Abstract
Objective:
Articular cartilage damage after joint trauma seldom heals and often leads to osteoarthritis (OA). We previously identified a migratory chondrogenic progenitor cell (CPC) population that responded chemotactically to cell death and rapidly repopulated the injured cartilage matrix, which suggested their potential for articular cartilage repair. To test that potential we determined whether SDF-1α, a potent CPC chemoattractant, would improve the quality of cartilage regeneration. We hypothesized that increased recruitment of CPCs by rhSDF-1α would promote the formation of cartilage matrix upon chondrogenic induction.
Methods:
Full-thickness bovine chondral defects were filled with hydrogel comprised of fibrin and hyaluronic acid and containing rhSDF-1α. Cell migration was monitored, followed by chondrogenic induction. Regenerated tissue was evaluated by histology, immunohistochemistry, and scanning electron microscopy. Push-out tests and unconfined compression tests were performed to assess the strength of tissue integration and the mechanical properties of regenerated cartilage.
Results:
rhSDF-1α dramatically improved CPCs recruitment to defects at 12 days. After 6 weeks under chondrogenic conditions, cell morphology, proteoglycan density, and ultrastructure of repair tissue, were similar to native cartilage. Neocartilage generated in rhSDF-1α-containing defects showed significantly greater interfacial strength than controls, and acquired mechanical properties comparable to native cartilage.
Conclusion:
This study showed that stimulating local CPCs recruitment prior to treatment with chondrogenic factors significantly improves the biochemical and mechanical properties of tissues formed in chondral defects. This simple approach may be implemented in vivo as a one-step procedure by staging the release of chemokine and chondrogenic factors from within the hydrogel using smart drug delivery systems.
1. Introduction
Stem cell-based tissue engineering treatments using bone marrow mesenchymal stem cells (BMSCs) [1], as well as adipose stem cells (ASCs) [2] for adult human articular cartilage repair have drawn great attention and been extensively studied [3]. Although substantial success has been achieved with BMSCs, low cell yields, and phenotypic alterations during prolonged in vitro cultivation remain problematic.. Moreover, the chondrogenic activity of BMSCs is age- and osteoarthritis (OA)-dependent. ASCs are more readily acquired than BMSCs, but generate repair tissue with mechanical properties that are inferior to hyaline cartilage. Even in youth, the chondrogenic potential of mesenchymal stem cells may be inferior to native chondrocytes, especially in an in vivo environment without growth factor supplementation and in the presence of pro-inflammatory cytokines. Stem cells may also display a hypertrophic phenotype upon chondrogenic induction, which is undesirable for restoring an articular surface [7]. In addition to MSCs, pluripotent progenitor cells from multiple joint tissues including synovium [4], infrapatellar fat pad [5], and meniscus [6], have shown chondrogenic potential. However, it remains to be seen if any of these strategies consistently regenerate stable hyaline cartilage that is well integrated with the surrounding matrix and biologically and mechanically similar to native cartilage. In addition, risks posed by these cell-based therapies like pathogen transmission and tumorigeneses, as well as complex ethical and regulatory issues, have limited clinical implementation [8, 9].
Articular cartilage tissue engineering by cell homing without cell transplantation is a provocative alternative, which has already achieved notable success [10]. In fact, a serial of studies have identified sub-populations of stem/progenitor cells in articular cartilage as well as from repair tissue of late-stage OA [11–13]. These cells, often referred to as chondrogenic progenitor cells (CPCs), respond to various chemokines and cytokines and migrate towards damaged cartilage tissue [14]. They also exhibit other characteristics of stem/progenitor cells including an apparent potential for repairing cartilage defects [13–15]. CPCs are thought to be a great candidates for regenerative therapy of OA [16], yet no studies have reported articular cartilage regeneration using CPCs.
Stromal cell-derived factor 1-alpha (SDF-1α) is a key chemokine regulating stem cell migration and homing to sites of tissue damage, where they participate in tissue or organ regeneration. SDF-1α exerts its effects through binding to the cell surface receptor, CXCR4 [17, 18]. Recently, Seol and colleagues reported that SDF-1α and CXCR4 expression were highly upregulated in a migratory progenitor cell population found on articular cartilage surfaces within a few days after focal impact [14], and progenitor cells responded vigorously to SDF-1α, which suggests that SDF-1α plays a role in in situ articular cartilage repair by recruiting endogenous stem/progenitor cells.
Fibrin and hyaluronic acid (HA) are classic biomaterials for articular cartilage regeneration. Their unique biocompatibility and highly hydrated structure can mimic natural tissues and deliver biochemical cues [19, 20]. A composite interpenetrating hydrogel network (IPN) composed of fibrin and HA has been shown to exhibit mechanical properties that are far superior to either polymer alone. The excellent cell affinity of fibrin and delayed degradation of HA results in mutually beneficial effects on cartilage extracellular matrix (ECM) synthesis [21].
Here, we attempted to repair full-thickness articular cartilage defects in a bovine osteochondral explant model by first enhancing the recruitment of migratory progenitor cells to IPN using recombinant human SDF-1α (rhSDF-1α) followed by treatments to initiate chondrogenic differentiation. We hypothesized that these sequential manipulations would result in near complete restoration of cartilage matrix within the defect and improved integration with host tissue compared with controls lacking one or both factors.
2. Materials and Methods
2.1. IPN hydrogel fabrication, drug release and biocompatibility
IPN hydrogel consisted of HA-thrombin (solution A) and fibrinogen (solution B). For Solution A, 10 mg/ml hyaluronate (GelOne®, Zimmer Inc., Warsaw, IN) was mixed with same volume of 40 U/ml thrombin (TISSEEL™, Baxter Healthcare Corp., Westlake Village, CA). Solution B was 25 mg/ml fibrinogen (TISSEEL™, Baxter Healthcare Corp.) in Dulbecco’s phosphate-buffered saline (DPBS, pH 7.4) with or without 400 ng/ml (or 200 ng/ml) rhSDF-1α (R&D Systems Inc., Minneapolis, MN). To form IPN, solution A and B were gently mixed together at a ratio of 1:1 at 4 °C. The final concentrations of HA, thrombin, fibrinogen, and rhSDF-1α were 2.5 mg/ml, 10 U/ml, 12.5 mg/ml, and 200ng/ml respectively.
