Abstract
Cuproptosis differs from other forms of cell death, such as apoptosis, necroptosis, and ferroptosis, in its unique molecular mechanisms and signaling pathways. In this review, we delve into the cellular metabolic pathways of copper, highlighting the role of copper in biomolecule synthesis, mitochondrial respiration, and antioxidant defense. Furthermore, we elucidate the relationship between cuproptosis-related genes (CRGs) and cancer prognosis, analyzing their expression patterns across various tumor types and their impact on patient outcomes. Our review also uncovers the potential therapeutic applications of copper chelators, copper ionophores, and copper-based nanomaterials in oncology. In addition, we discuss the emerging role of cuproptosis in remodeling the tumor microenvironment, enhancing immune cell infiltration, and converting “cold tumors” into “hot tumors” that respond better to immunotherapy. In short, this review underscores the pivotal importance of cuproptosis in cancer biology and highlights its translational potential as a novel therapeutic target.
Keywords: Cuproptosis, Copper metabolism, Cancer prognosis, Immunotherapy
Introduction
In recent years, the scientific community has witnessed a surge of interest in elucidating the diverse mechanisms underlying cell death, a fundamental biological process essential for tissue homeostasis and disease progression. Among the various modalities of programmed cell death, cuproptosis has emerged as a novel and intriguing concept, distinct from well-established pathways such as apoptosis, necroptosis, and ferroptosis. The discovery of cuproptosis by Tsvetkov et al. in 2022 highlights the complex interplay between copper ions and cellular metabolism, uncovering a new dimension in our understanding of cell demise.
Copper, an essential trace element in the human body, plays crucial roles in biomolecule synthesis, mitochondrial respiration, and antioxidant defense. However, excess copper can disrupt cellular homeostasis, leading to the induction of cuproptosis. This form of cell death is characterized by the aggregation of lipoylated proteins in the tricarboxylic acid (TCA) cycle, resulting in proteotoxic stress and ultimately triggering mitochondrial dysfunction and cell death.
The discovery of cuproptosis has significant implications for cancer biology and therapeutics. Cancer cells, known to have a higher copper dependency compared to normal cells, may be particularly susceptible to cuproptosis-inducing agents. This finding underscores the potential of targeting copper metabolism as a novel therapeutic strategy for cancer treatment.
In this review, we aim to provide a comprehensive overview of cuproptosis, delving into its molecular mechanisms, signaling pathways, and relationship with cancer prognosis. Furthermore, we will discuss the emerging therapeutic applications of cuproptosis, focusing on copper chelators, copper ionophores, and copper-based nanomaterials that have shown promise in inducing cuproptosis and inhibiting tumor growth. By elucidating the underlying biology of cuproptosis and its potential therapeutic value, we hope to stimulate further research in this exciting field and contribute to the development of innovative cancer treatments [1,2].
Molecular mechanisms of copper
Copper homeostasis and transport
Copper is an essential trace element required by nearly all living organisms, predominantly sourced from solid food and drinking water. Serving as a crucial cofactor, copper is naturally abundant in organ meats, shellfish, seeds, legumes, vegetables, and whole grains. Additionally, industrial products can influence copper intake [3]. The human body contains an estimated 100 to 150 milligrams of copper, distributed across various tissues, including the brain, skin, and others, with predominant concentrations in the liver, muscles, and bones [4].
The distribution of copper within the body occurs in two distinct phases [Owen, 1971]. During Phase I, albumin and transferrin facilitate the transportation of copper to the liver and kidneys. Subsequently, in Phase II, ceruloplasmin is responsible for distributing copper from the liver to other tissues and organs. The liver effectively eliminates excess copper through biliary excretion or by releasing metal ions into the feces. Other excretory routes, such as urine, sweat, and menstrual fluid, contribute minimally to copper excretion. Consequently, systemic copper homeostasis is primarily maintained through duodenal absorption and biliary excretion [5](Fig. 1).
Fig. 1.
Copper uptake and transport in the human body
Copper absorption, distribution, and excretion
The human body primarily absorbs copper in the duodenum and small intestine, facilitated mainly by the Cu transport protein 1 (CTR1), which is positioned at the apical membrane of intestinal epithelial cells [6]. This uptake process is also associated with the reduction of divalent Cu2+ to monovalent Cu+ by enzymes such as six-transmembrane epithelial antigen of prostate (STEAP) and duodenal cytochrome b(DCYTB) [7]. The CTR1 protein exhibits a conserved three-domain topology akin to members of the SLC31 family, featuring an amino-terminal exo-structural domain rich in Cu(I)-binding histidine and methionine sequences, along with three transmembrane domains. Biochemical studies reveal that SLC31 family members assemble into functional trimers, each comprising nine transmembrane domains, enabling the selective inward flow of Cu(I) along concentration gradients [8].
Copper-transporting ATPases, ATP7A and ATP7B, are membrane-bound proteins in human cells that play a pivotal role in digestion. ATP7A may aid in the exocytosis of Cu+ from the intestinal epithelium and its subsequent transport into the circulation, while ATP7B stores copper within intracellular vesicles to uphold intestinal epithelial homeostasis [9]. ATP7A is expressed in most tissues, with the liver being an exception, whereas ATP7B is exclusively present in hepatocytes, responsible for pumping Cu+ from the cytoplasm into the trans-Golgi network (TGN). When hepatocytes encounter excess copper, endolysosomal vesicles containing ATP7B transport it to the bile ducts, excreting the surplus copper ions into the bile [10]. Mutations in ATP7A are associated with copper metabolism disorders, leading to intracellular Cu+ accumulation in Menkes’ and Wilson’s diseases, while mutations in ATP7B are also related to Wilson’s disease [11]. In the bloodstream, copper ions are transported bound to proteins rather than in a free state. Ceruloplasmin (CP) binds approximately 75% of copper ions in a non-exchangeable manner, with each CP molecule binding six Cu+ ions. Human serum albumin (HSA) binds approximately 25% of copper ions exchangeably, and about 0.2% of the copper ions are bound to histidine [12].
A copper ion (Cu+) enters hepatocytes through CTR1, and within the mitochondria, it binds to cytochrome c oxidase (CCO), an enzyme involved in both the respiratory chain and redox reactions. The transport of Cu + to the mitochondria, and subsequently to CCO, is facilitated by copper chaperone proteins (COX17, COX19, and COX23) through the mitochondrial inner membrane proteins SCO1, SCO2, and COX11 [13].
The primary dependence for the transport of copper ions into mitochondria lies on COX17, also known as the cytochrome c oxidase copper chaperone [14]. As Cu(I) is shuttled from the cytoplasm to the mitochondrial membrane proteins SCO1 and SCO2, it is COX17 that facilitates the insertion of copper into MT-CO2/COX2, which is the mitochondrial-encoded cytochrome C oxidase II [15].
In addition to transporting copper from the cytoplasm to COX11, COX17 also aids in its transfer to the mitochondrial subunit of cytochrome c oxidase, namely MT-CO1/COX1. Complex IV comprises two copper-binding subunits, MT-CO1 and MT-CO2, which are responsible for transferring electrons from CYCS (somatic cytochrome c) and driving the electrochemical synthesis of ATP. Consequently, mitochondrial oxidative phosphorylation is intimately linked to the cellular copper pool. MTCO1 and MTCO2 serve as vital components of the mitochondrial respiratory chain, and COX17 plays a pivotal role in tumor growth, invasion, and metastasis. Hence, cancers originating from solid or hematologic tissues may potentially benefit from therapeutic interventions targeting COX17 [16].
ATOX1 transports copper to the trans-Golgi network (TGN) and facilitates the synthesis of copper-dependent enzymes, including lysine oxidase, tyrosinase, and ceruloplasmin [17]. ATOX1 can transport Cu+ into superoxide dismutase (SOD) to mitigate oxidative stress [18]. Within the nucleus, Cu+ has the ability to bind to transcription factors, thereby modulating gene expression (Figs. 2 and 3).
Fig. 2.
Mechanism of action of copper death in the mitochondria
Fig. 3.
Mechanism of action of copper death
Physiological functions of copper
Copper exhibits numerous functions at the physiological level, capitalizing on its capacity to exist in two distinct redox states: the oxidized state Cu(II) and the reduced state Cu(I) [19]. It serves as a catalytic cofactor in redox chemistry, participating in the regulation of various enzymatic activities. Some examples of these enzymes include superoxide dismutase, an antioxidant enzyme; phosphodiesterase 3B (PDE3B), which facilitates lipolysis; ferric oxide lyase, crucial for iron absorption; DBHdopamine hydroxylase (DBH), which converts dopamine into norepinephrine; and lysyl oxidase (LOX), which is essential for tissue regeneration and repair [20].
Maintaining copper homeostasis is crucial for cell survival due to the instability of copper’s redox potential. It is imperative to strictly regulate systemic copper levels to ensure optimal biochemical functioning.
Cuproptosis and other cell death pathways
Various physiological activities can lead to cell death, which is influenced by the cell type. The lifespan of cells varies, ranging from a few days to several years, depending on their specific type. In certain circumstances, cells can initiate immune responses and regulate their own life cycle. Regulatory cell death (RCD), also referred to as programmed cell death (PCD), exhibits distinct biochemical, morphological, and immunological features. Within the body, RCD plays a vital role in maintaining homeostasis and contributing to the progression of diseases [21]. Malignant tumor cells can evolve multiple mechanisms to escape these RCD pathways during treatment, so it is important to find a new RCD pathway.
