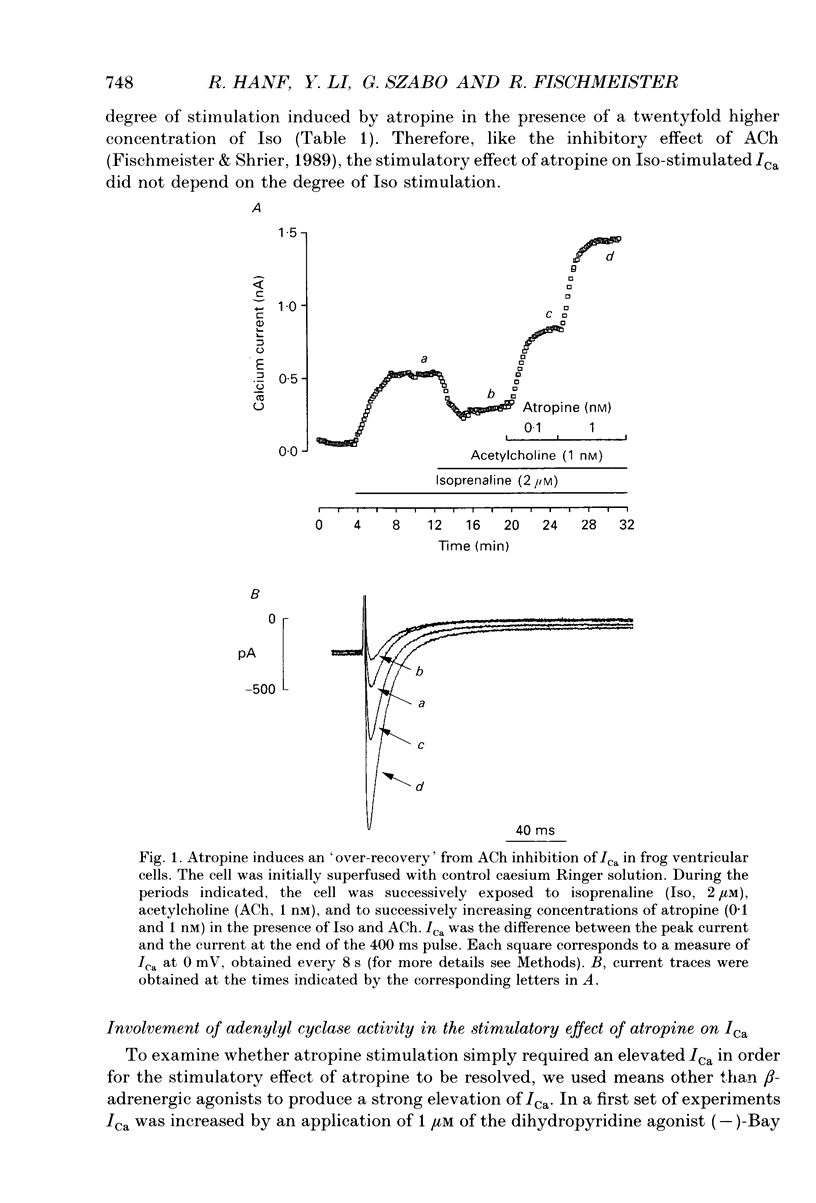
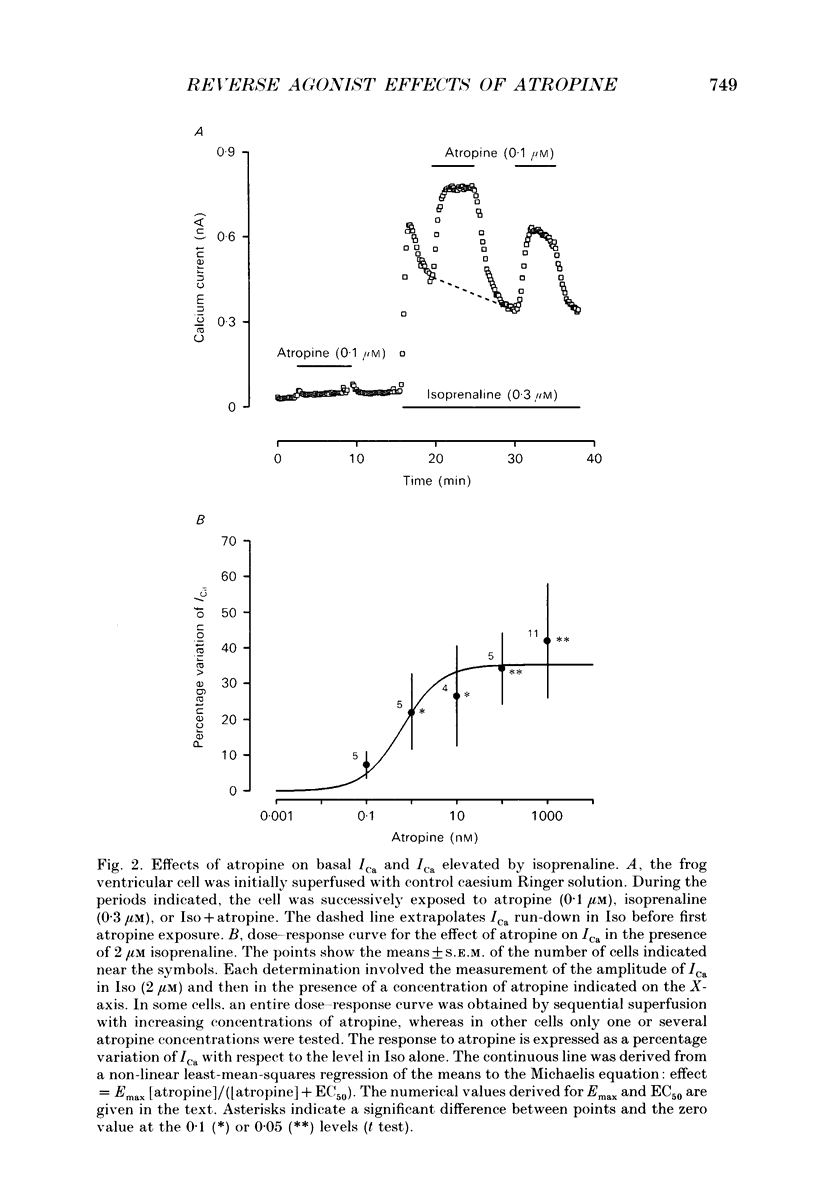
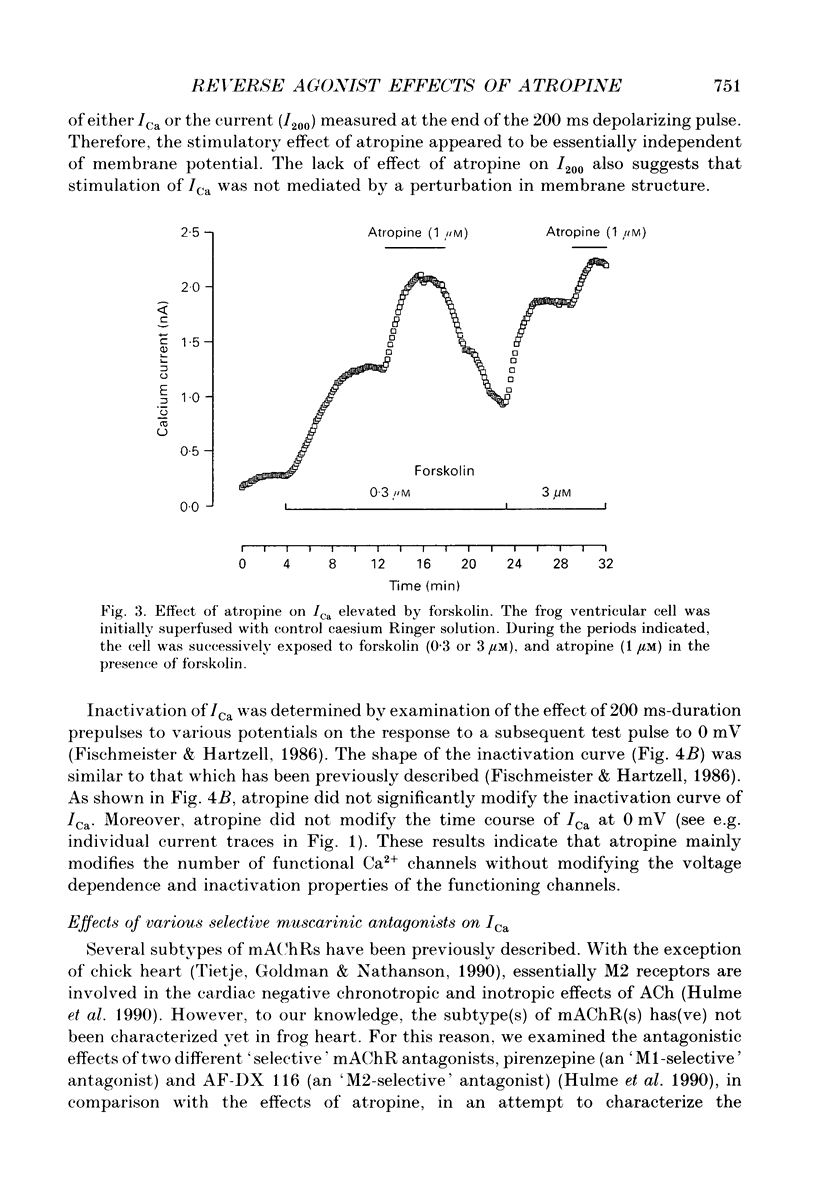
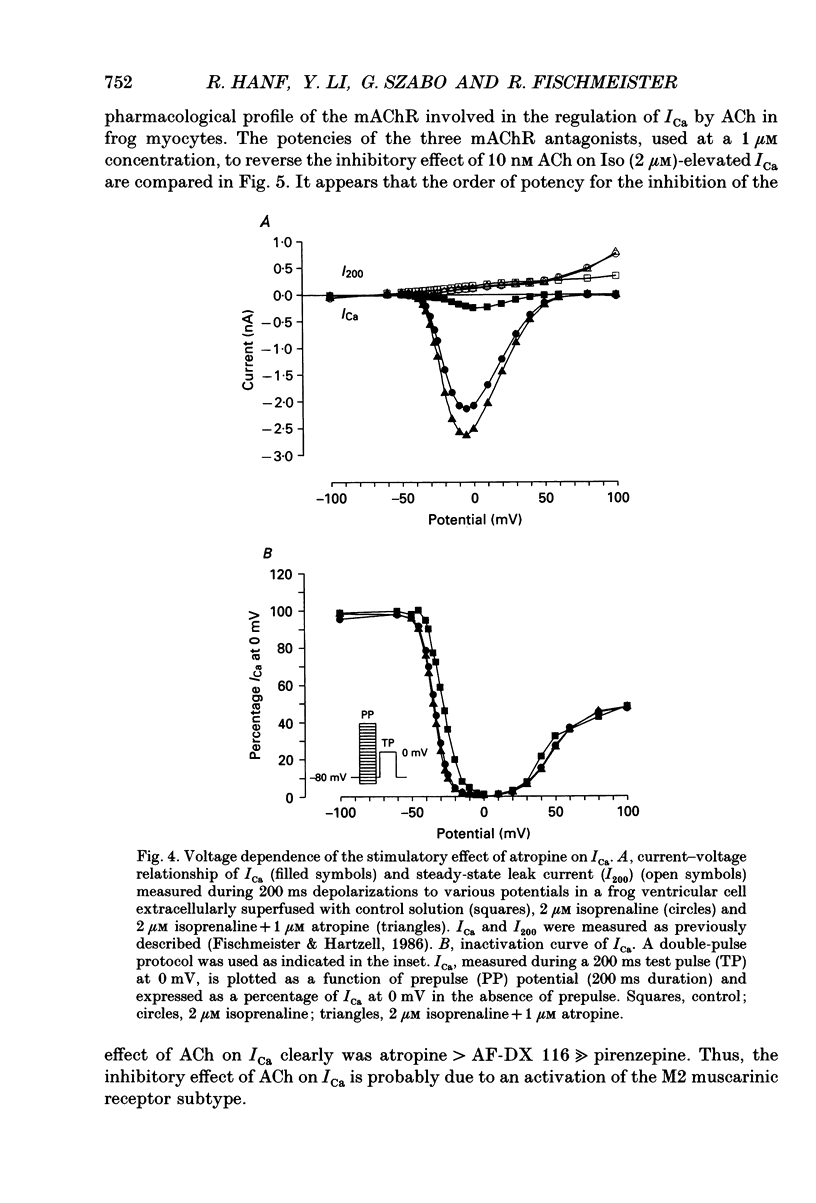
Abstract
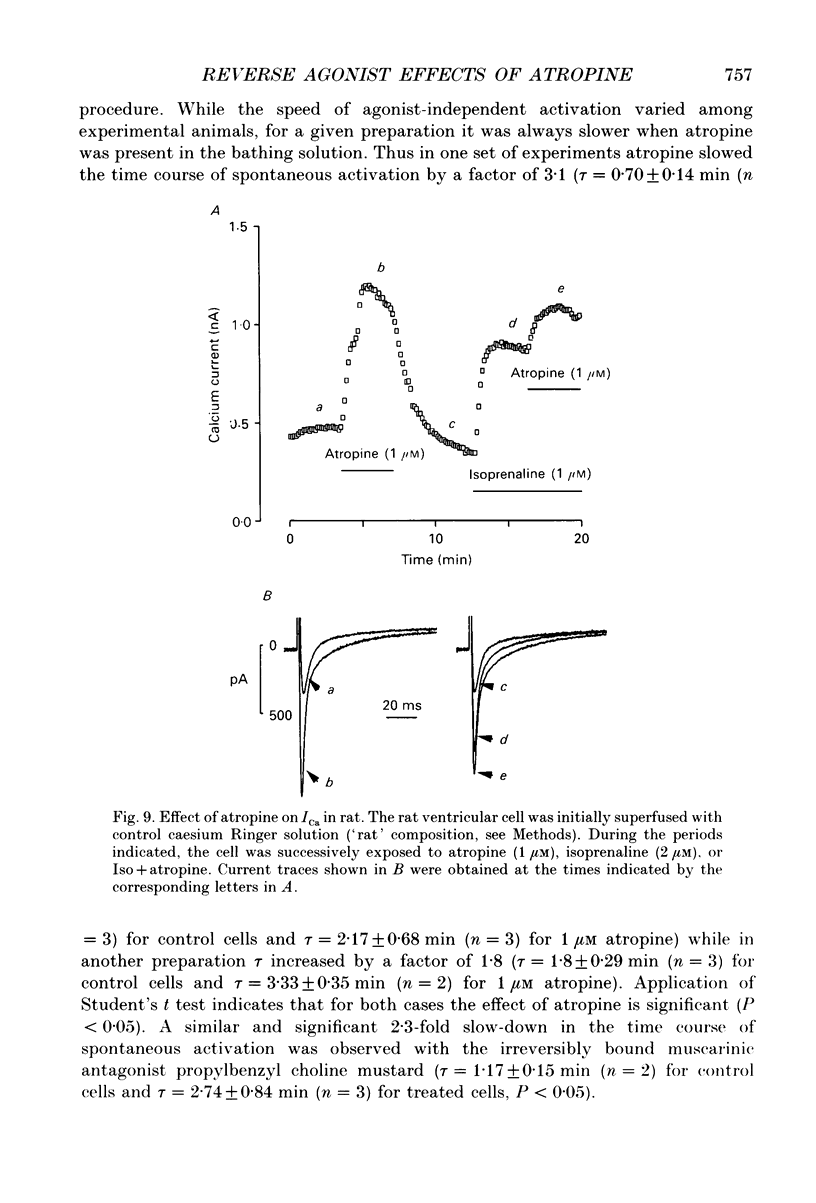
1. The whole-cell patch clamp and intracellular perfusion techniques were used for studying the effects of atropine and other muscarinic acetylcholine receptor (mAChR) antagonists on the L-type calcium currents (ICa) in frog and rat ventricular myocytes, and on the mAChR-activated K+ current (IK(ACh)) in frog atrial myocytes. 2. In frog ventricular myocytes, atropine (0.1 nM to 1 microM) reversed the inhibitory effect of acetylcholine (ACh, 1 nM) on ICa previously stimulated by isoprenaline (Iso, 2 microM), a beta-adrenergic agonist. However, in the concomitant presence of Iso, ACh and atropine, ICa was > 50% larger than in Iso alone. 3. The effects of atropine were then examined in the absence of mAChR agonists. After a preliminary stimulation of ICa with Iso (0.1 or 2 microM), atropine induced a dose-dependent stimulation of ICa. EC50 (i.e. the concentration of atropine at which the response was 50% of the maximum) and Emax (i.e. maximal stimulation of ICa expressed as percentage increase in ICa with respect to the level in Iso alone) were respectively 0.6 nM and 35%. The stimulatory effect of atropine on ICa was not voltage dependent. 4. Atropine (1 microM) had no effect on frog ICa (i) under basal conditions, (ii) upon stimulation of ICa by the dihydropyridine agonist (-)-Bay K 8644 (1 microM), or (iii) when ICa had been previously stimulated by intracellular perfusion with cyclic AMP (3 microM). However, atropine increased ICa after a stimulation by