Cylindrical shaped IPN hydrogel disks (2 mm in thickness and 4 mm in diameter) were fabricated in a plastic mold and kept in DPBS for future use. Protein release kinetics of rhSDF-1α was determined according to previous reported protocol [22]. Briefly, each IPN disk was placed in a well of a 24-well plate with 400μl of DPBS, and cultured at 37 °C. Supernatants were collected at each time point (day 2, 4, 6, 8, 10, 12, and 14). DPBS (400 μl) was added to replenish each well and samples were placed back for cultivation until next time point. Enzyme-linked immunosorbent assay (ELISA) was used for quantification according to the manufacturer`s instructions (MyBioSource, San Diego, CA).
To test the biocompatibility of IPN, CPCs were isolated as previously described [14] and were encapsulated in IPN hydrogel disks (5 × 106 cells/ ml) for in vitro viability assay using LIVE/DEAD staining as previously described [23] at different time point (day 1, 7, and 21).
2.2. SDF-1α and its receptor CXCR4 expression
To assess SDF-1α and its receptor CXCR4 expression upon cartilage focal injury, immunofluorescence staining was used for cell surface markers using monoclonal anti-SDF-1α antibody (Abcam, Cambridge, MA) and anti-CXCR-4 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX). A goat anti-mouse fluorescent secondary antibody (Alexafluor 488) was used for fluorescent labeling and detection (Jackson Immunoresearch, West Grove, PA) using confocal microscopy. Staining was performed on monolayer cultured CPCs, normal chondrocytes (NCs), as well as on cryosections of impacted articular cartilage, and non-impact fresh cartilage tissue as previously described [14]. SDF-1α and CXCR4 expression were also compared between CPCs and NCs by real time RT-PCR following previous method [24]. Each real-time PCR experiment was done with at least three replicates, and target gene expression is presented as normalized values to β-actin.
2.3. IPN scaffold implantation, cell migration, and in vitro chondrogenesis
Osteochondral explants (12 mm of diameter and 8–10 mm of thickness) were harvested from the bovine femoral condyle (12–18 months of age, 9 animals in total). After two days pre-equilibrium culture, full-thickness chondral defects (4 mm in diameter and ~2 mm thick) were created as previously described [14], and maintained in culture for overnight before IPN implantation. IPN (~60 ul) with or without rhSDF-1α (100 or 200 ng/ml) was implanted into defects slightly over the surface of the explants, which were then placed back to culture. To monitor cell migration, confocal microscopy was performed essentially as described [15]. Cell numbers were quantified by averaging automated cell counts from 6 random 20X images using ImageJ [25]. DNA content in IPN hydrogel was quantified following previous procedures [15]. Empty IPN gel was used as blank control.
Upon cell migration by day 12, explants were incubated in chondrogenic medium (DMEM containing 10 ng/ml TGF-β1, 100 ng/ml IGF-1, 0.1 μM dexamethasone, 25 μg/ml L-ascorbate, 100 μg/ml pyruvate, 50 mg/ml ITS+ Premix and antibiotics) at 5% CO2, 37 °C for up to 6 weeks. Regenerated tissue together with host cartilage were harvested from explants and analyzed for ECM formation using Safranin-O/fast green staining of either cryosections (3 weeks) or paraffin sections (6 weeks).
2.4. Immunohistochemical, biochemical and ultrastructural evaluation of cartilage repair
For immunohistochemistry analysis, deparaffinized sections from samples of 6 weeks were stained with type II collagen and aggrecan antibodies (Developmental Studies Hybridoma Bank, Iowa City, IA). A goat anti-mouse secondary antibody (Vector Laboratories, Inc., Burlingame, CA) was used for detection. The reaction products were visualized by Vectastain ABC kit and the DAB Peroxidase Substrate Kit (Vector laboratories, Inc.), according to the manufacturer`s instructions. Lubricin, an articular cartilage superficial zone protein, staining was also performed using a rabbit polyclonal antibody, and detected with a goat anti-rabbit secondary antibody (Vector Laboratories, Inc.). All negative controls were performed using same staining without using primary antibodies. Dimethyl methylene blue (DMMB) dye-binding assay was used for quantifying sulfated glycosaminoglycan (sGAG) content as previously described [15].
We also compared the water content between cartilage repair tissue and native cartilage, while blank IPN hydrogel was used as a negative control. All samples were measured for their wet weight with a bench top scale (Mettler-Toledo, LLC, Columbus, OH), as well as dry weight after lyophilization (Lobconco, Kansas City, MO) overnight at −45 °C. Water content was determined by the following calculation; water content = (wet weight - dry weight) / wet weight × 100%. The cartilage tissues were harvested at 6 weeks after chondrogenesis as well as freshly fabricated IPN gel. Scanning electron microscopy (SEM) samples were processed using the previous methods [26], and all SEM was performed at the University of Iowa, Central Microscopy Research Facility (CMRF).
2.5. Biomechanical assessment of tissue repair and material properties of regenerated tissue
In order to evaluate integration (interfacial) strength between repair and host cartilage tissues, we performed “push-out” tests for both SDF-treated groups (n=9) and non-treated groups (n=6) from 6 week-cultured samples. A customized cartilage fixation device rigidly held samples to measure integration strength (Figure 5B). Upon harvesting, the specimens were then placed in the fixation device while a LabVIEW (National Instruments Corporation, Austin, TX) controlled stepper motor (Ultra motion, Cutchogue, NY) depressed a cylindrical indenter (3.8 mm diameter) connected to a load cell (1 Kg Honeywell, 1 KHz sample rate) at a constant velocity of 0.1 mm/s (Figure 5B, dashed inset). The test proceeded through the full-thickness of the tissue, and the integration strength was determined by maximum force record divided by the area of integration.
