phenotypes, can drastically change the course and progression of a tumor over time by enabling seeding to distant sites (Cheung and Ewald, 2016; Liotta et al., 1977). We thus predict that even a single cell-cell fusion event could be a key distinguishing characteristic between pre- and post-metastatic patients on clinical presentation. Thus it is important to study cell fusion, as its occurrence could have potentially exponential effects on cancer progression and patient survival, despite its apparent rarity.
6. Discussion
Fundamentally, cell fusion is a rare event. Just how rare it is has yet to be measured, but current model-based estimates suggest that in the absence of selective pressure, it would need to be as common as one per 1000 cell divisions to matter in a tumor’s evolutionary progression, 100–1000 times more frequent than polymerase errors during DNA replication (Miroshnychenko, 2021; Preston et al., 2010). If fusion events were that common, there would likely be more evidence of its presence across cancer already in the literature. As a result, we predict that these estimates do not reflect the true frequency of cell-cell fusion events in human cancers. Even so, there have been an increasing number of review papers over the past decade that favor the progression of cell fusion research in the oncologic and broader scientific communities (Dittmar, 2022; Hass et al., 2021),(Hass et al., 2021; Wang, 2021; Dietz, 2021; Gast, 2018; Zhang, 2022; Sieler et al., 2021).
In this review, we described how the tumor microenvironment provides important biological context and evidence that fusion does matter, even if exceedingly rare. Even a single fusion event could have important effects on the course of a tumor’s progression since post-fusion hybrid phenotypes are likely heavily favored by the selective pressures of the TME.
Using an evolutionary oncology perspective, we can begin to understand how evolutionary rules define cancer behaviors and patterns of progression. Cancer is a fundamentally rare event after all, but its incidence continues to rise despite the biological rarity of the initiating mutations (Pepper et al., 2009; Yildirim and Sever, 2024). Fundamentally, cancer cells are always under selective pressure. At baseline, before clinical diagnosis, cancer cell survival is being selected for based on the combination of energetic needs, resource availability, etc. Following the administration of chemotherapy or radiation, modern medicine provides additional selective pressures to select for only the fittest cells, which are often those that are or are primed to become treatment resistant. This is a major concern of modern oncology research and treatment developement, and cell fusion could be the next big breakthrough that drives us closer to resolution. We need only to consider the increasingly large evidence base that cell-cell fusion in cancer enables the cancer hallmarks and can propagate the spread of treatment resistance through a tumor cell population.
There are several limitations that remain unaddressed in fusion research. The current lack of an appropriate in vitro system or in vivo animal model to measure cell fusion rates, especially within a tumor microenvironment, is the biggest hurdle cell fusion research faces. This is only worsened by the limited number of temporal studies of tumor cell populations over evolutionary time, as fusion is far more likely to be observed if cells are monitored over longer periods of time. Without these models to directly monitor and track cell-cell fusion events and outcomes over time, the field will continue to struggle to directly implicate cell-cell fusion in tumor initiation and progression. These are the key issues preventing the establishment of cell fusion as a cancer hallmark. In the meantime, there are a tremendous number of persisting research questions in this field that can be addressed with existing research methods. We highlight some of those now.
For one, there is a need for a more cohesive effort to look for fusogenic motifs and/or fingerprints across all cancer subtypes, through both in vitro (via improved models and model systems) and in vivo studies. Current evidence of cell-cell fusion in vitro and in vivo primarily relies on the use of fluorescent markers such as GFP or mCherry to isolate fused cells from their cellular lineages (Wang, 2020; Melzer et al., 2018), (Melzer et al., 2019; Huerta et al., 2006). The potential techniques that could characterize new and/or previously identified fusogens range from highly multiplexed immunohistochemistry of tumor biopsy samples to more computational methods such as gene expression profiling and transcriptomic studies. Another promising technique is that of short tandem repeat (STR) analyses, which has previously been used to identify cancer cell fusions with surrounding non-cancerous cells such as leuokocytes and macrophages in vivo (Pawelek and Chakraborty, 2008; Pawelek, 2020,Pawelek, 2014). Unfortunately with cancer cell-cell fusion, the more proximal lineages of the cells in question may make STR analyses hard to apply directly. Identifying the fusion of clonal cancer cell populations may have more similarities to forensically distinguishing monozygotic twins (Yuan, 2020). Furthermore, SNP analysis may fail as clonal lineage tracing may assume a fused clone is a progenitor of the clones that formed it (Tarabichi, 2021). Advanced genotypic analysis of clones within primary and recurrent tumor samples may eventually allow us to identify cancer-cancer fusion processes in patients (Tarabichi, 2021).
Additionally, there needs to be an improved understanding of the molecular biology triggered by fusogenic signaling and fusion should it result, and how the different outcomes of fusion (e.g. senescence, ploidy reduction, additional fusion etc.) are selected for by intrinsic and/or extrinsic signaling. There is the question of how each of these outcomes could potentially impact tumor evolution; a question best addressed by improving computational approaches to detect the occurrence of fusion and predict its impacts – whether by cancer modeling (Hanin, 2013; Maltas, 2024; King et al., 2024), bioinformatics, or other tools that are still being developed.