Cuproptosis
Background and significance of cuproptosis
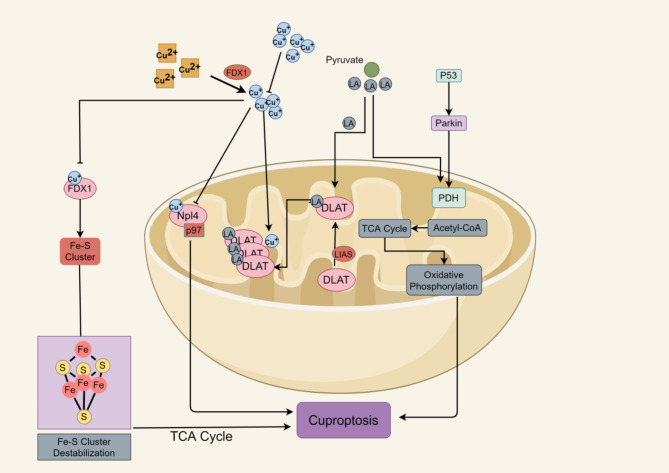
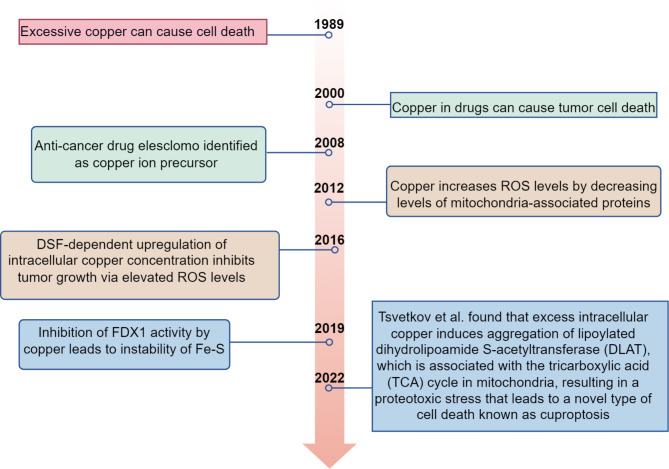
In 2019, Tsvetkov et al. described a form of Cu-dependent cell death in their investigation of eresclomol’s anticancer mechanism, where eresclomol serves as a Cu ion carrier [22]. In 2022, Tsvetkov et al. coined the term “Cuprotosis” to describe the unique copper-dependent cell death mechanism induced by eresclomol, further elucidating its underlying mechanism [23].This is a new RCD pattern with a copper-dependence. Copper-induced programmed cell death distinguishes itself from other cell death mechanisms triggered by oxidative stress, such as apoptosis, ferroptosis, and necroptosis. By directly binding to the fatty acylated portion of TCA cycle, copper initiates a death pathway that is independent of apoptosis, leading to the aggregation of fatty acylated proteins and the destabilization of Fe-S cluster proteins [22]. The distinctive mechanism of eresclomol indicates that it can both hinder the natural function of FDX1, a crucial element in the Fe-S cluster assembly pathway, and, when combined with copper, function as a novel substrate to induce a unique type of Cu-dependent cell death. Cuprotosis is a type of mitochondrial cell death characterized by the disruption of intracellular copper homeostasis. It exhibits several morphological features, including mitochondrial atrophy, cell membrane rupture, endoplasmic reticulum damage, and chromatin damage. When cuprotosis occurs, it impairs mitochondrial respiration and protein lipidation, resulting in membrane permeabilization, cellular destruction, and the activation of suicidal pathways [24] (Fig. 4).This form of cell death is dependent on disrupted mitochondrial respiration and the subsequent stress on mitochondrial proteins, rather than mitochondrial oxidative stress. Consequently, Cu-dependent cell death is mitigated by inhibitors of respiratory chain complexes I and III, specifically rotenone and antimycin A respectively, as well as by an inhibitor of the mitochondrial pyruvate transporter protein, UK5099 [16].
Fig. 4.
Time line of cuproptosis study
Molecular mechanisms of cuproptosis
To date, ten key genes associated with cuprotosis have been identified. These include positive regulators, such as ferredoxin 1 (FDX1), lipoic acid synthase (LIAS), lipotransferase 1 (LIPT1), dihydrolipoamide dehydrogenase (DLD), thiolipoamide S-acetyltransferase (DLAT), pyruvate dehydrogenase E1 subunit α 1 (PDHA1), and pyruvate dehydrogenase E1 subunit β (PDHB), as well as negative regulators, like metal-responsive transcription factor-1 (MTF1), glutaminase (GLS), and cyclin-dependent kinase inhibitor 2 A (CDKN2A) [25]. There are seven genes that safeguard cells against copper overload and mitochondrial dysfunction: LIAS, LIPT1, DLD, DLAT, PDHA1, PDHB, and FDX1. Conversely, three genes—MTF1, GLS, and CDKN2A—promote the proliferation of cells induced by copper [26]. Additionally, two copper transporter proteins play pivotal roles: ATP7B, which exports copper, and SLC31A1, which imports copper. Research indicates that genomic alterations and the miRNA-mRNA network are accountable for the ectopic expression of cuproptosis genes. These genes interact with other cancer-related pathways, suggesting that kidney renal clear cell carcinoma (KIRC) could be a potential cancer type influenced by cuproptosis [27].
The copper reductase enzyme encoded by FDX1 converts Cu2+ into Cu+, a form that is more cytotoxic. FDX1 positively regulates a number of metabolic pathways associated with cuproptosis. Both FDX1 and protein lipoylation are essential for copper ion carrier-induced cell death. FDX1 is responsible for protein thiolation, and when protein thiolation is absent, FDX1 becomes depleted. In four enzymes that regulate TCA cycle, thiolation represents a highly conserved post-translational modification of lysine. By binding directly to copper-dependent enzymes involved in the TCA cycle, inhibiting FDX1 function can prevent cellular cuproptosis [28–30].
Importantly, FDX1 is considered a central modulator in cuproptosis due to the fact that its absence leads to a total loss of protein lipoylation, a substantial decrease in cellular respiration, accumulation of pyruvate and α-ketoglutarate, reduction of succinate, and stabilization of Fe-S cluster proteins. In summary, cuproptosis arises from an excess of copper that aggregates lipidated TCA cycle proteins and destabilizes Fe-S cluster proteins, both processes being mediated by FDX1 [31].
Ferroptosis
In comparison to ferroptosis, cuproptosis remains relatively less understood. Although both processes are associated with mitochondria, the toxic mechanisms of copper in cells differ from those of other known regulated cell death mechanisms.
Ferroptosis is a form of regulated cell death that is dependent on iron, influenced by molecular pathways involving lipid peroxidation resulting from intracellular iron accumulation and inhibition of glutathione (GSH) synthesis [32,33]. To initiate this process, two main pathways must be targeted: the exogenous pathway, which encompasses SLC7A11, SLC38A1, NOXs, and TFRC; and the endogenous or enzyme-regulated pathway, which involves components such as ACSL4, ALOXs, GPX4, POR, GCH1, NOS, and AIFM2, as well as systems like TFRC [34]. A novel marker of ferroptosis, hyperoxidized PRDX3, has been identified by Jin’s team [35].
GPX4, the central suppressor of ferroptosis, is not the sole factor responsible for controlling lipid reactive oxygen species (ROS). In recent years, three systems have been uncovered that inhibit ferroptosis independently of GPX4. One such mechanism is Iron-dependent apoptosis suppressor protein-1 (FSP1), which inhibits ferroptosis by utilizing extra-mitochondrial ubiquitin or exogenous vitamin K and NAD(P)H/H+ as electron donors [36]. The FSP1 gene is regulated by upstream factors, including transcription factors and non-coding RNAs (ncRNAs), as well as epigenetic modifications that influence disease progression [37]. Furthermore, dihydroorotate dehydrogenase (DHODH) and GCH1/BH4 have been identified as independent regulators of ferroptosis, functioning without the involvement of GPX4 [38].
Due to ferroptosis, cells do not display the typical characteristics of apoptosis, such as chromatin condensation and the formation of apoptotic bodies. Rather, the morphological hallmarks of ferroptosis encompass a decrease in mitochondrial volume, rupture of the mitochondrial outer membrane, reduced or absent mitochondrial cristae, and a nucleus of normal size without condensation, setting it apart from other forms of cell death [39–41].
Ferroptosis is associated with various human diseases, including neurodegenerative disorders, diabetic nephropathy, cardiovascular diseases, and myocardial conditions. In animal models of traumatic brain injury (TBI), ferroptosis has been linked to acute central nervous system (CNS) damage, as demonstrated by findings related to glutathione peroxidase activity, lipid-reactive oxygen species, and mitochondrial atrophy [42]. Certain cancer cells are particularly prone to ferroptosis due to their unique metabolism, elevated levels of reactive oxygen species (ROS), and specific mutations. Drugs that have been approved by the FDA have demonstrated the ability to induce ferroptosis, sparking considerable interest as a potential new strategy for treating drug-resistant cancer [43,44]. Various tumor proteins, tumor suppressors like p53 and BRCA1-associated protein 1 (BAP1), and alterations in oncogenic signaling pathways within cancer cells may act as predictive biomarkers for the response to therapies that induce ferroptosis. Furthermore, ferroptosis-inducing therapies can be combined with other treatment modalities, such as immune checkpoint blockade and radiotherapy. The combination of immune checkpoint blockade with other therapeutic strategies is an area of ongoing research [45].
Cuproptosis differs from ferroptosis and other established cell death modalities
Recent decades have witnessed numerous studies revealing the intricate connection between cuproptosis, reactive oxygen species (ROS), and inflammation. Furthermore, cuproptosis has been shown to precede other forms of cell death, including apoptosis, pyroptosis, and ferroptosis [7].
It is worth noting that well-established modes of cell death, such as necroptosis (for instance, induced by necrostatin-1), apoptosis (inhibited by Z-VAD-FMK), oxidative stress (mitigated by N-acetylcysteine), and ferroptosis (inhibited by ferrostatin-1), have all failed to suppress cell death triggered by eresclomol [46]. Conversely, a copper chelator has demonstrated the ability to effectively inhibit cuproptosis, distinguishing it from other types of cell death [47] (Table 1).
Table 1.