Figure 5.
Assessment of cartilage tissue integration. (A) Typical macroscopic appearance of repair tissues formed in defects with and without SDF-1a [SDF (+), SDF (−) respectively]. The defect was still clearly visible in the SDF (−) defect (upper left), but not in the SDF (+) defect (lower left). Safranin-O staining showed continuous proteoglycan-rich matrix in repair tissue to with seamless connection host cartilage tissue in SDF (+) defects (lower middle), while SDF (−) defects contained matrix that showed spotty safranin-O staining and poor adhesion to native cartilage (upper middle); in SDF (+) groups, type II collagen showing well-organized strong intensity staining in the entire matrix of the interfacial area (lower right), while in SDF (−) groups (upper right), staining only presented partially at the tissue interface; (B) Apparatus and scheme (dashed inset) for push-out test; (C) Both peak force (p= 0.0004) and stress (p< 0.0001) were significantly higher (>20 fold) in SDF (+) (n= 9) than SDF (−) (n= 6) groups. (D) SEM images showed continuous cells ingrowth from the surface (I) and cross-section at the tissue interface (III), also interconnected extra cellular matrix (II) with entangled collagen fibers (IV). (*) indicates significant difference (P< 0.05).
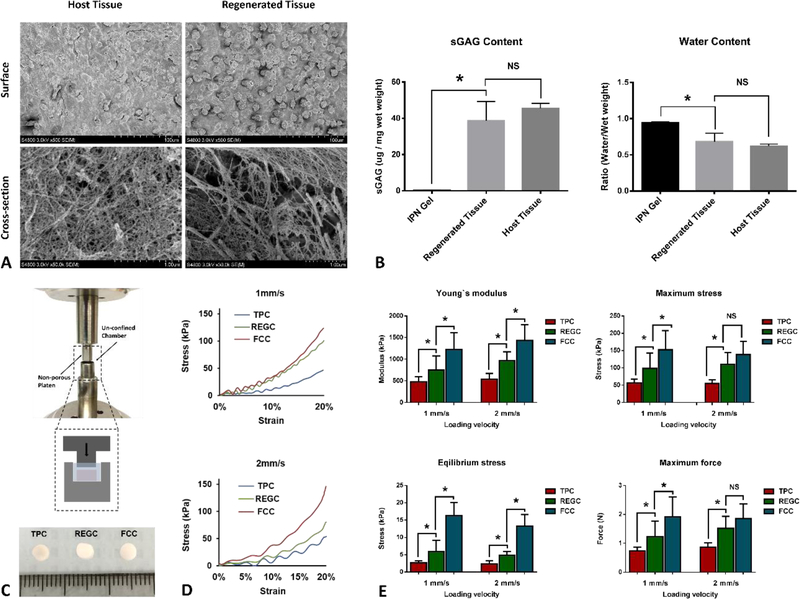
To further characterize the mechanical property of regenerated cartilage tissue, we used a materials testing machine (MTS Systems Corporation, Eden Prairie, MNA) to perform stress-relaxation tests on regenerated cartilage as well as native cartilage tissue harvested from the explants (Figure 6C top). Briefly, cartilage sample` thickness was measured by a laser measurement system (Keyene Corporation of America, Itasca, IL) and placed in an unconfined chamber. A non-porous platen was brought into contact with the tissue surface and the tissue was compressed to 20% strain at 1 mm/s or 2 mm/s velocity. A 10 N load cell recorded the load as compression to 20% strain was held for 20 minutes. Maximum stress, equilibrium stress, Young`s modulus, and maximum force were recorded or calculated. This test was applied to regenerated cartilage (REGC, n= 9) formed in defects filled with IPN contained SDF-1α and were cultured for 6 weeks. Native cartilage samples were harvested from tibial plateau (TPC, n=8) or femoral condyle (FCC, n=8) of healthy bovine knee joint, respectively (Figure 6C bottom).
Figure 6.
Biomechanical characterization of regenerated cartilage tissue. (A) SEM images showing morphology of cells and pattern of ECM fibers of host cartilage and regenerated cartilage tissue; (B) The sGAG content and water content of regenerated cartilage was similar to host cartilage, but differed significantly from empty IPN gel; (C) Apparatus and scheme (dashed inset) for stress-relaxation test, and gross appearance of three different cartilage tissue under test; (D) stress-strain curve for three kinds of tested cartilage tissue under 1 mm/s (upper) and 2 mm/s (lower) loading rate, respectively; (E) maximum force, maximum stress, equilibrium stress and Young`s modulus for TPC, REGC, FCC under 1 mm/s and 2 mm/s loading rate. Data presented are mean ± SD for 8–9 different samples for each group. (*) indicates significant differences (P< 0.05). NS: no significance.
2.6. Statistical analysis
All data are presented as the mean ± SD and were analyzed by GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) using Student`s t-test. P values less than 0.05 were considered significant.