The study of cell fusion in cancer serves as a reminder that there needs to be a greater consideration for potentially less obvious, but evolutionarily relevant sources of biological variation across tumors, of which cell-cell fusion is one. This concept of incorporating evolutionary principles into the oncology clinic is not a new one, and has led to the development of evolutionary oncology (Scarborough et al., 2023; Farrokhian et al, 2022; Nichol, 2015; Gluzman et al., 2020) as a research field and more notably the development of several new treatment regimes over the past few decades (Gatenby and Brown, 2020; Pepper et al., 2009; Niculescu, 2023; Enriquez-Navas et al., 2015). Thus, cell-cell fusion is an important area of research to consider as the oncology field continues on its path towards an evolution-informed approach to personalized medicine. As the evidence for cell-cell fusion in cancer continues to grow, we anticipate that the discussion of cell-cell fusion as a novel hallmark will return. There is a lot of work to be done to develop this research area before that can happen, but we are confident that cell-cell fusion will in fact be the next cancer hallmark in the years to come.
Table 1.
The primary fusogenic classes, their characteristics, a few example protiens, where they are found in biology, and evolutionary origins. Consolidated from (Harrison, 2008; Sapir et al., 2008; Yang and Margam, 2021; Yu, 2014).
| Class | Structural Motifs | Proteins | Where Found | Evolutionary Origins | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| I | 2 alpha-helices, fusion peptides by N-terminus | Hemagglutinin (HA), HIV-1, S-protein (spike protein) | Human placentae, HIV, Coronaviridae, Influenza, | Viral | |||
| II | Beta-sheets with internal fusion peptides | E/E1 | Dengue, yellow fever, West Nile | Viral | |||
| III | Mix of alpha-helices and beta-sheets; fusion occurs independent of fusion peptide exposure | HSV-1 gB, EBV gB, VSV G | Rabies, VSV, HSV, related viruses (herpes-viridae) | Viral | |||
| SNAREs | 3 single alpha-helix (t-) SNARE proteins combine into a complex that bind with a structurally distinct 4th (v-)SNARE | Syntaxin-1, SNAP-25 | Human neurons, Vesicle fusion (intracellular fusion) | Eukaryotic | |||
Box 2. Evolutionary Origins of Fusion: The Convergent Evolution of Fusogens.
The most well studied example of fusion in biology is that of viral fusion with host cells as the first step of the viral life cycle. Virus-cell fusion often relies on the interaction of viral surface and/or envelope proteins with membrane bound host-cell receptors. This interaction eventually leads to the structural and energetic changes that allow for pore opening and viral entry into the cell (Lazebnik, 2021; Petrany and Millay, 2019).
There are now four main classes of fusogens, of which there are three (I, II, III) with viral origins, separated by their structural motifs (Harrison, 2008; Sapir et al., 2008; Yang and Margam, 2021; Yu, 2014). A fourth fusogenic class (SNARE proteins) has been added due to the functional similarities and parallels in fusogenic processes, but they are evolutionarily distinct and can only be found in eukaryotes. These four classes are summarized below in Table 1.
The evolutionary development of several classes of fusogens – each with distinct structural motifs and viral classes associated with them – points to the convergent evolution of fusion over time (Chan, 2021; Fédry, 2017; Moi, 2022; Quinn, 2017; Sapir et al., 2008; Vance and Lee, 2020; Yang and Margam, 2021). All viruses need to be able to fuse with host cells in order to enter the replicative stage of the viral life cycle. However, based on the genetic and structural diversity that allow for fusion to occur across viral classes, it can be deduced that these evolved through genetically distinct lineages – an example of convergent evolution. For more detail on the evolution of fusogens please refer to (Chan, 2021; Fédry, 2017; Moi, 2022; Quinn, 2017; Sapir et al., 2008; Vance and Lee, 2020; Yang and Margam, 2021).
More interestingly, eukaryotic cells have also evolved this ability to fuse in several ways. The presence of the SNARE class of proteins that are unique to eukaryotes demonstrates yet another example of convergent evolution; novel protein structures evolved to enable membrane fusion, specifically vesicle fusion (Hernández and Podbilewicz, 2017; Wang, 2016; Pérez-Vargas, 2014; Yang and Margam, 2021). Similarly, myomaker and myomerger, the fusogen classes involved in human muscle formation and maturation, also evolved independently (Quinn, 2017; Yang and Margam, 2021; Zhang, 2022). Their unique structural properties exclude them from the primary fusogenic classes listed below (Quinn, 2017; Yang and Margam, 2021; Zhang, 2022).
Some of the eukaryotic fusion mechanisms are not evolutionary distinct and are in fact directly related to viral fusion. This is characteristic of the syncytins associated with placentation; they are directly related to retroviral envelope proteins that were introduced to eukaryotic genomes following retroviral infection and the resultant insertion of genetic material (Fei, 2019; Bjerregaard et al., 2006; Kloc et al., 2021; Soe, 2011).
Significance Statement.
Problem or issue
Intermittent auscultation (IA) is a central facet of midwifery practice, but there is very little evidence about women’s experience of IA.
What is already known
IA is recommended for fetal monitoring during labour for women with uncomplicated pregnancies. IA allows increased mobility in labour compared with continuous monitoring, and potentially better communication and holistic assessment of the labouring woman.
What this paper adds
Limited communication about fetal monitoring practice in labour from maternity care professionals in the antenatal period has an impact on women’s opportunities to make informed decisions about monitoring.