A comparative analysis of cell death mechanism
| Differentiation | Contributing factor | Key indicators | Morphological changes | Signaling pathway | Virulence | References |
|---|---|---|---|---|---|---|
| Cuproptosis | Intracellular copper accumulation |
Copper, a-Ketoglutaric, acidpyruvate,FDX1, DLAT, LIAS, |
Mitochondrial atrophy, cell membrane rupture, endoplasmic reticulum and chromatin damage | Lipoic acid pathway | Neurodegenerative diseases (AD, Wilson’s disease), STAD), hepatocellular carcinoma (HCC), and squamous carcinoma of the head and neck (HNSC) | [23–31,48–50] |
| Ferroptosis | Iron ion accumulation |
iron, MDA, ROS, LPO, GPX4, SLC7A1,ALOXs, FSP1,p53,BAP1 |
Reduced mitochondrial volume, breaks in the outer mitochondrial membrane, reduced or absent mitochondrial cristae, normal nuclear size, no nuclear concentration | Iron death inhibitor GPX4 and FSP1/E-cadherin-NF2- Hippo-YAP signaling pathway/AMPK (AMP-dependent protein kinase) signaling pathway | Neurodegenerative diseases, diabetic nephropathy, myocardial diseases, cardiovascular diseases, cancer | [51–55] |
| Pyroptosis | A programmed cell death caused by inflammatory vesicles | Caspase-1, pro-IL-1β,pro-IL-18,IL-1β,IL-18 | Cell swelling, pyknotic body formation, cell membrane rupture, DNA fragmentation, intact nucleus |
Classical caspasel-dependent pathways Caspase1/GSDM Non-classical pathway dependent on caspase4/5/11: caspase4/5/1/GSDM Performer: GSDM membrane perforation |
Infectious diseases, autoimmune diseases, cancer and neurodegenerative diseases | [56,57] |
| Necroptosis | Performed by the receptor-interacting protein kinase 1 (RIPK1)-RIPK3 mixed-spectrum kinase structural domain-like protein (MLKL) signaling cascade | Death receptor, FADD, caspase-8,MLKL, RIPK3 | Plasma membrane permeability, cell swelling, and loss of cell and organelle integrity |
TNF/TNFRI-TRADD/RIPI/RIP3-MLKL Performer: MLKL Perforation on membrane |
Atherosclerosis, CNS disease, inflammation, cancer, AD | [58–64] |
Molecular links between cuproptosis and cancer biology
Cuproptosis-related genes (CRGs) are upregulated in the majority of cancers. Higher expression levels of most CRGs are associated with better outcomes in renal cell carcinoma (KIRC), renal pelvis carcinoma (KIRP), lower-grade glioma (LGG), mesothelioma (MESO), and penile squamous cell carcinoma (PCPG). However, patients with adrenocortical cancer (ACC), liver hepatocellular carcinoma (LIHC), and endometrial carcinoma (UCEC) tend to have a poorer prognosis when CRGs are highly expressed. Pathway analysis has revealed that cuproptosis regulators are linked to metabolism-related pathways. Across nearly all tumor types, the expression levels of MTF1, NLRP3, and SLC31A1 exhibit a positive correlation with immune and stromal scores, including ImmuneScore, StromalScore, and ESTIMATEScore. Conversely, the expression of ATP7B, DLAT, DLD, LIAS, PDHA1, and PDHB is significantly negatively correlated with these scores. Additionally, a notable correlation has been observed between CRGs and various scores related to RNA stemness, DNA stemness, microsatellite instability, and tumor mutational burden [64]. An increasing amount of literature supports the role of CRGs as biomarkers for diseases such as HCC and gastric adenocarcinoma (STAD) [65]. Tumor tissues require higher levels of copper compared to healthy tissue. Therefore, we can assume that copper ions can influence tumor progression and occurrence. This newly discovered cell death pathway may have a crucial role in tumor therapy.
Key proteins involved in cuproptosis: FDX1
Evidence suggests that low expression of FDX1 is linked to a poor prognosis, as well as an increased risk of cancer development and metastasis. FDX1 plays a role in regulating lipidated proteins during cuproptosis, a process that has been implicated in the progression and metastasis of various cancer types. A comprehensive analysis across multiple cancers indicates that FDX1 is underexpressed in most tumors, including breast cancer, renal chromophobe cell carcinoma, and colorectal carcinoma. Furthermore, proteomic analysis of tumor samples has revealed differential expression of FDX1, with reduced levels observed in cancers such as colon adenocarcinoma (COAD), glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), lung adenocarcinoma (LUAD), and pancreatic adenocarcinoma (PAAD) compared to normal tissues [66,67].
Cox regression analysis reveals a positive correlation between FDX1 expression and the prognosis of adrenocortical carcinoma (ACC) as well as low-grade glioma (LGG). Conversely, it shows a negative correlation with the prognosis of COAD, hepatocellular carcinoma (LIHC), and thyroid carcinoma (THCA) [62–63]. There is supportive evidence indicating that elevated FDX1 expression facilitates cell death in LIHC, and patients with HCC who have high FDX1 expression exhibit increased immune cell infiltration compared to those with low expression [68]. In tissues affected by clear cell renal cell carcinoma (ccRCC), the expression level of the FDX1 gene is significantly reduced compared to normal tissues (P < 0.05). Furthermore, the levels of FDX1 gene expression are tightly linked to cancer grading and more advanced stages of lymph node metastasis in tumors [69].
Furthermore, FDX1 expression demonstrates significant correlations with immune cell infiltration, immune-related genes, the tumor microenvironment (TME), and drug resistance. In the majority of tumors, elevated FDX1 expression is associated with a potential rise in the levels of immune cell infiltration. Intriguingly, it has been found that FDX1 expression exhibits a negative correlation with immune cell infiltration specifically in STAD [70].
In conclusion, FDX1 appears to be a multifaceted protein with implications in cancer prognosis, progression, and immune response. Its differential expression across various cancers and its correlation with prognosis and immune cell infiltration suggest that FDX1 could be a potential therapeutic target or a biomarker for cancer management. Future research should focus on understanding the mechanistic role of FDX1 in cuproptosis and its interaction with the TME, as well as exploring the potential of FDX1-based therapies in preclinical and clinical settings. Additionally, larger-scale studies are needed to validate the prognostic value of FDX1 across different cancer types and to elucidate its role in drug resistance, which could have significant implications for personalized medicine.
LA pathway-related proteins LIAS, LIPT1 and DLD
LIPT1, LIAS, and DLD encode components of the lipoic acid pathway. LIAS is a member of the biotin and lipoic acid synthases family and functions as an iron-sulfur cluster mitochondrial enzyme. Lipopoic acid (LA) serves as a powerful antioxidant and also acts as a coenzyme for two mitochondrial energy metabolism enzymes: pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. LIAS is associated with mitochondrial energy metabolism and antioxidant defense, with mitochondria playing a role in electron transport chain transfer and oxidative phosphorylation through the TCA cycle [10]. Reports indicate that LIAS is underexpressed in numerous tumors, encompassing breast cancer (BRCA), COAD, kidney renal papillary cell carcinoma (KIRP), prostate adenocarcinoma (PRAD), rectal adenocarcinoma (READ), thyroid carcinoma (THCA), endometrial carcinoma (UCEC), acute myeloid leukemia (LAML), ovarian cancer (OV), and testicular germ cell tumors (TGCT). Conversely, LIAS expression is upregulated in cancers such as cholangiocarcinoma (CHOL), hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), and lung squamous cell carcinoma (LUSC). Patients with kidney renal clear cell carcinoma (KIRC), rectal adenocarcinoma, breast cancer, and ovarian cancers exhibiting high levels of LIAS tend to have a more favorable prognosis. However, lung cancer patients with elevated LIAS levels have a poorer prognosis. Notably, LIAS expression is significantly increased in lymphoid tumors, including diffuse large B-cell lymphoma (DLBCL), low-grade glioma (LGG), and thymoma (THYM). In other tumors, such as adrenocortical carcinoma (ACC), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), glioblastoma multiforme (GBM), pancreatic adenocarcinoma (PAAD), pheochromocytoma and paraganglioma (PCPG), sarcoma (SARC), and uterine carcinosarcoma (UCS), LIAS expression remains relatively unchanged [71].
LIPT1 is a protein-coding gene that is implicated in diseases resulting from LIPT1 deficiency and Leigh syndrome, which is often accompanied by white matter dystrophy. A mutation within the LIPT1 gene can lead to fatal disorders that are associated with impaired lipoylation of 2-ketoacid dehydrogenase [72,73].
Adenocarcinomas, endometriomas, breast cancer, squamous cervical cancer, and adenomas exhibit low levels of LIPT1 [74]. Patients diagnosed with KICH and KIRP who have high expression of LIPT1 tend to have a poorer prognosis. Conversely, high LIPT1 expression in bladder urothelial carcinoma (BLCA), KIRC, lung squamous cell carcinoma (LUSC), rectal adenocarcinoma (READ), and cutaneous melanoma (SKCM) is linked to a more favorable prognosis [75].
The expression level of LIPT1 is notably higher in HCC tissues compared to normal tissues, and this increased expression is associated with more aggressive pathological characteristics and poorer outcomes for patients with HCC. A Gene Set Enrichment Analysis (GSEA) reveals that LIPT1 expression is significantly associated with the PI3K-AKT signaling pathway and the Wnt/β-catenin pathway, both of which play roles in cancer. Further molecular experiments demonstrate that silencing LIPT1 inhibits PPAR expression and inactivates the AKT/GSK-3β/β-catenin signaling pathway [76].
As a constituent of the three-ketoacid dehydrogenase complexes, DLD, also known as dihydrolipoamide dehydrogenase, is predominantly found in mitochondria, with a minor occurrence in the nucleus. Mutations in the DLD gene have been linked to various diseases [77]. Apart from influencing the tumor’s TCA cycle and the underlying biochemical processes that facilitate tumor growth, DLD also serves as a novel positive regulatory modulator of copper carrier-induced cell death, impacting mitochondrial respiration-dependent copper ion carrier-mediated cell death. There is evidence suggesting that downregulation of DLD affects mitochondrial metabolism, leading to a decrease in the concentration of downstream metabolites generated by the TCA cycle. This, in turn, induces melanoma cell death and inhibits human tumor progression by stimulating reactive oxygen species (ROS) production and altering energy metabolism [78].
Elevated DLD expression is linked to a favorable prognosis in breast cancer (BRCA), KICH, and lung adenocarcinoma (LUAD). Conversely, high DLD expression is associated with a poorer prognosis in numerous other tumors, including colon adenocarcinoma, kidney renal clear cell carcinoma, and kidney renal papillary cell carcinoma. The levels of DLD expression correlate with pathological staging in KIRC, KIRP, LUAD, READ, thyroid carcinoma (THCA), and uterine carcinosarcoma (UCS) [79,80].
In conclusion, LIPT1, LIAS, and DLD play complex roles in cancer metabolism, antioxidant defense, and prognosis. The expression patterns of these genes are closely associated with the prognosis of specific cancers, suggesting their potential as therapeutic targets. Future research should delve deeper into the mechanisms by which these genes influence cancer development, as well as how they impact tumor metabolism and antioxidant status. Moreover, the role of antioxidants in cancer requires further investigation to determine their potential applications in cancer therapy. Understanding the intricate relationships between these genes and cancer biology is crucial for the development of novel diagnostic and treatment strategies.