3. Results
3.1. Fabrication and characterization of IPN scaffold
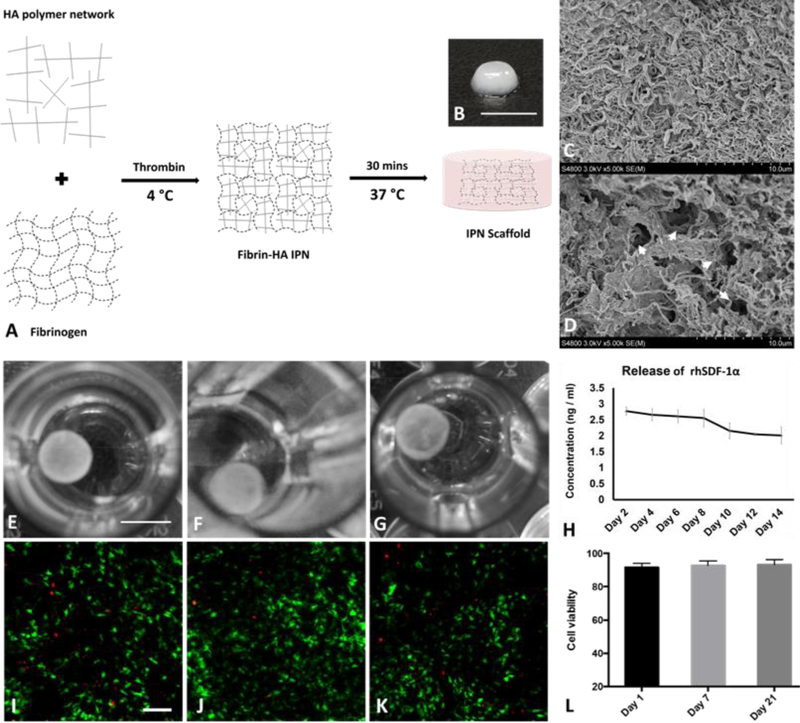
IPN hydrogel can be readily formed by thrombin initiated cross-linking of fibrinogen to become fibrin fibers, and fully polymerized with defined shape under physiological temperature (37 °C) with HA network fully penetrated the pores among fibrin fibers (Figure 1A). After polymerization, the IPN scaffold displayed an opaque appearance, and a well-defined disk shape (Figure 1B). SEM images showed HA network was fully distributed within fibrin fibers with great homogeneity and interconnected pore (arrow heads), both from the surface (Figure 1C) and the cross-section (Figure 1D). This porous structure would allow cells to attach and migrate both along the surface, and within implanted IPN scaffold.
Figure 1.
Fabrication and characterization of IPN hydrogel. (A) A schematic presentation of IPN hydrogel fabrication. Fibrin hydrogel and HA polymer were blended and cross-linked to form interpenetrating polymer network; Macroscopic view of IPN scaffold (B) showed white color and SEM images showed interpenetrated polymer fibers (C) and interconnected pores (D, arrow heads). RhSDF-1α loaded IPN scaffold maintained its integrity in PBS during drug release study for 14 days (E-G); rhSDF-1α protein continued to release from IPN over 14 days (H). Data was presented as mean ± SD (n= 4 for each time point). Encapsulated CPCs were largely viable (green fluorescence) at day 1 (L), 7 (J), (21), with minimal number of dead cells presented (red fluorescence). Average cell viability maintained over 90% for different time points (L). (n= 6 for each time point) Scale bar, B: 5 mm, E-G: 4 mm, and I-K: 500 μm.
IPN scaffold maintained its integrity in PBS as long as 2 weeks without noticeable changes (Figure 1E–G). The time-dependent release curve showed that rhSDF-1α could be released over 14 days (Figure 1H), with daily protein concentration maintained at over 2.0 ng/ml, and still with a continuous releasing trend. CPCs were encapsulated in IPN scaffold to check their biocompatibility in term of cell viability. Confocal images showed minimal number of dead cells (red fluorescence), while most of the cells are viable (green fluorescence) (Figure 1I–K). The initial encapsulation process yielded a cell viability of 91.6 ± 2.4 at day 1, and cell viability continued to maintain in high level (≥ 90%) during 21 days (Figure 1L). These data suggested IPN scaffolds are easy to fabricate, able to support sustained release of rhSDF-1α, and biocompatible.
3.2. SDF-1α/CXCR4 expression and rhSDF-1α guided CPCs migration.
Immunofluorescence staining showed high expression of SDF-1α protein in CPCs with over 90% cells positively stained (Figure 2A, upper right). In contrast, the SDF-1α protein expression was barely detectable in NCs (Figure 2A, upper left). A similar pattern was observed for CXCR4 (Figure 2A, middle). For impacted cartilage, SDF-1α also showed significantly increased expression (Figure 2A, lower right) compared with non-injured freshly isolated cartilage (Figure 2A, lower left) throughout the full depth of the tissue, with stronger expression on the superficial/middle zone (arrow pointing from superficial to deep zone). For RT-PCT, SDF-1α and CXCR4 mRNA expression was 13-fold and 3.5-fold higher in the CPCs compared with NCs, respectively (P = 0.0004).
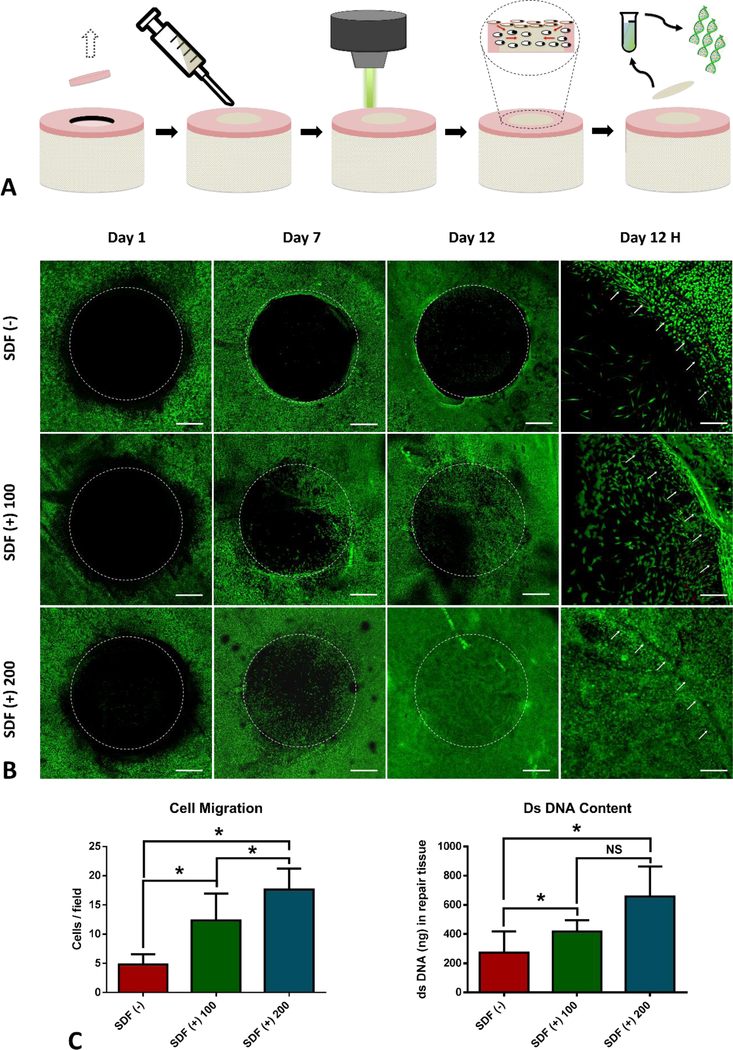
Figure 2.