Acknowledgements
The authors are grateful for the T32 and R37 grants that financially supported and continue to support this work. JGS and PVS were supported by NIH 5R37CA244613–04 (https://www.cancer.gov/). PVS was supported by NIH 3T32GM007250–46S1 (https://www.nigms.nih.gov/). JGS was supported by American Cancer Society Research Scholar Grant RSG-20–096-01 (https://www.cancer.org/). DST was supported by the Norwegian Research Council (NRC) with grant 325628/IAR (https://www.forskningsradet.no/en/). The funders had no role in the decision to publish, or the preparation of the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Paulameena V. Shultes, Jacob G. Scott reports financial support was provided by National Institute of Health. Jacob G. Scott reports financial support was provided by American Cancer Society. Dagim S. Tadele reports financial support was provided by Norwegian Research Council (NRC). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Paulameena V. Shultes: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation. Davis T. Weaver: Writing – review & editing, Conceptualization. Dagim S. Tadele: Writing – review & editing, Conceptualization. Rowan J. Barker-Clarke: Writing – review & editing, Conceptualization. Jacob G. Scott: Writing – review & editing, Supervision, Funding acquisition.
Data availability
No data was used for the research described in the article.
References
- [1]Aneuploidy May Help Tumors Become Resistant to Treatment - NCI (2021). Archive Location: nciglobal,ncienterprise. Aneuploidy May Help Tumors Become Resistant to Treatment - NCI (2021). Archive Location: nciglobal,ncienterprise. [Google Scholar]
- Aguilar PS, et al. , 2013. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29 (7), 427–437. 10.1016/j.semcdb.2020.02.004. ISSN: 01689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh K, Vanoppen M, Rose CD, Matthys P, Wouters CH, 2022. Multinucleated giant cells: current insights in phenotype, biological activities, and mechanism of formation. Front. Cell Dev. Biol. 10, 873226 10.3389/fcell.2022.873226 (Publisher: Frontiers). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimov SA, Molotkovsky RJ, Kuzmin PI, Galimzyanov TR, Batishchev OV, 2020. Continuum models of membrane fusion: evolution of the theory. Int. J. Mol. Sci. 21, 3875. 10.3390/ijms21113875. Number: 11 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anatskaya OV, Vinogradov AE, 2022. Polyploidy as a fundamental phenomenon in evolution, development, adaptation and diseases. Int. J. Mol. Sci. 23, 3542. 10.3390/ijms23073542. Number: 7 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti M, 2022. Polyploidy as an adaptation against loss of heterozygosity in cancer. Int. J. Mol. Sci. 23, 8528. 10.3390/ijms23158528. Number: 15 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena GO, et al. , 2023. Horizontal transfer of malignant traits and the involvement of extracellular vesicles in metastasis. Cells 12, 1566 (Publisher: MDPI). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilés JM, Azcón-Bieto J, 1988. The metabolic environment of cancer. Mol. Cell. Biochem. 81, 3–17. 10.1007/BF00225648. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bullough F, Murphy S, 2000. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth 1. Cancer Res. 60, 1492–1497. [PubMed] [Google Scholar]
- Bates M, et al. , 2023. Circulating tumour cells: the good, the bad and the ugly. Biochim. Et. Biophys. Acta (BBA) Rev. Cancer 1878, 188863. 10.1016/j.bbcan.2023.188863. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Amon A, 2020. Context is everything: aneuploidy in cancer. Nat. Rev. Genet. 21, 44–62. 10.1038/s41576-019-0171-x (Publisher: Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- Ben-Shoshan SO, et al. , 2014. Induction of polyploidy by nuclear fusion mechanism upon decreased expression of the nuclear envelope protein LAP2β in the human osteosarcoma cell line U2OS. Mol. Cytogenet. 7, 9. 10.1186/1755-8166-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerregaard B, Holck S, Christensen IJ, Larsson LI, 2006. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 63, 1906–1911. 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T, 2008. Concerning the origin of malignant tumours by theodor boveri. translated and annotated by henry harris. J. Cell Sci. 121, 1–84. 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- Brukman NG, Li X, Podbilewicz B, 2022. Fusexins, HAP2/GCS1 and evolution of gamete fusion. Front. Cell Dev. Biol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brukman NG, Uygur B, Podbilewicz B, Chernomordik LV, 2019. How cells fuse. ISSN: 15408140 J. Cell Biol. Vol. 218 (5), 1436–1451. 10.1083/jcb.201901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler A, et al. , 2022. When Mech. Stress Matter.: Gener. Polyploid Giant Cancer Cells Tumor- Micro. 10.1101/2022.09.22.508846. Pages: 2022.09.22.508846 Section: New Results. [DOI] [Google Scholar]