Genes encoding PDH complex-related proteins DLAT, PDHA1, and PDHB
DLAT is overexpressed in most tumors and is the sole cancer-related gene (CRG) with prognostic significance in pancreatic adenocarcinoma (PAAD). In PAAD, T-helper (Th) cells, particularly Th2 cells, exhibit the strongest correlation with DLAT expression. Th1 and Th2 cells have opposing roles in modulating immune responses: Th1 cells stimulate and sustain antitumor cytotoxic T lymphocyte (CTL) responses, whereas Th2 cells impair Th1-driven immunity and facilitate cancer progression. Patients with PAAD who have elevated DLAT expression demonstrate resistance to multiple drugs [81].
Researchers have discovered that DLAT has the ability to acetylate the K76 site of 6-phosphogluconate dehydrogenase (6PGD), thereby enhancing the proliferation and growth of lung cancer cells H1299 [82]. A strong correlation exists between DLAT expression and immune status, indicating that patients with high DLAT expression may derive greater benefits from targeted therapies and immunotherapy [83].
PDHA1 protein encodes for pyruvate dehydrogenase E1, a crucial enzyme in energy metabolism. Disorders associated with PDHA1 encompass sudden infant death syndrome and pyruvate dehydrogenase E1-deficiency [84].
A high expression level of PDHA1 is linked to a more favorable prognosis in LUSC, and PDHA1 can serve as a biomarker for the tumor microenvironment in LUSC [85]. Conversely, increased PDHA1 expression in uveal melanoma (UVM) patients is associated with decreased disease-specific survival (DSS) and overall survival (OS) [86].
PDHB, which is localized in the mitochondria, catalyzes the transformation of glucose-derived acetyl-CoA. As part of the PDH complex, PDHB participates in the decarboxylation of pyruvate and is involved in the glycolytic pathway, thereby modulating cellular energy metabolism. PDHB may contribute to the alteration of metabolic reprogramming in tumors. PDHB expression is downregulated in various cancers, including COAD, head and neck squamous cell carcinoma (HNSC), KIRC, LUSC, rectal adenocarcinoma (READ), and thyroid carcinoma (THCA). However, PDHB is overexpressed in hepatocellular carcinoma (LIHC). Renal cancer patients with low PDHB expression have a poorer prognosis for survival. A positive correlation exists between high PDHB expression and patient prognosis in lung cancer, gastric cancer, and breast cancer, whereas a negative correlation is observed between high PDHB expression and patient prognosis in ovarian cancer. In LUSC, PDHB is frequently deleted and is associated with worse overall survival (OS) in non-small cell lung cancer [87–89].
In conclusion, the roles of DLAT, PDHA1, and PDHB in cancer are multifaceted, involving energy metabolism, immune response, and prognosis.
Understanding the intricate relationships between these genes and cancer biology is crucial for the development of novel diagnostic and treatment strategies.
CRGs suppressor MTF1, GLS, CDKN2A
Upon exposure to heavy metals, MTF1 accumulates in the nucleus as a protein-coding gene. It binds to metal response elements (MREs) within the promoters of metallothionein (MT) genes, thereby activating their transcription. The MTF1 protein structure comprises six conserved zinc finger domains that modulate transcriptional activity through binding to free zinc ions and DNA within the cell. MTF1 plays crucial roles in metal homeostasis, embryogenesis, and hematopoiesis, and can be activated by cytokines, growth factors, and redox reactions. As an important CRG, MTF1 is downregulated in KIRC, leading to enhanced proliferation and migration of KIRC cells [90].
MTF1 is typically downregulated in tumors, with exceptions in cholangiocarcinoma (CHOL) and LIHC, where its expression exceeds that in normal tissues. Elevated MTF1 expression is associated with a poor prognosis in LIHC and lower-grade glioma (LGG), but is correlated with a favorable prognosis in KIRC, as well as lung, ovarian, and breast carcinomas. The level of MTF1 positively correlates with immune cell infiltration, and knocking down MTF1 in vivo can accelerate tumor growth [91].
Glutamine transporters facilitate the entry of glutamine into the mitochondria, where it is converted into glutamate, stoichiometric ammonia, and additional glutamate. The enzyme GLS catalyzes the conversion of glutamine to glutamate. Glutamate plays a role in ATP production through the TCA cycle and is vital for the synthesis of amino acids and lipids, highlighting its importance in bioenergetics and biosynthesis [92].
Pan-cancer analysis reveals abnormal overexpression of GLS in various types of cancer, particularly in breast cancer, where GLS expression exhibits a high ROC-AUC value for diagnostic purposes. There is a strong correlation between GLS expression and tumor growth as well as metastasis. Furthermore, it exerts a direct influence on the immunotumor microenvironment [93].
CDKN2A, a member of the INK4 family of tumor suppressor genes, stimulates cell proliferation and angiogenesis in the liver. In HCC, the expression of CDKN2A is significantly elevated compared to adjacent normal tissues. Elevated CDKN2A expression is tightly linked to the levels of immune cell infiltration. Methylation of the CDKN2A promoter is associated with an increased risk of HCC and plays a pivotal role in its progression [94]. In head and neck squamous cell carcinomas (HNSCCs), CDKN2A expression is significantly higher in stage T2 tumors compared to stages T3 and T4, suggesting higher expression in early malignant tumors and lower expression in advanced cancers. According to Kaplan-Meier (KM) curves, clinical outcomes are more favorable when CDKN2A expression is high [95]. Across all histologic subtypes of renal cell carcinoma (RCC), deletion of CDKN2A, either due to chromosome 9p21.3 deletion or promoter hypermethylation, is associated with poorer survival [96].
In conclusion, the article provides valuable insights into the roles of MTF1, GLS, and CDKN2A in cancer. Further research is needed to fully understand their mechanisms of action and to develop targeted therapies that can exploit these pathways for cancer treatment.
Copper transporter protein ATP7B, SLC31A1
ATP7B expression is linked to a favorable prognosis in LUAD [97]. Conversely, the ATP7B gene is downregulated in low-grade gliomas (LGG) [98].
SLC31A1 encodes CTR1, a member of the copper transporter protein family, which is essential for regulating copper homeostasis and affects the uptake of chemotherapeutic agents like cisplatin and carboplatin by human cells. Studies have shown that SLC31A1 mRNA levels are significantly elevated in colorectal cancer samples, along with increased expression of copper metabolism-related genes ATP7A, SCO1, and COX11 [99]. SLC31A1 expression is upregulated in various cancers, including prostate cancer, colorectal cancer, breast cancer, and melanoma. Decreasing SLC31A1 expression inhibits cancer cell growth [100]. Patients with high SLC31A1 gene expression generally have a poorer prognosis compared to those with low expression [101]. There is a notable positive correlation between SLC31A1 and tumor-infiltrating immune cells in breast cancer. Furthermore, there is a positive correlation between SLC31A1 and CD274 (PD-L1) as well as CTLA4, indicating that modulating SLC31A1 may enhance the effectiveness of immunotherapy in breast cancer [102].
In summary, the research on ATP7B and SLC31A1 adds to the growing body of evidence that highlights the importance of metal homeostasis in cancer biology. It is our hope that continued research in this field will lead to the discovery of new therapeutic targets and strategies, ultimately contributing to the conquest of cancer.
Therapeutic applications of cuproptosis cuproptosis for anti-tumor
In recent years, there has been a surge in cancer incidence, posing a substantial threat to human health and becoming a prominent global public health concern. Cancer remains an unresolved challenge to this day. The most prevalent clinical treatment methods currently include surgery, radiotherapy, chemotherapy, and immunotherapy, each with its inherent limitations. There is evidence suggesting that deregulated copper metabolism plays a significant role in cancer development. Dysregulation of copper homeostasis can lead to oxidative stress, cytotoxicity, and even tumor development. High concentrations of copper can inhibit the activity of proteasomes and induce apoptosis in human cancer cells. Tumor cells require more copper to maintain the energy requirements of rapid cell division, and manipulation of the Cu content in tumor cells is a novel cancer therapeutic strategy. Furthermore, a growing number of studies indicate that cancer cells require more copper than other tissues. Consequently, targeting copper metabolic pathways could serve as an alternative therapeutic approach in oncology.
Copper chelators and copper ionophores
Copper is recognized for its role in promoting tumor growth, angiogenesis, and metastasis. There are two main strategies to target copper in cancer therapy: decreasing copper bioavailability using copper chelators and elevating intracellular copper levels through copper ionophores.
A copper ion carrier is a lipophilic Cu2+-binding molecule that aids in the transfer of copper ions into cells, thereby triggering copper reduction. Examples of such carriers include eresclomol, NSC-319,726, and disulfiram [103].Eresclomol binds specifically to the FDX1 α2/α3 helix and β5 strand, inhibiting FDX1-mediated iron-sulfur cluster biosynthesis. Additionally, it functions as an oxidative stress inducer, ultimately resulting in cancer cell death. However, ion carriers may lead to non-selective accumulation. Conversely, nanoparticles can accumulate at the tumor site due to the enhanced permeability and retention (EPR) effect. They can also be engineered to respond to the tumor microenvironment, releasing metal ions selectively for therapeutic use [104].
Copper chelators can efficiently capture excess copper ions and have excellent biocompatibility and biodegradability. Unbound unstable copper ions can produce ROS or play cytotoxic effects in cells, chelating intracellular Cu (I) proteins.The application of copper chelators can suppress tumor proliferation, metastasis, and angiogenesis by inhibiting crucial proteins and pathways, including MEK1/2, ATP7A, ATOX1, Cu/Zn superoxide dismutase (SOD1), hypoxia-inducible factor-1 (HIF-1), and nuclear factor-κB (NF-κB) [105].
Yoshida et al. found that CDPT selectively induces copper chelation in tumor tissues without decreasing copper concentrations in normal brain tissue before cuproptosis occurs [106]. Brady et al. suggested that copper chelators, which inhibit MEK1/2 kinase activity, can serve as an adjunctive therapy for treating BRAF mutation-positive cancers and cancers that are resistant to BRAFV600E or MEK inhibitors [107]. Numerous copper chelators have been proposed as potential solutions to overcome resistance to BRAF-MEK1/2 inhibition in melanoma and colon cancer. Pediatric patients with Wilson’s disease can benefit from copper chelation therapy for managing neurological symptoms, and this therapy is also being investigated for use in various types of cancer [108].