SDF-1α expression and in vitro cell migration. (A) Monolayer-cultured CPCs were positively stained (red fluorescence) for SDF-1α and CXCR4, while NCs were largely negative for both markers and positive only for nuclear DAPI staining (blue fluorescence); Positive SDF-1α staining was present in impacted cartilage tissue sections, but not in those from healthy un-impacted cartilage; RT-PCT showed profound upregulation of SDF-1α (> 13-fold) and CXCR4 (> 3.5 fold) for CPCs in comparison with NCs. (B) Schematic representation of experimental design. Stacked confocal images from different time points showed that rhSDF-1α initiated dramatic cell migration in comparison with PBS control in a concentration and time dependent manner. (C) Quantification of high magnitude images (Day 12H) confirmed significantly higher (P< 0.0001, n= 8) number of progenitor cells migrated in response to rhSDF-1α, and DNA quantification also suggested much higher (P= 0.0227, n=8) dsDNA content in rhSDF-1α loaded IPN compared with controls. Scale bar, A: 200 μm and C: 500 μm. (*) indicates significant difference (P< 0.05). NS: no significance.
Upon creation of full-thickness articular cartilage defect and implantation of IPN in the absence (DPBS) or presence of rhSDF-1α (100 ng/ml and 200 ng/ml), we monitored cell migration at different time points by confocal microscopy (Figure 2B). As clearly shown in Figure 2C, in explants implanted with rhSDF-1α free IPN, very few cells migrated into the defect area over 12 days, and the migrated cells were mainly at the defect edge, leaving the majority of the defect empty. For explants implanted with rhSDF-1α loaded IPN, significant number of cells migrated from the peripheral area to the center of the defect at day 7 and more cells at day 12. Cell migration also displayed an rhSDF-1α concentration dependent manner, with increased number of migrating cells in higher dose (200 ng/ml) of rhSDF-1α either at day 7 or day 12. Thus, 200 ng/ml rhSDF-1α was used in future studies for full-thickness cartilage repair.
To further quantify the effect of rhSDF-1α on progenitor cells migration, high magnification confocal images from Day 12 (Day 12H) were used for automated cell counting. IPN loaded with rhSDF-1α (200 ng/ml) attracted over 250% (P < 0.0001) as many cells as that in IPN scaffold without rhSDF-1α. Similarly, dsDNA content on day 12 was over 2-fold increase in rhSDF-1α (200 ng/ml) loaded IPN compared with rhSDF-1α free IPN (Figure 2D), while not significantly higher than rhSDF-1α (100 ng/ml) group. These observations suggest that exogenous rhSDF-1α could act as a chemotactic cue for initiation of progenitor cells homing to repopulate full-thickness cartilage defect filled with IPN.
3.3. Histology and immunohistochemistry of repaired cartilage tissue
Histological evaluation of repaired cartilage defects for cartilage ECM production was carried out at the end of 3 weeks and 6 weeks. Three weeks after chondrogenic induction, a substantially higher amount of proteoglycan deposition was observed in rhSDF-1α loaded IPN scaffold with strong positive staining for Safranin-O (Figure 3D) compared with IPN only scaffold, which mainly displayed fast-green staining only (Figure 3A). Stronger Safranin-O staining was observed on the superficial zone of regenerated cartilaginous tissue and gradually decreased to the deep zone (Figure 3E). Most of the migrated cells still displayed a spindle-like morphology (Figure 3C&F), more similar to CPCs than chondrocytes [14]. Six weeks after chondrogenic differentiation, both IPN-only scaffold and rhSDF-1α loaded IPN scaffold showed increased proteoglycan deposition and stronger staining for Safranin-O (Figure G&J) compared with those at 3 weeks. The rhSDF-1α loaded IPN scaffold yielded evenly distributed cells and more intense Safranin-O positive staining for both pericellular and inter-territorial ECM nearly throughout whole depth of regenerated tissue (Figure 3K). In contrast, rhSDF-1α free IPN scaffold showed rather uneven cell distribution with moderately positive safranin-O staining, mainly in the pericellular ECM (Figure 3H). The cells took on a chondrocyte-like spherical morphology,a sign of complete differentiation (Figure 3I&L), with cells in the rhSDF-1α loaded IPN scaffold having more similarity to host chondrocytes (Figure 3L).
Figure 3.
Histological and quantitative analysis for cartilage tissue regeneration. (A-L) Safranin-O/fast green staining of regenerated cartilage tissue sections. Stronger Safranin-O positive staining and more organized proteoglycan deposition presented in rhSDF-1α-treated group at both three weeks (3W) (D-F) and six weeks (6W) (J-L). At 3W, cells displayed the spindle shape characteristic of migrating CPCs in both groups (C&F), while at 6W the cells were more chondrocyte-like (spherical) in shape (I&L). HT indicates host tissue, and RT indicates regenerated tissue. sGAG content was normalized to wet weight (μg/mg), and cell density is reported as cells per field (n= 8 for each group). Scale bar, A, D, G, J: 1 mm; B, E, H, K: 200 μm; C, F, I, L: 50 μm. (*) indicates significant difference (P< 0.05).