- Cairns J, 1975. Mutation selection and the natural history of cancer. Nature 255, 197–200. 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Casotti MC, et al. , 2023. Computational biology helps understand how polyploid giant cancer cells drive tumor success. Genes 14, 801. 10.3390/genes14040801. Number: 4 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KMC, et al. , 2021. Evolutionarily related small viral fusogens hijack distinct but modular actin nucleation pathways to drive cell-cell fusion. Proc. Natl. Acad. Sci. 118, e2007526118 10.1073/pnas.2007526118 (Publisher: Proceedings of the National Academy of Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Ewald AJ, 2016. A collective route to metastasis: seeding by tumor cell clusters. Science 352, 167–169. 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari K, Somarelli JA, Kumar S, Townsend JP, 2021. The somatic molecular evolution of cancer: Mutation, selection, and epistasis. Prog. Biophys. Mol. Biol. 165, 56–65. 10.1016/j.pbiomolbio.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, de Lange T, 2012. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell 21, 765–776. 10.1016/j.ccr.2012.03.044 (Publisher: Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespaul L, et al. , 2020. Cell-cell fusion of mesenchymal cells with distinct differentiations triggers genomic and transcriptomic remodelling toward tumour aggressiveness. Sci. Rep. 10 10.1038/s41598-020-78502-z (Publisher: Nature Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demin S, Berdieva M, Goodkov A, 2022. Cell-cell fusions and cell-in-cell phenomena in healthy cells and cancer: lessons from protists and invertebrates. Semin. Cancer Biol. 81, 96–105. 10.1016/j.semcancer.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Deneke VE, Pauli A, 2021. The fertilization enigma: how sperm and egg fuse. Annu. Rev. Cell Dev. Biol. 37, 391–414. 10.1146/annurev-cellbio-120219-021751. [DOI] [PubMed] [Google Scholar]
- Dietz MS, et al. , 2021. Relevance of circulating hybrid cells as a non-invasive biomarker for myriad solid tumors. Sci. Rep. 11, 1–13. 10.1038/s41598-021-93053-7. Number: 1 Publisher: Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T, 2022. Generation of cancer stem/initiating cells by cell-cell fusion. Int. J. Mol. Sci. 23 (9) 10.3390/ijms23094514. ISSN: 14220067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T, Hass R, 2022. Extracellular events involved in cancer cell-cell fusion. Int. J. Mol. Sci. 23 (24) 10.3390/ijms232416071. ISSN: 14220067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T, Hass R, 2023. Intrinsic signalling factors associated with cancer cell-cell fusion. Cell Commun. Signal. 21, 68. 10.1186/s12964-023-01085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T, Sieler M, Hass R, 2023. Why do certain cancer cells alter functionality and fuse? Biol. Chem. 404, 951–960. 10.1515/hsz-2023-0162 (Publisher: De Gruyter). [DOI] [PubMed] [Google Scholar]
- Dittmar T, Weiler J, Luo T, Hass R, 2021. Cell-cell fusion mediated by viruses and HERV-derived fusogens in cancer initiation and progression. Cancers 13, 5363. 10.3390/cancers13215363. Number: 21 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin J, Wu L, Mangalmurti NS, 2023. The ultimate tradeoff: how red cell adaptations to malaria alter the host response during critical illness. Am. J. Physiol. -Lung Cell. Mol. Physiol. 324, L169–L178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhkova I, Ignatova N, Shirmanova M, 2023. Cell-in-cell structures in gastrointestinal tumors: biological relevance and clinical applications. J. Pers. Med. 13, 1149. 10.3390/jpm13071149. Number: 7 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson A, Anuj A, Barnea-Zohar M, Reuven N, 2022. The origins and formation of bone-resorbing osteoclasts. Bone 164, 116538. 10.1016/j.bone.2022.116538. [DOI] [PubMed] [Google Scholar]
- Emans N, et al. , 1993. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 120, 1357–1369. 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA, 2015. Application of evolutionary principles to cancer therapy. Cancer Res. 75 (22) 10.1158/0008-5472.CAN-15-1337. ISSN: 15387445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhian N, et al. , 2022. Measuring competitive exclusion in non-small cell lung cancer. Sci. Adv. 8, eabm7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fédry J, et al. , 2017. The ancient gamete fusogen HAP2 Is a eukaryotic class II fusion protein. Cell 168, 904–915. 10.1016/j.cell.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei F, et al. , 2019. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer Med. 8, 3047–3058. 10.1002/cam4.2173 (Publisher: Blackwell Publishing Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, 2010. Somatic evolutionary genomics: Mutations during development cause highly variable genetic mosaicism with risk of cancer and neurodegeneration. Proc. Natl. Acad. Sci. 107, 1725–1730. 10.1073/pnas.0909343106 (Publisher: Proceedings of the National Academy of Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galofré C, et al. , 2020. Centrosome reduction in newly-generated tetraploid cancer cells obtained by separase depletion. Sci. Rep. 10, 9152. 10.1038/s41598-020-65975-1 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast CE, et al. , 2018. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 4, 7828–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Brown JS, 2020. Integrating evolutionary dynamics into cancer therapy. Nat. Rev. Clin. Oncol. 17 (11), 675–686. 10.1038/s41571-020-0411-1, 7594782. [DOI] [PubMed] [Google Scholar]
- Gerstung M, et al. , 2020. The evolutionary history of 2,658 cancers. Nature 578, 122–128. 10.1038/s41586-019-1907-7 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DE, Pyle AD, 2023. Muscle fusogens go viral for gene delivery to skeletal muscle. Cell 186, 2041–2043. 10.1016/j.cell.2023.04.021 (Publisher: Elsevier). [DOI] [PubMed] [Google Scholar]
- Gingerich PD, 2019. Rates of Evolution: A Quantitative Synthesis. Cambridge University Press. [Google Scholar]