An experimental model of TH-MYCN neuroblastoma demonstrated that TEPA decreased tumor growth and PD-L1 expression, while increasing immune cell infiltration [109]. Similar to lenvatinib and sorafenib, TM inhibits MEK1/2 kinase activity and PTC cell growth, and it enhances the antitumor activity of both sorafenib and vemurafenib [110]. Furthermore, copper chelators inhibit the EMT process by downregulating the TGF-β signaling pathway in tumors. There is evidence suggesting that TEPA downregulates multiple TGF-signaling pathways and decreases the expression of EMT markers, thereby inhibiting tumor invasion and metastasis in TNBC, NB, and DIPG cell lines [111,112].
Zhou et al. synthesized a copper-chelated comb-shaped block copolymer, RGD-PEG-b-PGA-g-(TETA-DTC-PHis) (RPTDH), for the preparation of nanoparticles loaded with the TLR7 and TLR8 agonist resiquimod (R848). This combines anti-angiogenic and immune-activating therapies for the treatment of breast cancer [113]. Nanoparticles containing RPTDH/R848 may hold promise in the treatment of advanced and metastatic breast cancer. Additionally, PSP-2, a newly developed high-affinity Cu(I) chelator with a low zeptomolar dissociation constant, exhibits notable antiangiogenic properties [114].
Design and synthesis of nanomaterials for cuproptosis induction
There has been significant interest in the use of nanoparticles for cancer therapy, drug delivery, and disease imaging. Owing to their nanoscale composition, nanoparticles can be easily modified on their surface. By modifying them with targeting ligands or membranes, nanoparticles can actively target tumor tissues while minimizing their distribution in healthy tissues. As of now, a range of copper-containing nanoparticles have been developed for oncology treatments. These nanoparticles utilize copper ions to deplete glutathione within tumor cells, thereby initiating Fenton-like reactions that are fatal to the cancer cells [115,116].
Nanomaterials applied to chemodynamic therapy
Chemodynamic therapy (CDT) selectively eliminates tumor cells in situ by producing hydroxyl radicals (-OH) via Fenton or Fenton-like reactions. Copper, with its excellent biocompatibility and role as a cofactor for numerous intracellular enzymes, serves as a highly efficient Fenton-like transition metal. Its catalytic activity remains relatively unaffected by strong acids within the pH range of 2 to 4. CDT has emerged as one of the most promising cancer therapies due to its high specificity, efficiency, and minimal side effects. However, it is undeniable that an excess of copper in the body can be harmful and potentially life-threatening. To alleviate these risks, copper-based nanomaterials have been developed, harnessing the catalytic properties of copper ions for CDT. Furthermore, CDT can be combined with other therapeutic approaches to enhance overall therapeutic outcomes [117–121].
A copper peroxide nanodot represents the inaugural Fenton-type metal perovskite nanomaterial capable of augmenting CDT by self-supplying H2O2. These copper-based nanomaterials can alleviate tumor hypoxia and weaken antioxidant defenses, thereby achieving photo-enhanced CDT and improved photodynamic therapy (PDT) [122].Yu et al. designed a nanocascade system mediated by Cu+ and DNA enzymes for copper apoptosis-based synergistic cancer therapy [123]. This system targets and triggers cuproptosis in tumor cells both in vitro and in vivo, notably inhibiting breast cancer lung metastasis and boosting the count of central memory T cells in peripheral blood, aiding in the prevention of tumor recurrence. By facilitating a cyclic transformation between Cu+ and Cu2+ through glutathione (GSH) and Fenton-like reactions, CDT synergizes with cuproptosis in the treatment of pancreatic cancer. Recently, strategies that enhance in situ OH generation and ROS-induced oxidative damage to cancer cells have been documented, such as elevating local H2O2 concentrations and inhibiting or depleting GSH synthesis. Furthermore, the integration of CDT with other therapeutic modalities, including chemotherapy/CDT, photothermal therapy (PTT)/CDT, and PDT/CDT, based on meticulously designed copper-containing nanoplatforms, has been shown to optimize antitumor effects through various mechanisms, ultimately enhancing therapeutic outcomes [124].
While CDT holds great promise, there are challenges that need to be addressed. The bioavailability and clearance of copper ions, the potential for off-target effects, and the development of resistance to ROS-induced cell death are areas that require further research. Additionally, the optimization of nanomaterial design to ensure selective targeting of cancer cells and minimal impact on healthy tissues is crucial.
In conclusion, CDT, particularly with copper-based nanomaterials, offers a promising avenue for cancer treatment. The synergistic approach with other therapies has the potential to overcome resistance and enhance the overall efficacy of cancer treatment. Future research should focus on improving the selectivity and reducing the side effects of CDT while exploring new combinations with other therapeutic modalities to achieve the best possible patient outcomes.
Nanomaterials applied to photochemotherapy
Photochemodynamic therapy (PDT) stands as a non-invasive, low-toxicity approach for tumor treatment, effectively amalgamating photosensitizers, benign light, and molecular oxygen. Nevertheless, treatment efficacy has been hindered by restricted tissue penetration depth and the inadequate performance of conventional transducers and photosensitizers. This challenge has spurred the development of combination therapies aimed at achieving enhanced synergistic effects [125]. PDT marks the first drug-device combination therapy endorsed by the U.S. Food and Drug Administration (FDA) for exerting toxic reactive oxygen species effects on both nonmalignant and neoplastic conditions. The PDT system encompasses three fundamental elements: oxygen, light, and a photosensitizer. Since the inception of Photofrin®, the first clinically applied photosensitizer in cancer therapy, concerted efforts have been made to develop ideal photosensitizers that are single-component, stable, cost-effective to produce, biocompatible, and highly sensitive. Copper-based nanomaterials have been extensively investigated for this purpose [105].Ju et al. [117] revealed that the combination of Cu2+ and g-C3N4 augments the effectiveness of PDT in cancer cells [126]. This enhancement is attributed to Cu2+-induced ROS generation and the reduction of glutathione (GSH) levels. This metal-inorganic hybrid system introduces a novel approach to decreasing intracellular glutathione levels and boosting PDT efficiency. In another study, Han et al. devised a therapeutic system that integrates hollow copper nanoparticles modified with BSA-FA with a phototherapeutic agent (ICG) [127].Their findings demonstrated that Polyethylenimine (PEI) can conjugate indocyanine green with copper nanoparticles via amine functionalization. Bharathiraja et al. developed copper nanoparticles sensitized with Ce6 as a multifunctional agent for combined photothermal and photodynamic treatments [128]. In treated breast cancer cells, Cu-Ce6 nanoparticles exhibited both photothermal and photodynamic toxicity, and the combination of PDT with photothermal therapy (PTT) significantly diminished cell viability.
As a contemporary cancer treatment strategy, Photodynamic Therapy (PDT) has evolved from its inception to its current medical applications, expanding into various fields including oncology, dermatology, and dentistry. Future research should focus on enhancing the selectivity and efficiency of PDT, as well as developing new photosensitizers and nanomaterials to improve the therapeutic effects and broaden the scope of PDT applications. Additionally, exploring the integration of PDT with other treatment modalities is essential to achieve more effective cancer treatment strategies.
Preclinical and clinical studies of nanomaterials in cancer therapy
Guo et al. devised a reactive oxygen species (ROS)-sensitive polymer (PHPM) for the co-encapsulation of esculomol (ES) and copper, resulting in the formation of nanoparticles (NP@ESCu) ([129]. Upon entry into cancer cells, the high intracellular ROS levels trigger the prompt release of ES and Cu. NP@ESCu facilitates targeted accumulation and release of the encapsulated ES and Cu within tumor cells. On one hand, it suppresses tumor growth via cuproptosis, stimulates dendritic cell (DC) maturation, augments CD8+ T-cell infiltration, enhances the tumor microenvironment (TME), and converts immunologically “cold” tumors into “hot” tumors. On the other hand, NP@ESCu notably elevates PD-L1 expression in tumor cells and effectively enhances the response rate to αPD-L1, enabling copper-based therapy to synergize with immunotherapy in cancer treatment. This research presents the inaugural instance of how the combination of nanomedicines and copper can amplify the effectiveness of tumor immunotherapy. Liu et al. created disulfiram (DSF)-loaded hollow copper sulfide nanoparticles (DSF@PEG-HCuSNPs) tailored for the tumor microenvironment (TME)-activated in situ generation of cytotoxic Cu(DTC)[2 [130]]. This strategy is employed for near-infrared (NIR)-II-induced, photonic hyperthermia-enhanced, and DSF-initiated cancer chemotherapy, with the objective of achieving TME-triggered in situ formation of the cytotoxic Cu(DTC)2 complex to enhance DSF-based chemotherapy.
Jun et al. introduced a straightforward in situ sacrificial growth approach and successfully engineered pH-sensitive Cu2O@CuBTC-DSF@HA nanocomposites (CCDHs), characterized by a swift Cu(I) release profile. This nanomaterial is capable of efficiently delivering an abundant amount of Cu(I) into cells, thereby triggering copper reduction within tumor cells. As a result, it attains substantial therapeutic efficacy while also guaranteeing biosafety ([131].
The research by Guo et al., Liu et al., and Jun et al. underscores the potential of copper-based nanomedicines in enhancing the effectiveness of tumor immunotherapy and chemotherapy. These studies highlight the importance of targeted drug delivery, immunomodulation, and the exploitation of the TME for cancer therapy. Future research should focus on optimizing the bioavailability and clearance of copper ions, minimizing off-target effects, and developing multifunctional nanoplatforms that can integrate various therapeutic modalities. The exploration of copper-based nanomedicines in combination with other treatments, such as immunotherapy and chemotherapy, holds great promise for the development of more effective and personalized cancer therapies.
Conclusions and perspectives
Long admired for its fundamental biological importance, copper has recently emerged as a versatile asset in the development of groundbreaking cancer therapies. Recent advancements have shed light on copper’s pivotal role in cancer biology, revealing its intricate involvement ranging from basic cellular mechanisms to innovative treatment approaches. This review highlights the emerging concept of cuproptosis, wherein copper-mediated oxidative stress selectively pinpoints and induces apoptosis in cancer cells, thereby presenting a promising avenue for cancer treatment.
Cuproptosis leverages copper’s ability to catalyze Fenton-like reactions inside cancer cells, producing ROS that lead to cellular harm and apoptosis. This focused toxicity offers potential benefits over traditional therapies, as it precisely targets cancerous cells while sparing healthy tissues. Additionally, insights into copper-driven pathways, including the modulation of MEK1/2 kinase activity, redox signaling, and immune responses within the tumor microenvironment (TME), underscore copper’s versatility in disrupting oncogenic mechanisms. This multi-dimensional activity highlights its potential as both a therapeutic target and a diagnostic biomarker.