Further quantification of sulfated glycosaminoglycan (sGAG) by DMMB assay showed that rhSDF-1α loaded IPN scaffold yielded nearly 8-fold (P= 0.0055) higher sGAG content than rhSDF-1α-free IPN scaffold (Figure 3M left). Moreover, regenerated cartilage tissue from rhSDF-1α loaded IPN scaffold had significantly (P= 0.0242) lower water content than that from rhSDF-1α free IPN scaffold (Figure 3M middle). Quantification of cell density for each high showed over twice (P< 0.0001) as many cells in IPN + rhSDF-1α group as that in IPN only group (Figure 3M right). Interestingly, we observed higher cell density in cartilage repair tissue compared with native cartilage from histology images (Figure 3D and 4J), and cell density in repair tissue gradually decreased from superficial/middle zone to deep zone. This may by attributable to the fact that most CPCs are located in the upper third of the ECM. [14].
Figure 4.
Immunohistochemical examination for articular cartilage-specific proteins. Type II collagen (COL2A; A-C), and aggrecan (AGC; D-F) immunohistochemical staining. Significant staining for rhSDF-1α treated group (C&F) in comparison with IPN only groups with the absence of rhSDF-1α (B&E); zonally organized lubricin staining (LUB; I) in SDF (+) groups, while not in SDF (−) groups (H); SDF (+) groups showed continuous staining for all three proteins between host cartilage and regenerated cartilage tissue, especially at the superficial zone (C, F& I, insets). All negative controls without primary antibodies were only lightly stained (A, D & G). Scale bar, 200 μm and 1 mm (insets).
Immunohistochemistry showed intense positive staining for type II collagen as well as aggrecan throughout the repair tissue from rhSDF-1α loaded IPN, nearly identical to native cartilage tissue (Figure 4C&F). In contrast, repair tissue from rhSDF-1α free IPN displayed uneven and isolated areas of collagen type II and aggrecan staining, which was mainly pericellular and in the superficial zone. (Figure 4B&E). RhSDF-1α-loaded IPN scaffold yielded regenerated tissue with strong positive staining for lubricin in, which was mainly in the superficial zone withrelatively fewer positively-stained cells in the middle and deep zone, largely similar to that in native cartilage (Figure 4I). In contrast, epair tissue from SDF-free IPN only had disordered lubricin staining cluttered within ECM (Figure 4H). A great continuity of all three type of staining across the surface of native tissue and repair tissue was also observed in rhSDF-1α loaded IPN (insets of Figure 4C, F, and I), indicating possible potential of restoring defected articular cartilage surface. All negative controls were only lightly stained for the background (Figure 4A, D, and G).
3.4. Integration of repair tissue with native cartilage
Macroscopic, ultrastructural, and histological analyses of the junction between defect and host tissue at week 6 showed that rhSDF-1α-loaded defects were nearly seamlessly integrated with host cartilage, a milestone of successful repair. Defects without SDF were not well-integrated. Safranin-O/fast green and collagen type II images showed significantly improved repair-host tissue connection upon rhSDF-1α treatment with subsequent chondrogenesis (Figure 5A). Push-out tests showed dramatically different integration strength between SDF (+) and SDF (−) groups. Both stress and peak force were significantly higher in rhSDF-1α treated groups than in untreated control groups (158.0 ± 26.04 kPa vs. 7.56 ± 1.34 kPa; 3.23 ± 0.53 N vs. 0.15 ± 0.03 N, respectively) (Figure 5C). In addition, SEM images of SDF (+) groups showed integration of regenerated tissue with host cartilage both for cell ingrowth and ECM fibers cross-linking. The defect line was largely closed by interconnected ECM fibers from both native and regenerated tissue in SDF (+) group (Figure 5D).
3.5. Biochemical and mechanical properties of regenerated cartilage tissue
We further compared the ultrastructure, sGAG content, water content, and various material properties of regenerated and native cartilage. SEM images showed that cells in regenerated tissue were not as closely connected with the surrounding ECM as cells in host cartilage Also, cell density was relatively higher in regenerated tissue in comparison to native tissue (Figure 6A, upper panel). Collagen fibers formed a less compacted network in regenerated cartilage compared with native cartilage (Figure 6A, lower panel), which may result in differential mechanical properties of two cartilage tissues. DMMB assay showed that sGAG content significantly (P= 0.0016) increased in regenerated tissue in regard to control IPN scaffold, but was not significant different (P= 0.2607) from host cartilage. Similarly, water content was significantly decreased in regenerated tissue compared with control IPN scaffold (P= 0.0016), but was not significantly different from host cartilage.
In terms of mechanical properties (maximum, equilibrium stress, Young`s modulus, and maximum force), regenerated cartilage (REGC) showed higher values than tibial plateau cartilage (TPC), and lower values than femoral condyle cartilage (FCC) at two testing speeds. The properties of IPN-only gel were too low to measure using our current testing system. REGC presented Young`s moduli of 746.7 ± 82.3 kPa (1 mm/s) and 965.4 ± 78.9 (2 mm/s), which were notably higher than that of TPC (475.6 ± 42.9 (1 mm/s) and 542.8 ± 46.1 (2 mm/s), respectively). The Young`s moduli for REGC reached only 70% of the values for FCC. These results indicated that the mechanical properties of REGC measured using physiologic loading rates were well within the range for native bovine cartilage. Notably, REGC showed an increased Young`s modulus with higher loading speed, similar to TPC and FCC.