- Gluzman M, Scott JG, Vladimirsky A, 2020. Optimizing adaptive cancer therapy: dynamic programming and evolutionary game theory. Proc. R. Soc. B 287, 20192454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouirand V, Guillaumond F, Vasseur S, 2018. Influence of the tumor microenvironment on cancer cells metabolic reprogramming. Front. Oncol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, et al. , 2023. Mitochondrial transfer in hematological malignancies. Biomark. Res. 11, 89. 10.1186/s40364-023-00529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. 10.1016/j.cell.2011.02.013 (Publisher: Elsevier). [DOI] [PubMed] [Google Scholar]
- Hanin L, 2013. Seeing the Invisible: How Mathematical Models Uncover Tumor Dormancy, Reconstruct the Natural History of Cancer, and Assess the Effects of Treatment. Springer, New York, New York, NY, pp. 261–282. [DOI] [PubMed] [Google Scholar]
- Harrison SC, 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698. 10.1038/nsmb.1456. Number: 7 Publisher: Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R, Ohe JVD, Dittmar T, 2021. Hybrid formation and fusion of cancer cells in vitro and in vivo. Cancers 13 (17). 10.3390/cancers13174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R, vonderOhe J, Dittmar T, 2021. CanceR Cell Fusion and Post-hybrid Selection Process (PHSP). Cancers 13, 4636. 10.3390/cancers13184636. Number: 18 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R, vonderOhe J, Ungefroren H, 2020. Impact of the tumor microenvironment on tumor heterogeneity and consequences for cancer cell plasticity and stemness. Cancers 12, 3716. 10.3390/cancers12123716. Number: 12 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández JM, Podbilewicz B, 2017. The hallmarks of cell-cell fusion. Development 144 (24), 4481–4495. 10.1242/dev.155523. [DOI] [PubMed] [Google Scholar]
- Huang S, 2021. Reconciling non-genetic plasticity with somatic evolution in cancer. Trends Cancer 7, 309–322. 10.1016/j.trecan.2020.12.007 (Publisher: Elsevier). [DOI] [PubMed] [Google Scholar]
- Huerta L, López-Balderas N, Larralde C, Lamoyi E, 2006. Discriminating in vitro cell fusion from cell aggregation by flow cytometry combined with fluorescence resonance energy transfer. J. Virol. Methods 138, 17–23. 10.1016/j.jviromet.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Jang G, et al. , 2022. Direct cell-to-cell transfer in stressed tumor microenvironment aggravates tumorigenic or metastatic potential in pancreatic cancer. NPJ Genom. Med. 7, 1–17. 10.1038/s41525-022-00333-w. Number: 1 Publisher: Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemaá M, et al. , 2023. Tetraploidization Increases the Motility and Invasiveness of Cancer Cells. Int. J. Mol. Sci. 24, 13926. 10.3390/ijms241813926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemaa M, 2021. Mitotic spindle as therapeutic target for tetraploid cancer cells. Eurasia J. Med. Oncol. 10.14744/ejmo.2021.13872. [DOI] [Google Scholar]
- Johnson G, et al. , 2008. Cancer cachexia: measured and predicted resting energy expenditures for nutritional needs evaluation. Nutrition 24, 443–450. 10.1016/j.nut.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Kaufmann TL, et al. , 2022. MEDICC2: whole-genome doubling aware copy-number phylogenies for cancer evolution. Genome Biol. 23, 241. 10.1186/s13059-022-02794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King ES, Tadele DS, Pierce B, Hinczewski M, Scott JG, 2024. Diverse mutant selection windows shape spatial heterogeneity in evolving populations. PLOS Comput. Biol. 20, 1–22. 10.1371/journal.pcbi.1011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, et al. , 2022. Giant multinucleated cells in aging and senescence–an abridgement. Biology 11, 1121. 10.3390/biology11081121. Number: 8 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, Subuddhi A, Uosef A, Kubiak JZ, Ghobrial RM, 2022. Monocyte-macrophage lineage cell fusion. Int. J. Mol. Sci. 23, 6553 (Publisher: MDPI). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, Uosef A, Kubiak JZ, Ghobrial RM, 2021. Exaptation of retroviral syncytin for development of syncytialized placenta, its limited homology to the SARS-CoV-2 spike protein and arguments against disturbing narrative in the context of COVID-19 vaccination. Biology 10, 238. 10.3390/biology10030238. Number: 3 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr I, et al. , 2005. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 579, 3991–3998. 10.1016/j.febslet.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Komarova NL, Sadovsky AV, Wan FYM, 2008. Selective pressures for and against genetic instability in cancer: a time-dependent problem. J. R. Soc. Interface 5, 105–121. 10.1098/rsif.2007.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutova O, Pospelov A, Balalaeva I, 2021. Multifaceted Role Connexins Tumor Microenviron. Initiat. Maint. 10.20944/preprints202112.0262.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y, 2021. Cell fusion as a link between the SARS-CoV-2 spike protein, COVID-19 complications, and vaccine side effects. Oncotarget 12, 2476–2488. 10.18632/oncotarget.28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HO, Davidson JM, Duronio RJ, 2009. Endoreplication: polyploidy with purpose. Genes Dev. 23, 2461–2477. 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehka L, Rędowicz MJ, 2020. Mechanisms regulating myoblast fusion: a multilevel interplay. Semin. Cell Dev. Biol. 104, 81–92. 10.1016/j.semcdb.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Leroy H, et al. , 2020. Virus-mediated cell-cell fusion. Int. J. Mol. Sci. 21 (24), 1–28. 10.1016/j.jmb.2021.167280. ISSN: 14220067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-Y, et al. , 2010. GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol. Reprod. 83, 387–395. 10.1095/biolreprod.110.083915. [DOI] [PubMed] [Google Scholar]