The incorporation of copper into nanomaterials has greatly expanded therapeutic possibilities, enhancing drug delivery, targeting, and overall treatment effectiveness. Copper-laden nanoparticles serve as a testament to these advancements, demonstrating synergistic effects when paired with chemotherapy, phototherapy, or immunotherapy. These innovations carry the potential to surmount therapeutic resistance and improve patient outcomes.
Moreover, copper-based therapies exert a profound impact on TME remodeling, promoting immune cell infiltration and reprogramming immunosuppressive conditions. Nanomaterial formulations not only trigger cuproptosis but also enhance the efficacy of immune checkpoint inhibitors, effectively converting immunologically “cold” tumors into responsive “hot” tumors.
Recent advancements in the understanding of cuproptosis and the development of copper-based nanomaterials have emphasized their revolutionary potential in cancer therapy. How to use the characteristics of copper in the tumor microenvironment, and the influence in the development of tumors to treat tumors. By integrating copper’s unique biochemical properties with nanotechnology, these approaches are pioneering new avenues to address the challenges of cancer treatment and advancing personalized, effective medical interventions.
Future research should explore the molecules, proteins, and signaling cascades involved in cuproptosis and cellular defense responses, as well as the genetic and epigenetic factors that may affect an individual’s susceptibility to this novel form of cell death. Additionally, to facilitate the advancement of clinical trials and research, the identification of sensitive copper-dependent biomarkers and copper ionophore biomarkers becomes crucial. As a newly discovered form of cell death, cuproptosis currently lacks specific biomarkers, which limits our ability to assess its role in human diseases. This deficiency significantly hinders the development of therapeutic strategies targeting cuproptosis. Therefore, the identification of precise and sensitive cuproptosis-specific biomarkers in different diseases is essential for the development of targeted clinical applications and for enhancing our understanding of the pathological conditions affected by cuproptosis. The effectiveness of copper chelators in alleviating copper-induced cell death underscores the importance of copper deprivation strategies in managing excessive intracellular copper levels. However, the application of copper ionophores in clinical therapy has also shown a range of side effects, including hepatotoxicity, neurotoxic symptoms, and psychiatric symptoms, which can significantly affect the quality of life of patients and may limit their use in clinical treatment. Therefore, future research needs to closely monitor these effects, optimize dosing regimens, and develop strategies to mitigate these reactions.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82060503) and the Project of the Guizhou Provincial Department of Science and Technology (Grant No. QKH-JC-ZK-2022-622 and No. QKH-JC-2019-1334).
Abbreviations
- ACC
Adrenocortical carcinoma
- ATOX1
Antioxidant protein 1
- ATPases
Adenosine triphosphatase
- CCO
Cytochrome c oxidase
- CDKN2A
Cyclin-dependent kinase inhibitor 2 A
- COX17
Cytochrome C oxidase copper chaperone cox 17
- COX19
Cytochrome C oxidase copper chaperone cox 19
- COX23
Cytochrome C oxidase copper chaperone cox 23
- CP
Ceruloplasmin
- CRGs
Cuproptosis-related genes
- CTR1
Cu transport protein 1
- CYCS
Cytochrome C, somatic
- DCYTB
Duodenal cytochrome B
- DHODH
Dihydroorotate dehydrogenase
- DLD
Dihydrolipoamide dehydrogenase
- DLAT
Drolipoamide S-acetyltransferase
- FDX1
Ferredoxin 1
- FSP1
Ferroptosis suppressor protein 1
- GLS
Glutaminase
- GSH
Glutathione
- HCC
Hepatocellular carcinoma
- HNSC
head and neck squamous cell carcinoma
- HAS
Human serum albumin
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney Renal Papillary Cell Carcinoma
- LGG
Lower-grade glioma
- LIAS
Lipoic acid synthase
- LIHC
Liver hepatocellular carcinoma
- LIPT1
Lipotransferase 1
- MESO
Mesothelioma; MT-CO2/COX2 mitochondria-encoded cytochrome C oxidase II
- MTF1
Metal-responsive transcription factor-1
- PCPG
Penile squamous cell carcinoma
- PDHA1
Pyruvate dehydrogenase E1 subunit α 1
- PDHB
Pyruvate dehydrogenase E1 subunit β;SCO1, Synthesis of cytochrome C oxidase 1
- SCO2
Synthesis of cytochrome C oxidase 2
- SLC31A1
Solute carrier family 31 member 1
- SOD
Superoxide dismutase
- STAD
Stomach adenocarcinoma
- STEAP
Six-transmembrane epithelial antigen of prostate
- TCA
Tricarboxylic acid cycle
- TGN
Trans golgi network
- UCEC
Uterine corpus endometrial carcinoma
Author contributions
Conceptualization, Yating Cong, Na Li and Hailong Zhao; writing—original draft preparation, Yating Cong and Na Li; writing—review and editing, Zixin Zhang and Yan Shang; visualization, Hailong Zhao; supervision, Hailong Zhao; project administration, Hailong Zhao; funding acquisition, Hailong Zhao. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China. (Grant No. 82060503) and the Project of the Guizhou Provincial Department of. Science and Technology (Grant No. QKH-JC-ZK-2022-622 and No. QKH-JC-2019-1334).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
All authors declare that there are no interest conflicts and agree to publish this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LIU Y, CHEN G, YOU X et al. Cuproptosis nanomedicine: clinical challenges and opportunities for anti-tumor therapy [J]. Chem Eng J, 2024, 495.
- 2.Tang D, Kroemer G. Targeting cuproplasia and cuproptosis in cancer [J]. Nat Reviews Clin Oncol. 2024;21(5):370–88. [DOI] [PubMed] [Google Scholar]
- 3.C Z, J Y. Copper metabolism and hepatocellular carcinoma: current insights [J]. Front Oncol. 2023;13:1186659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TP C. Copper metabolism in man [J]. Biochem J. 1935;29(2):476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swedik SM, Madola A, Cruz M A et al. Th17-Derived Cytokines Synergistically Enhance IL-17 C Production by the Colonic Epithelium [J]. Journal of immunology (Baltimore, Md: 1950), 2022, 209(9): 1768-77. [DOI] [PMC free article] [PubMed]
- 6.Z CB. Copper homeostasis and cuproptosis in tumor pathogenesis and therapeutic strategies [J]. Front Pharmacol. 2023;14:1271613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.X C, Q C. R L, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies [J]. Volume 14. Cell death & disease; 2023. p. 105. 2. [DOI] [PMC free article] [PubMed]
- 8.VC S. Copper metabolism as a unique vulnerability in cancer [J]. Biochim et Biophys acta Mol cell Res. 2021;1868(2):118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NL SL, MY B. Function and regulation of human copper-transporting ATPases [J]. Physiol Rev. 2007;87(3):1011–46. [DOI] [PubMed] [Google Scholar]
- 10.Q Z. The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression [J]. Front Oncol. 2023;13:1117164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GM Y, L X. Structures of the human Wilson disease copper transporter ATP7B [J]. Cell Rep. 2023;42(5):112417. [DOI] [PubMed] [Google Scholar]
- 12.J X, Y Y, Y G, et al. Cuproptosis: mechanisms and links with cancers [J]. Mol Cancer. 2023;22(1): 46. [DOI] [PMC free article] [PubMed]
- 13.LM R. Role of copper on mitochondrial function and metabolism [J]. Front Mol Biosci. 2021;8:711227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.W M-K AV. Oxidative switches in functioning of mammalian copper chaperone Cox17 [J]. Biochem J. 2007;408(1):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LA CA, BE Y. Yeast cox17 solution structure and copper(I) binding [J]. J Biol Chem. 2004;279(51):53584–92. [DOI] [PubMed] [Google Scholar]
- 16.Q X, DJ RK. Copper metabolism in cell death and autophagy [J]. Autophagy. 2023;19(8):2175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.X Z, GR W. Memo1 binds reduced copper ions, interacts with copper chaperone Atox1, and protects against copper-mediated redox activity in vitro [J]. Proc Natl Acad Sci USA. 2022;119(37):e2206905119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GJ MG. The copper chaperone CCS facilitates copper binding to MEK1/2 to promote kinase activation [J]. J Biol Chem. 2021;297(6):101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Y A, S L, X H, et al. The role of copper homeostasis in Brain Disease [J]. Int J Mol Sci, 2022; 23(22). [DOI] [PMC free article] [PubMed]
- 20.H Z, C E. Mitochondrial copper homeostasis and its derailment in Wilson disease [J]. Int J Biochem Cell Biol, 2018, 102: 71–5. [DOI] [PubMed]
- 21.Q SC. Role of cuproptosis in understanding diseases [J]. Hum Cell. 2023;36(4):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.P T, A D, K C, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress [J]. Nat Chem Biol, 2019;15(7): 681–9. [DOI] [PMC free article] [PubMed]
- 23.P T. S C, B P, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins [J]. Volume 375. Science (New York, NY); 2022. pp. 1254–61. 6586. [DOI] [PMC free article] [PubMed]
- 24.PA C, Cuproptosis DCB. Cellular and molecular mechanisms underlying copper-induced cell death [J]. Mol Cell. 2022;82(10):1786–7. [DOI] [PubMed] [Google Scholar]
- 25.M Y, H Z, K X, et al. A novel signature to guide osteosarcoma prognosis and immune microenvironment: cuproptosis-related lncRNA [J]. Front Immunol, 2022;13: 919231. [DOI] [PMC free article] [PubMed]
- 26.K RW. Cuproptosis engages in c-Myc-mediated breast cancer stemness [J]. J Translational Med. 2023;21(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.H L. Pan-cancer profiles of the cuproptosis gene set [J]. Am J cancer Res, 2022;12(8): 4074–81. [PMC free article] [PubMed]
- 28.C X. Prognostic and immunological role of cuproptosis-related protein FDX1 in pan-cancer [J]. Front Genet. 2022;13:962028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.V S, S B, SA F, et al. Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2 [J]. Nat Chem Biol, 2023, 19(2): 206–17. [DOI] [PMC free article] [PubMed]
- 30.M Z Ans. FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery [J]. Proc Natl Acad Sci USA. 2023;120(10):e2216722120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.C X. Cuproptosis: p53-regulated metabolic cell death? [J]. Cell Death Differ. 2023;30(4):876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Z L. H M, Z L. The role of Ferroptosis and cuproptosis in curcumin against Hepatocellular Carcinoma [J]. Molecules, 2023, 28(4). [DOI] [PMC free article] [PubMed]
- 33.N K. Stimuli-responsive ferroptosis for cancer therapy [J]. Chem Soc Rev. 2023;52(12):3955–72. [DOI] [PubMed] [Google Scholar]