4. Discussion
The development of novel cartilage repair strategies based on stimulating endogenous cell homing is of substantial clinical interest. In this study, we show for the first time that fullthickness cartilage defects can be repaired entirely by endogenous progenitor cells from articular cartilage, without requiring cells from other sources [22, 27, 28]. Demonstrating that intrinsic cartilage healing potential can be enhanced by a two-step strategy to first initiate progenitor cell chemotaxis with rhSDF-1α, followed by stimulation of chondrogenesis with growth factors.
The expression of SDF-1α and CXCR4 upon cartilage injury supports the involvement of the SDF-1α/CXCR4 axis in migration of CPCs to the site of cartilage defect. SDF-1α also significantly increased progenitor cell migration from surrounding cartilage into IPN scaffolds, clearly demonstrating its ability to direct progenitor cell homing. These results are consistent with a number of published studies [6, 29–32]. Subsequent chondrogenic induction further stimulated type II collagen and aggrecan deposition, resulting in proteoglycan-rich cartilage matrix. The distribution of lubricin staining, which tended to be stronger towards the surface, suggests the potential for regenerating stratified articular cartilage with zone-specific properties. REGC and native cartilage showed great similarities, in terms of sGAG and water content, as well as in terms of ultrastructural collagen fiber alignment and cell-ECM interaction, which are all essential elements needed to support articular cartilage function.
The bonding of engineered cartilage with surrounding native tissue determines integration strength [33]. Our study showed that rhSDF-1α dramatically increased integration strength, over SDF (−) controls. The average value for REGC of 158.0 ± 26.04 kPa was more than three times higher than that reported in comparable studies [34–36]. This may indicate that increased CPC migration enhanced tissue integration, which is consistent with the results of Lu et al. showing that cell migration at the interface of engineered cartilage and surrounding cartilage could result in dramatically stronger host-graft tissue integration after autologous chondrocyte implantation [37]. It is also worth noting that the collagen fiber networks of the regenerated and host tissues in the fully treated defects were extensively entangled with each other, which might explain the gain in integration strength.
Regeneration of mechanically functional cartilage tissue is key to the success of any cartilage repair strategy. Although engineering cartilage with primary chondrocytes has reached physiological equivalence with native cartilage for compressive moduli, cartilage engineered from stem/progenitor cells have achieved no more than 50% of that value. In our study, the Young`s moduli of tissue formed in large full-thickness chondral defects exceeded those of tibial plateau cartilage and rivaled those of femoral condyle cartilage. Moreover, this was accomplished in a relatively short time compared to other studies.. Further improvement of mechanical performance may require loading stimulation, which has been shown to enhance Young`s modulus of engineered cartilage [38]. For in vivo translation, the IPN gel may not be able to withstand initial mechanical stresses like repetitive loading, thus certain immobilization procedures may be needed during the early stages of neocartilage development, after which physiological loading would be beneficial for further maturation.
Although the results are promising, there are certainly limitations within this study. The healthy young cows may have superior regenerative capacity compared with aged animals. This may limit the translatability of our strategy, especially for aged OA patients, since CPCs from OA patients may have limited chondrogenic potential either due to altered phenotype, or to the unfriendly environment they reside in. Various inflammatory factors, like IL-1β, TNF-α, nitric oxide (NO), etc. could also inhibit the migration activity of CPCs in OA [39]. More strategies could be developed to not only incorporate chemotactic factors for cell homing, but to modify scaffolds by introducing anti-inflammatory agents, which would certainly have profound benefits for cartilage neo-genesis. In terms of in vivo translation, approaches of efficient delivery and retention of these factors at sites of damage will need to be carefully designed. This can be achieved encapsulating chemokines, growth factors [40] or genetic materials [41], within polymer microspheres to achieve sustained, or multi-phase release from the scaffold.
5. Conclusion
We have developed a cartilage repair strategy that exploits the regenerative potential of endogenous chondrogenic progenitor cells. The matrix formed by these cells is similar in composition to native cartilage and strongly adheres to surrounding tissues. Regenerated cartilage tissue possesses mechanical properties within the physiological range for functional native cartilage. Optimization of this strategy could lead to a new minimally invasive, single-step procedure for cartilage repair.
Acknowledgements:
This work was supported by the Department of Defense (W81XWH-10–1-0702a) and by a grant from the American Arthritis Society. The authors would like to thank Mr. Jianqiang Shao from CMRF of University of Iowa for the help with SEM imaging and Dr. Anneliese Heiner from Orthopaedic Biomechanics Laboratory of University of Iowa for the help with mechanical testing.