- Liberti MV, Locasale JW, 2016. The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218. 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Basile JR, Mallya S, Lin Y-L, 2023. The impact and outcomes of cancer-macrophage fusion. BMC Cancer 23, 497. 10.1186/s12885-023-10961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Delisi C, Saidel G, Kleinerman J, 1977. Micrometastases formation: a probabilistic model. Cancer Lett. 3, 203–208. 10.1016/S0304-3835(77)95675-0. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ikawa M, 2022. Eukaryotic fertilization and gamete fusion at a glance. J. Cell Sci. 135, jcs260296. 10.1242/jcs.260296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie D Small Cell-Cell Fusogens Activate a PI3K-mTORC2-Akt Signalling Axis and Macropinocytosis for Efficient Cell Fusion. (2023). Accepted: 2023–09-08T10:37:40Z. [Google Scholar]
- Maley CC, Szabo E, Reid BJ, 2011. Somatic Evolution in Neoplastic Progression and Cancer Prevention (Springer, New York, NY,). In: Pre-Invasive Disease: Pathogenesis and Clinical Management, pp. 111–127. 10.1007/978-1-4419-6694-0_7. [DOI] [Google Scholar]
- Maltas J, et al. , 2024. Frequency-dependent ecological interactions increase the prevalence, and shape the distribution, of preexisting drug resistance. PRX Life 2, 023010. 10.1103/PRXLife.2.023010. [DOI] [Google Scholar]
- Mandelia M Targeting aneuploidy, CIN and mechanisms of DNA content reduction in cancer. (2021). Publisher: Institute of Cancer Research; (University Of London). [Google Scholar]
- Melzer C, Ohe JVD, Hass R, 2018. In vitro fusion of normal and neoplastic breast epithelial cells with human mesenchymal stroma/stem cells partially involves tumor necrosis factor receptor signaling. Stem Cells 36, 977–989. 10.1002/stem.2819 (Publisher: Wiley-Blackwell). [DOI] [PubMed] [Google Scholar]
- Melzer C, vonderOhe J, Hass R, 2019. In vivo cell fusion between mesenchymal stroma/stem-like cells and breast cancer cells. Cancers 11, 185. 10.3390/cancers11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo LMF, Pepper JW, Reid BJ, Maley CC, 2006. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935. 10.1038/nrc2013 (Publisher: Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- Miroshnychenko D, et al. , 2021. Spontaneous cell fusions as a mechanism of parasexual recombination in tumour cell populations. Nat. Ecol. Evol. 5, 379–391. 10.1038/s41559-020-01367-y (Publisher: Nature Research). [DOI] [PubMed] [Google Scholar]
- Mirzayans R, Murray D, 2023. Intratumor heterogeneity and treatment resistance of solid tumors with a focus on polyploid/senescent giant cancer cells (PGCCs). Int. J. Mol. Sci. 24, 11534. 10.3390/ijms241411534. Number: 14 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, et al. , 2022. A phylogenetic approach to study the evolution of somatic mutational processes in cancer. Commun. Biol. 5, 1–11. 10.1038/s42003-022-03560-0 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y Class II Fusion Proteins.In: Madame Curie Bioscience Database [Internet] Landes Bioscience, (2013). [Google Scholar]
- Moi D, et al. , 2022. Discovery of archaeal fusexins homologous to eukaryotic HAP2/GCS1 gamete fusion proteins. Nat. Commun. 13, 3880. 10.1038/s41467-022-31564-1 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D, et al. , 2015. Steering evolution with sequential therapy to prevent the emergence of bacterial antibiotic resistance. PLoS Comput. Biol. 11, e1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu VF Understanding Cancer from an Evolutionary Perspective; DSCD Cells as Cell-of-Origin in Non-Mutational Sporadic Cancers, 10.20944/preprints202308.1688.v (2023). [DOI] [Google Scholar]
- Nowak MA, Michor F, Iwasa Y, 2003. The linear process of somatic evolution. Proc. Natl. Acad. Sci. 100, 14966–14969. 10.1073/pnas.2535419100 (Publisher: Proceedings of the National Academy of Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell PC, 1976. The clonal evolution of tumor cell populations. Science 194, 23–28. 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Nunes SC, 2020. Tumor Microenvironment - Selective Pressures Boosting Cancer Progression (Springer International Publishing, Cham,). In: Tumor Microenvironment: The Main Driver of Metabolic Adaptation, Advances in Experimental Medicine and Biology, pp. 35–49. 10.1007/978-3-030-34025-4_2. [DOI] [PubMed] [Google Scholar]
- Ogle BM, Cascalho M, Platt JL, 2005. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 6, 567–575. 10.1038/nrm1678. Number: 7 Publisher: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Olafsson S, Anderson CA, 2021. Somatic mutations provide important and unique insights into the biology of complex diseases. Trends Genet. 37, 872–881. 10.1016/j.tig.2021.06.012 (Publisher: Elsevier). [DOI] [PubMed] [Google Scholar]
- Otto SP, 2007. The evolutionary consequences of polyploidy. Cell 131, 452–462. 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Parris GE, 2013. Historical perspective of cell-cell fusion in cancer initiation and progression. Crit. Rev. Oncog. 18 10.1615/CritRevOncog.v18.i1-2.20 (Publisher: Begel House Inc). [DOI] [PubMed] [Google Scholar]
- Pawelek JM, 2014. Fusion of bone marrow-derived cells with cancer cells: metastasis as a secondary disease in cancer. Chin. J. Cancer 33, 133–139. 10.5732/cjc.013.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek JM, 2020. Commentary on “Leukocyte-cancer cell fusion-genesis of a deadly journey. J. Dermatol. Ski. Sci. 2. [Google Scholar]