- 34.X DT. Ferroptosis: molecular mechanisms and health implications [J]. Cell Res. 2021;31(2):107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.S C, A G, Y D, et al. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases [J]. Mol Cell, 2023, 83(21): 3931–e95. [DOI] [PMC free article] [PubMed]
- 36.C TN, A S D M H, et al. Phase separation of FSP1 promotes ferroptosis [J]. Nature. 2023;619(7969):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.W L, L L, S L, et al. FSP1: a key regulator of ferroptosis [J]. Trends Mol Med, 2023, 29(9): 753–64. [DOI] [PubMed]
- 38.BR S. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications [J]. Cell, 2022, 185(14): 2401–21. [DOI] [PMC free article] [PubMed]
- 39.M G, J Y, J Z, et al. Role of Mitochondria in ferroptosis [J]. Mol Cell, 2019, 73(2): 354 – 63.e3. [DOI] [PMC free article] [PubMed]
- 40.S J. Mitochondria and ferroptosis [J]. Curr Opin Physiol, 2022, 25. [DOI] [PMC free article] [PubMed]
- 41.H W, X J. cGASing mitochondria to fend off ferroptosis [J]. Cell Res, 2023, 33(4): 263–4. [DOI] [PMC free article] [PubMed]
- 42.J Y, S H, Y B, et al. Targeting cell death: pyroptosis, Ferroptosis, apoptosis and necroptosis in osteoarthritis [J]. Front cell Dev Biology, 2021, 9: 789948. [DOI] [PMC free article] [PubMed]
- 43.B H PV. Targeting ferroptosis to Iron Out Cancer [J]. Cancer Cell. 2019;35(6):830–49. [DOI] [PubMed] [Google Scholar]
- 44.G L, L Z, B G. Targeting ferroptosis as a vulnerability in cancer [J]. Nat Rev Cancer, 2022, 22(7): 381–96. [DOI] [PMC free article] [PubMed]
- 45.X J, BR S. Ferroptosis: mechanisms, biology and role in disease [J]. Nat Rev Mol Cell Biol. 2021;22(4):266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.X DT. Cuproptosis: a copper-triggered modality of mitochondrial cell death [J]. Cell Res. 2022;32(5):417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Y Y, Q F. Exploring cuproptosis as a mechanism and potential intervention target in cardiovascular diseases [J]. Front Pharmacol. 2023;14:1229297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos G P, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases [J]. Mayo Clinic proceedings, 2019, 94(1): 155 – 65. [DOI] [PMC free article] [PubMed]
- 49.Yongqiang W, Fangfang Z. Fangfang Z. Cuproptosis: a new form of programmed cell death [J]. Cellular & Molecular Immunology; 2022. [DOI] [PMC free article] [PubMed]
- 50.KałUżna A, Olczyk P, Komosińska-Vassev K. The role of Innate and Adaptive Immune cells in the Pathogenesis and development of the inflammatory response in Ulcerative colitis [J]. J Clin Med, 2022, 11(2). [DOI] [PMC free article] [PubMed]
- 51.Sean K R, Cathryn L U, Anne-Sophie R, et al. Therapeutic inhibition of ferroptosis in neurodegenerative disease [J]. Trends in Pharmacological Sciences; 2023. [DOI] [PubMed]
- 52.Hui W, Bin Dongweil. Z, et al. Emerging role of ferroptosis in Diabetic kidney disease: Molecular mechanisms and Therapeutic opportunities [J]. International Journal of Biological Sciences; 2023. [DOI] [PMC free article] [PubMed]
- 53.Xuexian F, Hossein A, Junxia M, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease [J]. Nature Reviews Cardiology; 2022. [DOI] [PMC free article] [PubMed]
- 54.Hai W, Yongxin Canl. Z, Mitochondria regulation in ferroptosis [J]. Eur J Cell Biol, 2020. [DOI] [PubMed]
- 55.Sufang Z, Wenjin C, Bo-Cheng W, et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis [J]. Redox Biology; 2023. [DOI] [PMC free article] [PubMed]
- 56.Wei X, Feng X, Xiaoxue Z, et al. Role of pyroptosis in inflammation and cancer [J]. Cellular & Molecular Immunology; 2022. [DOI] [PMC free article] [PubMed]
- 57.Fan Y, Sahana B Markss, et al. Pyroptosis and pyroptosis-inducing cancer drugs [J]. Acta Pharmacologica Sinica; 2022. [DOI] [PMC free article] [PubMed]
- 58.R W, A O, HM B, et al. Necroptosis in development, inflammation and disease [J]. Nat Rev Mol Cell Biol, 2017;18(2): 127–36. [DOI] [PubMed]
- 59.YW JS. Necroptosis molecular mechanisms: recent findings regarding novel necroptosis regulators [J]. Exp Mol Med. 2021;53(6):1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.W P. Diversity and complexity of cell death: a historical review [J]. Exp Mol Med. 2023;55(8):1573–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.T Z YW. Necroptosis pathways in tumorigenesis [J]. Sem Cancer Biol. 2022;86(Pt 3):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.M P, P V. Necroptosis and its role in inflammation [J]. Nature, 2015, 517(7534): 311–20. [DOI] [PubMed]
- 63.S S, B M, S A, et al. MicroRNAs in cancer cell death pathways: apoptosis and necroptosis [J]. Free radical biology & medicine, 2019, 139: 1–15. [DOI] [PubMed]
- 64.C Z. Regulation, genomics, and clinical characteristics of cuproptosis regulators in pan-cancer [J]. Front Oncol. 2022;12:934076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.X S, Z P. Identification and immunological characterization of cuproptosis-related molecular clusters in idiopathic pulmonary fibrosis disease [J]. Front Immunol. 2023;14:1171445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.X L, Z D. Characterization of the functional effects of ferredoxin 1 as a cuproptosis biomarker in cancer [J]. Front Genet. 2022;13:969856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.B C ZH. The core genes of cuproptosis assists in discerning prognostic and immunological traits of clear cell renal cell carcinoma [J]. Front Oncol. 2022;12:925411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Y Q, W L, R Y, et al. Tumor cuproptosis and immune infiltration improve survival of patients with hepatocellular carcinoma with a high expression of ferredoxin 1 [J]. Front Oncol, 2023, 13: 1168769. [DOI] [PMC free article] [PubMed]
- 69.T W, Y L, Q L, et al. Cuproptosis-related gene FDX1 expression correlates with the prognosis and tumor immune microenvironment in clear cell renal cell carcinoma [J]. Front Immunol, 2022, 13: 999823. [DOI] [PMC free article] [PubMed]
- 70.Y LY. Ferredoxin 1 is a cuproptosis-key gene responsible for tumor immunity and drug sensitivity: a pan-cancer analysis [J]. Front Pharmacol. 2022;13:938134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Y C, Q H. Comprehensive analysis of the potential cuproptosis-related biomarker LIAS that regulates prognosis and immunotherapy of pan-cancers [J]. Front Oncol. 2022;12:952129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.RC S, Q S. LIPT1 deficiency presenting as early infantile epileptic encephalopathy, Leigh disease, and secondary pyruvate dehydrogenase complex deficiency [J]. Am J Med Genet Part A. 2018;176(5):1184–9. [DOI] [PubMed] [Google Scholar]
- 73.X F-C FT. Mutations in the lipoyltransferase LIPT1 gene cause a fatal disease associated with a specific lipoylation defect of the 2-ketoacid dehydrogenase complexes [J]. Hum Mol Genet. 2014;23(7):1907–15. [DOI] [PubMed] [Google Scholar]
- 74.Z W. C L, F Z, TIGD1 function as a potential cuproptosis Regulator following a Novel cuproptosis-related gene risk signature in Colorectal Cancer [J]. Cancers, 2023;15(8). [DOI] [PMC free article] [PubMed]
- 75.Peipei Y, YE L, Wenjie H, et al. Pan-cancer analyses confirmed the cuproptosis-related gene LIPT1 as an immunotherapy predictor and prognostic biomarker [J]. Research Square (Research Square); 2022. [DOI] [PMC free article] [PubMed]
- 76.D JL. Aberrant expression of cuproptosis–related gene LIPT1 is associated with metabolic dysregulation of fatty acid and prognosis in hepatocellular carcinoma [J]. J Cancer Res Clin Oncol. 2023;149(17):15763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiahui L, Guowei W, SHA C, et al. Pan-cancer analysis of the cuproptosis-related gene DLD [J]. Research Square (Research Square); 2023.
- 78.Lina F, Ning L, Lingmin Z, et al. High expression of cuproptosis-related gene DLD in relation to good prognosis and immune cells infiltration in colon cancer [J]. Research Square (Research Square); 2023. [DOI] [PMC free article] [PubMed]
- 79.W Y, Q G. Comprehensive analysis of the cuproptosis-related gene DLD across cancers: a potential prognostic and immunotherapeutic target [J]. Front Pharmacol. 2023;14:1111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.H Q, D Z. Oncogenic role of copper–induced cell death–associated protein DLD in human cancer: a pan–cancer analysis and experimental verification [J]. Oncol Lett, 2023;25(5): 214. [DOI] [PMC free article] [PubMed]
- 81.W ZF. Cuproptosis-related gene DLAT as a Novel Biomarker correlated with prognosis, Chemoresistance, and Immune Infiltration in pancreatic adenocarcinoma: a preliminary study based on Bioinformatics analysis [J]. Curr Oncol (Toronto Ont). 2023;30(3):2997–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.C SP. Identification of cuproptosis-related subtypes in lung adenocarcinoma and its potential significance [J]. Front Pharmacol. 2022;13:934722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D H, Y W, X S, et al. Genetic landscape and clinical significance of cuproptosis-related genes in liver hepatocellular carcinoma [J]. Genes Dis, 2024, 11(2): 516–9. [DOI] [PMC free article] [PubMed]
- 84.X JJ. Artificial intelligence reveals dysregulation of osteosarcoma and cuproptosis-related biomarkers, PDHA1, CDKN2A and neutrophils [J]. Sci Rep. 2023;13(1):4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Y Z, J Z, H L, et al. Prediction of risk and clinical outcome of cuproptosis in lung squamous carcinoma [J]. BMC Pulm Med, 2023, 23(1): 205. [DOI] [PMC free article] [PubMed]
- 86.Y C, X C, X W. Identification of a prognostic model using cuproptosis-related genes in uveal melanoma [J]. Front cell Dev Biology, 2022, 10: 973073. [DOI] [PMC free article] [PubMed]
- 87.F Z YY. A combined analysis of bulk and single-cell sequencing data reveals metabolic enzyme, pyruvate dehydrogenase E1 subunit beta (PDHB), as a prediction biomarker for the tumor immune response and immunotherapy [J]. Heliyon. 2023;9(2):e13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.JY L, LP L. The role of cuproptosis-related gene in the classification and prognosis of melanoma [J]. Front Immunol. 2022;13:986214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.S L, Y W, Y C, et al. Pan-cancer analysis of cuproptosis regulation patterns and identification of mTOR-target responder in clear cell renal cell carcinoma [J]. Biol Direct, 2022, 17(1): 28. [DOI] [PMC free article] [PubMed]
- 90.Weipu M, Zhiyong D, Wang K, et al. Cuproptosis-related MTF1 inhibits kidney renal clear cell carcinoma progression by suppressing proliferation and regulating immune cell infiltration [J]. Acta Materia Medica; 2023.