Appendix 1: Abbreviations, Acronyms and Symbols
- OA
Osteoarthritis
- CPCs
Chondrogenic progenitor cells
- NCs
Normal chondrocytes
- rhSDF-1α
recombinant human stromal cell-derived factor 1 alpha
- BMSCs
Bone marrow mesenchymal stem cells
- ASCs
Adipose stem cells
- CXCR4
chemokine (C-X-C motif) receptor 4
- HA
Hyaluronic acid
- IPN
Interpenetrating polymer network
- ECM
Extra cellular matrix
- DPBS
Dulbecco`s phosphate-buffered saline
- ELISA
Enzyme-linked immunosorbent assay
- RT-PCR
Reverse transcription polymerase chain reaction
- DMEM
Dulbecco`s Modified Eagle Medium
- DNA
Deoxyribonucleic Acid
- TGF-β1
Transforming growth factor beta 1
- IGF-1
Insulin-like growth factor 1
- DMMB
Dimethylmethylene
- sGAG
Sulphated glycosaminoglycans
- SEM
Scanning electron microscopy
- REGC
Regenerated cartilage
- TPC
Tibial plateau cartilage
- FCC
Femur condyle cartilage
- mRNA
Messenger ribonucleic acid
- COL2A
Type II collagen
- AGC
Aggrecan
- LUB
lubricin
- iPSCs
Induced pluripotent stem cells
- BMP
bone morphogenetic protein
- TKA
Total knee arthroplasty
- TNF-α
Tumor necrosis factor alpha
- IL-1β
Interleukin 1 beta
- NO
Nitric oxide
- MMP
Matrix metalloprotease
6. References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 2.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, Mauck RL: High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta biomaterialia 2012, 8(8):3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuan RS: Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis and rheumatism 2006, 54(10):3075–3078. [DOI] [PubMed] [Google Scholar]
- 4.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP: Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and rheumatism 2001, 44(8):1928–1942. [DOI] [PubMed] [Google Scholar]
- 5.Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F: Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clinical orthopaedics and related research 2003(412):196–212. [DOI] [PubMed] [Google Scholar]
- 6.Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, Heng BC, Yin Z, Chen X, Ji J et al. : Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem cells translational medicine 2014, 3(3):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE et al. : Tissue engineering for articular cartilage repair--the state of the art. European cells & materials 2013, 25:248–267. [DOI] [PubMed] [Google Scholar]
- 8.Fodor WL: Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate. Reproductive biology and endocrinology : RB&E 2003, 1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prockop DJ: Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Molecular therapy : the journal of the American Society of Gene Therapy 2009, 17(6):939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ: Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 2010, 376(9739):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B et al. : The surface of articular cartilage contains a progenitor cell population. Journal of cell science 2004, 117(Pt 6):889–897. [DOI] [PubMed] [Google Scholar]
- 12.Alsalameh S, Amin R, Gemba T, Lotz M: Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis and rheumatism 2004, 50(5):1522–1532. [DOI] [PubMed] [Google Scholar]
- 13.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N: Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell stem cell 2009, 4(4):324–335. [DOI] [PubMed] [Google Scholar]
- 14.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA et al. : Chondrogenic progenitor cells respond to cartilage injury. Arthritis and rheumatism 2012, 64(11):3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seol D, Yu Y, Choe H, Jang K, Brouillette MJ, Zheng H, Lim TH, Buckwalter JA, Martin JA: Effect of short-term enzymatic treatment on cell migration and cartilage regeneration: in vitro organ culture of bovine articular cartilage. Tissue engineering Part A 2014, 20(13–14):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caroline N: Chondrogenic progenitors for cartilage repair and osteoarthritis treatment. Rheumatology: Current Research 2012. [Google Scholar]
- 17.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S et al. : Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001, 97(11):3354–3360. [DOI] [PubMed] [Google Scholar]
- 18.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M: Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature medicine 2000, 6(11):1229–1234. [DOI] [PubMed] [Google Scholar]
- 19.Klein TJ, Rizzi SC, Reichert JC, Georgi N, Malda J, Schuurman W, Crawford RW, Hutmacher DW: Strategies for zonal cartilage repair using hydrogels. Macromolecular bioscience 2009, 9(11):1049–1058. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA: Hydrogels in regenerative medicine. Adv Mater 2009, 21(32–33):3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rampichova M, Filova E, Varga F, Lytvynets A, Prosecka E, Kolacna L, Motlik J, Necas A, Vajner L, Uhlik J et al. : Fibrin/hyaluronic acid composite hydrogels as appropriate scaffolds for in vivo artificial cartilage implantation. ASAIO J 2010, 56(6):563–568. [DOI] [PubMed] [Google Scholar]
- 22.Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A: Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue engineering Part A 2012, 18(9–10):934–945. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Zhang Y, Martin JA, Ozbolat IT: Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. Journal of biomechanical engineering 2013, 135(9):91011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seol D, Choe H, Zheng H, Jang K, Ramakrishnan PS, Lim TH, Martin JA: Selection of reference genes for normalization of quantitative real-time PCR in organ culture of the rat and rabbit intervertebral disc. BMC research notes 2011, 4:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nature methods 2012, 9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swords WE, Chance DL, Cohn LA, Shao J, Apicella MA, Smith AL: Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infection and immunity 2002, 70(8):4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Chen JL, Tao JD, Jiang YZ, Hu CC, Huang L, Ji JF, Ouyang HW: The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials 2013, 34(3):713–723. [DOI] [PubMed] [Google Scholar]
- 28.Mendelson A, Frank E, Allred C, Jones E, Chen M, Zhao W, Mao JJ: Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011, 25(10):3496–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L: The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31(14):3997–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schantz JT, Chim H, Whiteman M: Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue engineering 2007, 13(11):2615–2624. [DOI] [PubMed] [Google Scholar]
- 31.Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, Ouyang HW: The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31(28):7239–7249. [DOI] [PubMed] [Google Scholar]
- 32.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T: Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis and rheumatism 2009, 60(3):813–823. [DOI] [PubMed] [Google Scholar]
- 33.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G: Integration of engineered cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2001, 19(6):1089–1097. [DOI] [PubMed] [Google Scholar]
- 34.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F: Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proceedings of the National Academy of Sciences 2012, 109(47):19172–19177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam HK, Srivastava A, Colwell CW Jr., D’Lima DD: In vitro model of full-thickness cartilage defect healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2007, 25(9):1136–1144. [DOI] [PubMed] [Google Scholar]
- 36.Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA: Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clinical orthopaedics and related research 2011, 469(10):2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Xu Y, Yin Z, Yang X, Jiang Y, Gui J: Chondrocyte migration affects tissue-engineered cartilage integration by activating the signal transduction pathways involving Src, PLCgamma1, and ERK1/2. Tissue engineering Part A 2013, 19(21–22):2506–2516. [DOI] [PubMed] [Google Scholar]
- 38.Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT: Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Engineering Part A 2010, 16(5):1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE: Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis research & therapy 2013, 15(5):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eswaramoorthy R, Chang CC, Wu SC, Wang GJ, Chang JK, Ho ML: Sustained release of PTH(1–34) from PLGA microspheres suppresses osteoarthritis progression in rats. Acta biomaterialia 2012, 8(6):2254–2262. [DOI] [PubMed] [Google Scholar]
- 41.Ha CW, Noh MJ, Choi KB, Lee KH: Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012, 14(2):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]