- Pawelek JM, Chakraborty AK, 2008. The cancer cell-leukocyte fusion theory of metastasis. Adv. Cancer Res. 101, 397–444. 10.1016/S0065-230X(08)00410-7. [DOI] [PubMed] [Google Scholar]
- Pepper JW, Findlay CS, Kassen R, Spencer SL, Maley CC, 2009. Cancer research meets evolutionary biology. Evolut. Appl. 2, 62–70. 10.1111/j.1752-4571.2008.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Vargas J, et al. , 2014. Structural basis of eukaryotic cell-cell fusion. Cell 157, 407–419. 10.1016/j.cell.2014.02.020 (Publisher: Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- Petrany MJ, Millay DP, 2019. Cell fusion: merging membranes and making muscle. Trends Cell Biol. 29, 964–973. 10.1016/j.tcb.2019.09.002 (Publisher: Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta KJ, et al. , 2022. Cancer cells employ an evolutionarily conserved polyploidization program to resist therapy. Semin. Cancer Biol. 81, 145–159. 10.1016/j.semcancer.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Potapova TA, Zhu J, Li R, 2013. Aneuploidy and chromosomal instability: a vicious cycle driving cellular evolution and cancer genome chaos. Cancer Metastas-.−. Rev. 32, 377–389. 10.1007/s10555-013-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston BD, Albertson TM, Herr AJ, 2010. DNA replication fidelity and cancer. Semin. Cancer Biol. 20, 281–293. 10.1016/j.semcancer.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SA, Elliott SA, Baracos VE, Chu QSC, Prado CM, 2016. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur. J. Clin. Nutr. 70, 1230–1238. 10.1038/ejcn.2016.96. Number: 11 Publisher: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Quinn ME, et al. , 2017. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 8, 15665. 10.1038/ncomms15665 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MM, Bernier A, Buchrieser J, Schwartz O, 2022. The mechanism and consequences of SARS-CoV-2 spike-mediated fusion and syncytia formation. J. Mol. Biol. 434, 167280 10.1016/j.jmb.2021.167280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhok AI, DeGregori J, 2020. The three dimensions of somatic evolution: integrating the role of genetic damage, life-history traits, and aging in carcinogenesis. Evolut. Appl. 13, 1569–1580. 10.1111/eva.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano APC, et al. , 2022. Fusion cell markers in circulating tumor cells from patients with high-grade ovarian serous carcinoma. Int. J. Mol. Sci. 23, 14687. 10.3390/ijms232314687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A, Avinoam O, Podbilewicz B, Chernomordik LV, 2008. Viral and developmental cell fusion mechanisms: conservation and divergence. Dev. Cell 14, 11–21. 10.1016/j.devcel.2007.12.008 (Publisher: Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough JA, Eschrich SA, Torres-Roca J, Dhawan A, Scott JG, 2023. Exploiting convergent phenotypes to derive a pan-cancer cisplatin response gene expression signature. NPJ Precis. Oncol. 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter ED, 2016. Myoblast fusion: experimental systems and cellular mechanisms. Semin. Cell Dev. Biol. 60, 112–120. 10.1016/j.semcdb.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Scott J, Marusyk A, 2017. Somatic clonal evolution: A selection-centric perspective. Biochim. Et. Biophys. Acta (BBA) Rev. Cancer 1867, 139–150. 10.1016/j.bbcan.2017.01.006 (Evolutionary principles - heterogeneity in cancer?). [DOI] [PubMed] [Google Scholar]
- Scott JG, Fletcher AG, Anderson ARA, Maini PK, 2016. Spatial metrics of tumour vascular organisation predict radiation efficacy in a computational model. PLOS Comput. Biol. 12, 1–24. 10.1371/journal.pcbi.1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles SC, Santosa EK, Bui JD, 2018. Cell-cell fusion as a mechanism of DNA exchange in cancer. Oncotarget 9 (5), 6156–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, et al. , 2021. Colon cancer cells acquire immune regulatory molecules from tumor-infiltrating lymphocytes by trogocytosis. Proc. Natl. Acad. Sci. 118, e2110241118 10.1073/pnas.2110241118 (Publisher: Proceedings of the National Academy of Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Z, Row S, Deng W-M, 2018. Endoreplication: the good, the bad, and the ugly. Trends Cell Biol. 28, 465–474. 10.1016/j.tcb.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieler M, Weiler J, Dittmar T, 2021. Cell-cell fusion and the roads to novel properties of tumor hybrid cells. Cells 10, 1465. 10.3390/cells10061465. Number: 6 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokos EA, Charokopos A, Khan K, Wanjala J, Kyriakides TR, 2011. Lack of TNF-α -induced MMP-9 production and abnormal E-cadherin redistribution associated with compromised fusion in MCP-1-null macrophages. Am. J. Pathol. 178, 2311–2321. 10.1016/j.ajpath.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe K, et al. , 2011. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone 48, 837–846. 10.1016/j.bone.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Stern DL, 2013. The genetic causes of convergent evolution. Nat. Rev. Genet. 14, 751–764. 10.1038/nrg3483. [DOI] [PubMed] [Google Scholar]
- Steven J, Kirkwood B, 2015. Chapter 10 - predicting correlated responses in quantitative traits under selection: A linear algebra approach. In: Robeva RS. (Ed.), Algebraic and Discrete Mathematical Methods for Modern Biology. Academic Press, Boston, pp. 237–259. 10.1016/B978-0-12-801213-0.00010-1. [DOI] [Google Scholar]