- 91.L S, R Z, K Y, et al. The biological significance of cuproptosis-key gene MTF1 in pan-cancer and its inhibitory effects on ROS-mediated cell death of liver hepatocellular carcinoma [J]. Discover Oncol, 2023, 14(1): 113. [DOI] [PMC free article] [PubMed]
- 92.L ZL. Identification of GLS as a cuproptosis-related diagnosis gene in acute myocardial infarction [J]. Front Cardiovasc Med. 2022;9:1016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D Z. GLS as a diagnostic biomarker in breast cancer: in-silico, in-situ, and in-vitro insights [J]. Front Oncol. 2023;13:1220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Z Z, Y Z DL, et al. Prognostic and immune correlation evaluation of a novel cuproptosis-related genes signature in hepatocellular carcinoma [J]. Front Pharmacol. 2022;13:1074123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.K F, Y D, T L, et al. Cuproptosis-associated CDKN2A is targeted by plicamycin to regulate the microenvironment in patients with head and neck squamous cell carcinoma [J]. Front Genet, 2022, 13: 1036408. [DOI] [PMC free article] [PubMed]
- 96.B W, Q S, Y W, et al. Comprehensive investigation into cuproptosis in the characterization of clinical features, molecular characteristics, and immune situations of clear cell renal cell carcinoma [J]. Front Immunol, 2022, 13: 948042. [DOI] [PMC free article] [PubMed]
- 97.Xin X, Xuehua Qianzil. Z, et al. Comprehensive analysis of cuproptosis-related genes in immune infiltration and prognosis in lung adenocarcinoma [J]. Computers in Biology and Medicine; 2023. [DOI] [PubMed]
- 98.Jia-Hao B, Weicheng L. HAO D, et al. Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas [J]. Frontiers in Immunology; 2022. [DOI] [PMC free article] [PubMed]
- 99.Yuhui Y, Yun W, Ende Y, et al. Cuproptosis-related gene – SLC31A1, FDX1 and ATP7B – polymorphisms are Associated with risk of Lung Cancer [J]. Pharmacogenomics and Personalized Medicine; 2022. [DOI] [PMC free article] [PubMed]
- 100.Z Y. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death [J]. Cell Prolif. 2019;52(2):e12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.J W, S L, Y G, et al. Cuproptosis-related gene SLC31A1 expression correlates with the prognosis and tumor immune microenvironment in glioma [J]. Functional & integrative genomics, 2023, 23(3): 279. [DOI] [PMC free article] [PubMed]
- 102.X L, Z M. Cuproptosis-related gene SLC31A1 is a potential predictor for diagnosis, prognosis and therapeutic response of breast cancer [J]. Am J cancer Res. 2022;12(8):3561–80. [PMC free article] [PubMed] [Google Scholar]
- 103.MA C, HB P. Increasing intracellular bioavailable copper selectively targets prostate cancer cells [J]. ACS Chem Biol. 2013;8(7):1621–31. [DOI] [PubMed] [Google Scholar]
- 104.D W, Z T. P Z, et al. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease [J]. Volume 163. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2023. p. 114830. [DOI] [PubMed]
- 105.Wensheng X, Zhenhu G, Lingyun Z et al. The copper age in cancer treatment: from copper metabolism to cuproptosis [J]. Prog Mater Sci, 2023.
- 106.Y I DY. S N. Copper chelation inhibits tumor angiogenesis in the experimental 9L gliosarcoma model [J]. Neurosurgery, 1995, 37(2): 287 – 92; discussion 92 – 3. [DOI] [PubMed]
- 107.DC B, MS C, ML T, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis [J]. Nature. 2014;509(7501):492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filip M, Bell J, Federica S, et al. DIPG-20. Copper chelation therapy targets S-adenosylmethionine (SAM) metabolism and epigenetic regulators in diffuse intrinsic pontine glioma (DIPG) [J]. Neuro-Oncology; 2022.
- 109.F V, E V, L L, et al. Intratumoral Copper modulates PD-L1 expression and influences Tumor Immune evasion [J]. Cancer Res, 2020, 80(19): 4129–44. [DOI] [PubMed]
- 110.DE MXMC. Copper chelation as targeted therapy in a mouse model of oncogenic BRAF-Driven papillary thyroid Cancer [J]. Clin cancer Research: Official J Am Association Cancer Res. 2018;24(17):4271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.EM P. Copper chelation suppresses epithelial-mesenchymal transition by inhibition of canonical and non-canonical TGF-β signaling pathways in cancer [J]. Cell Bioscience. 2023;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ensieh M P, Daniele M, Prahlad R, et al. Copper chelation inhibits TGF-βpathways and suppresses epithelial-mesenchymal transition in cancer [J]. bioRxiv (Cold Spring Harbor Laboratory); 2022.
- 113.P Z, J Q, C Z, et al. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer [J]. Biomaterials, 2019, 195: 86–99. [DOI] [PubMed]
- 114.DM H, S H. High-affinity cu(I) chelator PSP-2 as potential anti-angiogenic agent [J]. Sci Rep. 2019;9(1):14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noh D, Lee H, Lee S et al. Copper-based nanomedicines for cuproptosis-mediated effective Cancer treatment [J]. Biomaterials Res, 2024, 28. [DOI] [PMC free article] [PubMed]
- 116.Ren X, Luo X, Wang F et al. Recent advances in copper homeostasis-involved tumor theranostics [J]. Asian J Pharm Sci, 2024, 19(5). [DOI] [PMC free article] [PubMed]
- 117.Shengxin H, Yong E G, Xianbin M et al. Tumor microenvironment responsive biomimetic copper peroxide nanoreactors for drug delivery and enhanced chemodynamic therapy [J]. Chem Eng J, 2021.
- 118.Ze H, ZN JL. One-Pot synthesis-biocompatible copper-tripeptide complex as a Nanocatalytic Medicine to Enhance Chemodynamic therapy [J]. ACS Biomaterials Sci Eng. 2021;7(4):1394–402. [DOI] [PubMed] [Google Scholar]
- 119.Yan Z, Zhang H, Chen J et al. Cu(II)-doped mesoporous polydopamine as biodegradable nanoplatforms for photothermal-enhanced multi-mode anti-tumor therapy [J]. Colloids Surf a, 2024, 685.
- 120.Xu Q, Li J, Liu B et al. Biodegradable copper-doped hollow mesoporous polydopamine nanoparticles for chemo/photothermal/chemodynamic synergistic therapy [J]. J Drug Deliv Sci Technol, 2023, 89.
- 121.Zhou X, Feng S, Xu Q, et al. Current advances in nanozyme-based nanodynamic therapies for cancer [J]. Acta Biomaterialia; 2024. [DOI] [PubMed]
- 122.W YL. All-in-one theranostic nanoagent with enhanced reactive Oxygen species Generation and modulating Tumor Microenvironment ability for effective tumor eradication [J]. ACS Nano. 2018;12(5):4886–93. [DOI] [PubMed] [Google Scholar]
- 123.Q Y. DNAzyme-Mediated Cascade Nanoreactor for cuproptosis-promoted pancreatic Cancer synergistic therapy [J]. Adv Healthc Mater. 2023;12(28):e2301429. [DOI] [PubMed] [Google Scholar]
- 124.S Y YS. Multifaceted roles of copper ions in Anticancer Nanomedicine [J]. Adv Healthc Mater. 2023;12(23):e2300410. [DOI] [PubMed] [Google Scholar]
- 125.CA SG et al. F, F C,. Activatable Hybrid Nanotheranostics for Tetramodal Imaging and Synergistic Photothermal/Photodynamic Therapy [J]. Advanced materials (Deerfield Beach, Fla), 2018, 30(6). [DOI] [PMC free article] [PubMed]
- 126.E J, K D, Z C, et al. Copper(II)-Graphitic Carbon Nitride Triggered Synergy: Improved ROS Generation and reduced glutathione levels for enhanced photodynamic therapy [J]. Angewandte Chemie (International ed in English), 2016, 55(38): 11467–71. [DOI] [PubMed]
- 127.L H, Y Z, XW C, et al. Protein-modified hollow copper sulfide nanoparticles carrying indocyanine green for photothermal and photodynamic therapy [J]. J Mater Chem B, 2016, 4(1): 105–12. [DOI] [PubMed]
- 128.MS SBPM. Chlorin e6 conjugated copper sulfide nanoparticles for photodynamic combined photothermal therapy [J]. Photodiagn Photodyn Ther. 2017;19:128–34. [DOI] [PubMed] [Google Scholar]
- 129.B G, F Y, L Z, et al. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy [J]. Advanced materials (Deerfield Beach, Fla), 2023, 35(22): e2212267. [DOI] [PubMed]
- 130.H WL. Nanomedicine enables drug-potency activation with Tumor Sensitivity and Hyperthermia Synergy in the Second Near-Infrared biowindow [J]. ACS Nano. 2021;15(4):6457–70. [DOI] [PubMed] [Google Scholar]
- 131.Jun Z, Xiang Z, Yuan W et al. In situ sacrificial growth of metastable copper-enriched nanomedicine for cuproptosis-based synergistic cancer therapy [J]. Chem Eng J, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.