- Storchova Z, Kuffer C, 2008. The consequences of tetraploidy and aneuploidy. J. Cell Sci. 121, 3859–3866. 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- Strick R, et al. , 2007. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-β. J. Mol. Med. 85, 23–38. 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- Su Y, et al. , 2015. Somatic cell fusions reveal extensive heterogeneity in basal-like breast cancer. Cell Rep. 11, 1549–1563. 10.1016/j.celrep.2015.05.011 (Publisher: Elsevier). [DOI] [PubMed] [Google Scholar]
- Tanaka K, et al. , 2018. Tetraploidy in cancer and its possible link to aging. Cancer Sci. 109, 2632–2640. 10.1111/cas.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabichi M, et al. , 2021. A practical guide to cancer subclonal reconstruction from DNA sequencing. Nat. Methods 18, 144–155. 10.1038/s41592-020-01013-2 (Publisher: Nature Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufaily C, Lokossou AG, Vargas A, Rassart Barbeau, B.A., 2015. CRE/AP-1-like motif is essential for induced syncytin-2 expression and fusion in human trophoblast-like model. PloS One 10, e0121468. 10.1371/journal.pone.0121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova MS, Subbalakshmi AR, Menyailo ME, Jolly MK, Denisov EV, 2022. Tumor hybrid cells: nature and biological significance. Front. Cell Dev. Biol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygur B, et al. , 2019. Interactions with muscle cells boost fusion, stemness, and drug resistance of prostate cancer cells. Mol. Cancer Res. 17, 806–820. 10.1158/1541-7786.MCR-18-0500 (Publisher: American Association for Cancer Research Inc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance TD, Lee JE, 2020. Virus and eukaryote fusogen superfamilies. Curr. Biol. 30, R750–R754. 10.1016/j.cub.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB, 2009. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, et al. , 2011. Illicit survival of cancer cells during polyploidization and depolyploidization. Cell Death Differ. 18, 1403–1413. 10.1038/cdd.2010.145 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoria MA, Quinton RJ, Ganem NJ, 2023. Whole-genome doubling in tissues and tumors. Trends Genet. 0 10.1016/j.tig.2023.08.004 (Publisher: Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling WF, Greetham J, Bennett DC, 1994. Efficient spontaneous fusion between some co-cultured cells, especially murine melanoma cells. Cell Biol. Int. 18, 207–210. 10.1006/cbir.1994.1063. [DOI] [PubMed] [Google Scholar]
- Wang H-F, et al. , 2021. Cell fusion in cancer hallmarks: current research status and future indications (Review). Oncol. Lett. 22, 530. 10.3892/ol.2021.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, et al. , 2020. Cancer-stromal cell fusion as revealed by fluorescence protein tracking. Prostate 80, 274–283. 10.1002/pros.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. , 2016. SNARE-mediated membrane fusion in autophagy. Semin. Cell Dev. Biol. 60, 97–104. 10.1016/j.semcdb.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier NM, et al. , 2023. Understanding cancer stem cells and plasticity: towards better therapeutics. Eur. J. Cell Biol. 102, 151321 10.1016/j.ejcb.2023.151321. [DOI] [PubMed] [Google Scholar]
- Weiler J, Mohr M, Zänker KS, Dittmar T, 2018. Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α -induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells. Cell Commun. Signal. CCS 16, 14. 10.1186/s12964-018-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Wang J, Liu L, 2016. Chemotherapy promotes tumour cell hybridization in vivo. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37, 5025–5030. 10.1007/s13277-015-4337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TL, et al. , 2017. Up-regulation of syncytin-1 contributes to TNF-α -enhanced fusion between OSCC and HUVECs partly via Wnt/β -catenin-dependent pathway. Sci. Rep. 7 10.1038/srep40983 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Margam NN, 2021. Structural insights into membrane fusion mediated by convergent small fusogens. Cells 10, 160. 10.3390/cells10010160. Number: 1 Publisher: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M and Sever N Journal of Oncological Sciences.2024. [Google Scholar]
- Yuan L, et al. , 2020. Identification of the perpetrator among identical twins using next-generation sequencing technology: a case report. Forensic Sci. Int.: Genet. 44, 102167 10.1016/j.fsigen.2019.102167. [DOI] [PubMed] [Google Scholar]
- Yubero P, Poyatos JF, 2021. Dissecting the fitness costs of complex mutations. Mol. Biol. Evol. 38, 4520–4531. 10.1093/molbev/msab193. ⟨https://academic.oup.com/mbe/article-pdf/38/10/4520/40449379/msab193.pdf⟩. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, et al. , 2014. Mutations in 3’-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene 33, 3947–3958. 10.1038/onc.2013.366 (Publisher: Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- Zack TI, et al. , 2013. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140. 10.1038/ng.2760 (Publisher: Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2022. Cell fusion-related proteins and signaling pathways, and their roles in the development and progression of cancer. Front. Cell Dev. Biol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. , 2014. Generation of cancer stem-like cells through formation of polyploid giant cancer cells. Oncogene 33. 10.1038/onc.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