forskolin (0.3 microM). Therefore, an increased adenylyl cyclase activity was required for atropine to produce its stimulatory effect on ICa. 5. The order of potency of mAChR antagonists to reverse the inhibitory effect of ACh on Iso elevated ICa in frog ventricle was atropine > AF-DX 116 >> pirenzepine. In the absence of ACh, mAChR antagonists produced their stimulatory effect on Iso elevated ICa with the same order of potency. 6. Intracellular substitution of Gpp(NH)p (5'-guanylylimidiphosphate) for GTP (420 microM) induced a strong inhibition of frog ICa in the presence of Iso (2 microM). This effect was attributed earlier to the spontaneous and irreversible activation of the GTP-binding regulatory protein (G protein), Gi, responsible for adenylyl cyclase inhibition. Atropine (1 microM) slowed down by a factor of 2 the rate of ICa inhibition induced by Gpp(NH)p. 7. In frog atrial myocytes, intracellular perfusion with 1 mM Gpp(NH)p induces spontaneous activation of IK(ACh). This effect was attributed earlier to the spontaneous and irreversible activation of the G protein, GK.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





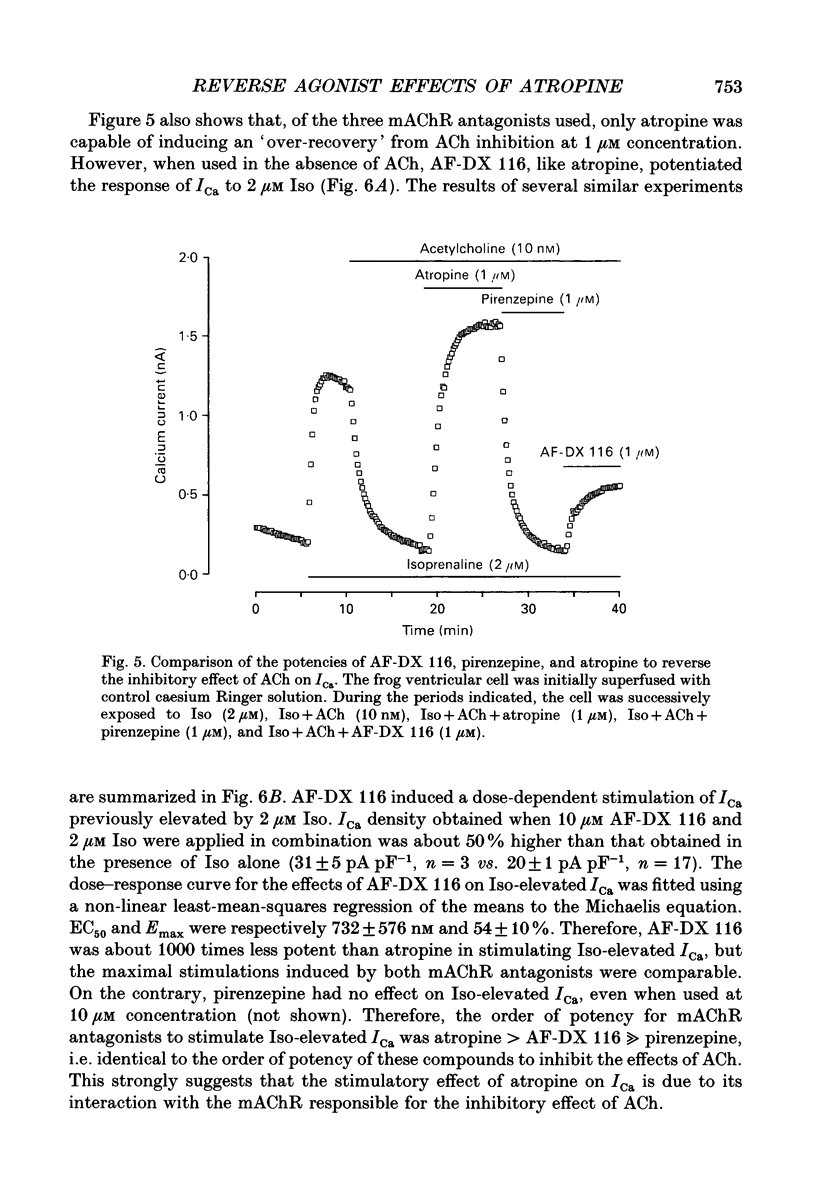
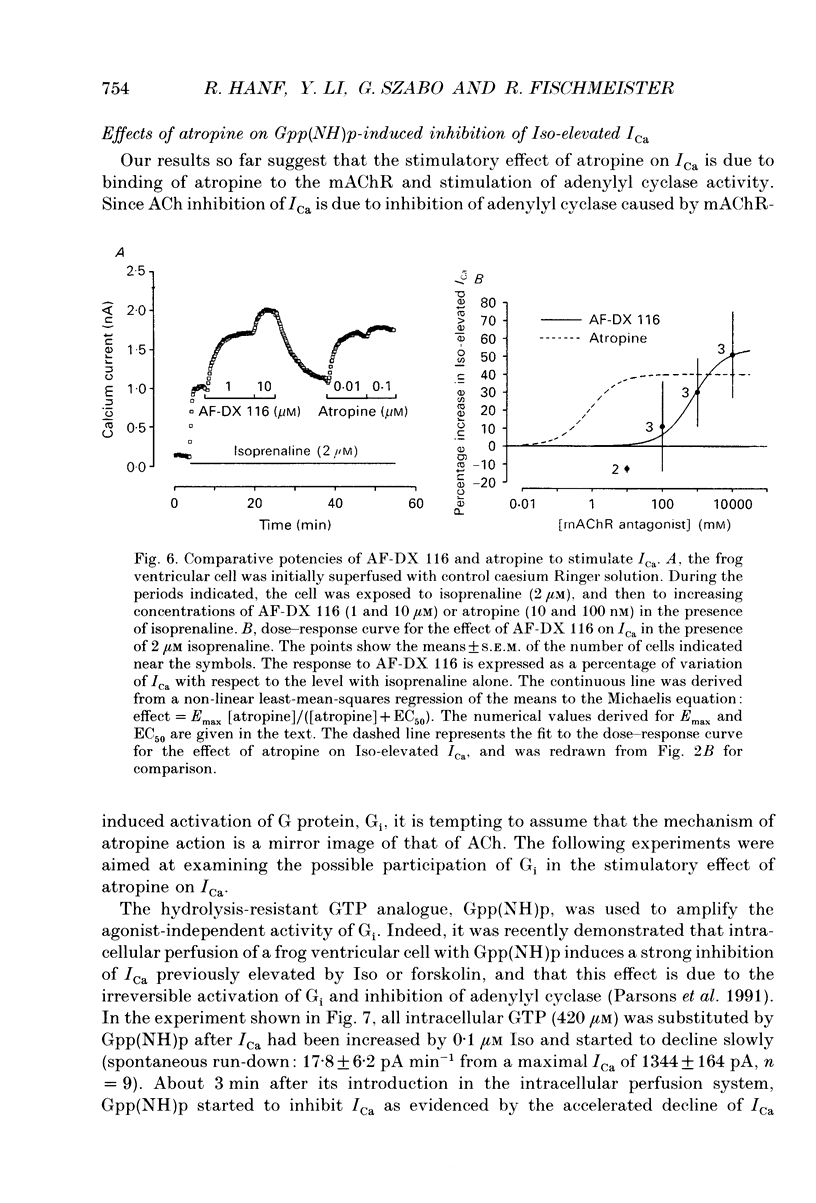
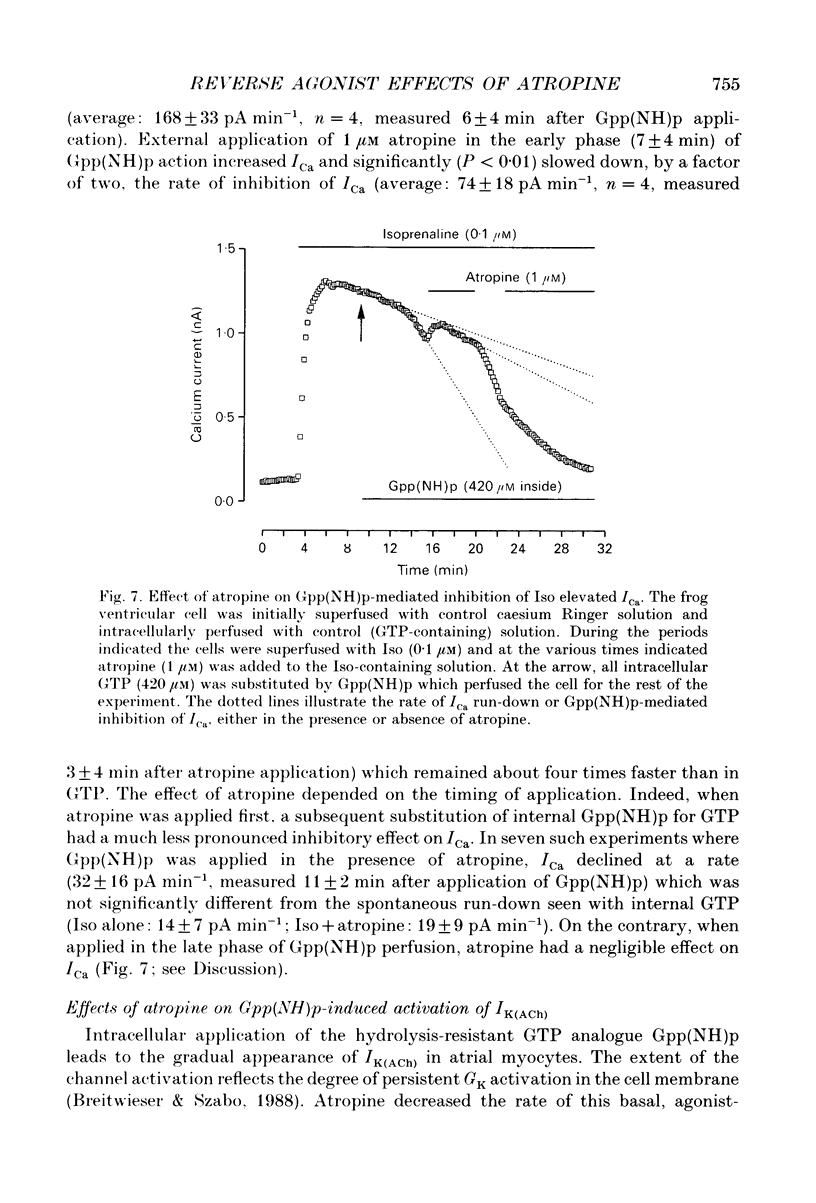
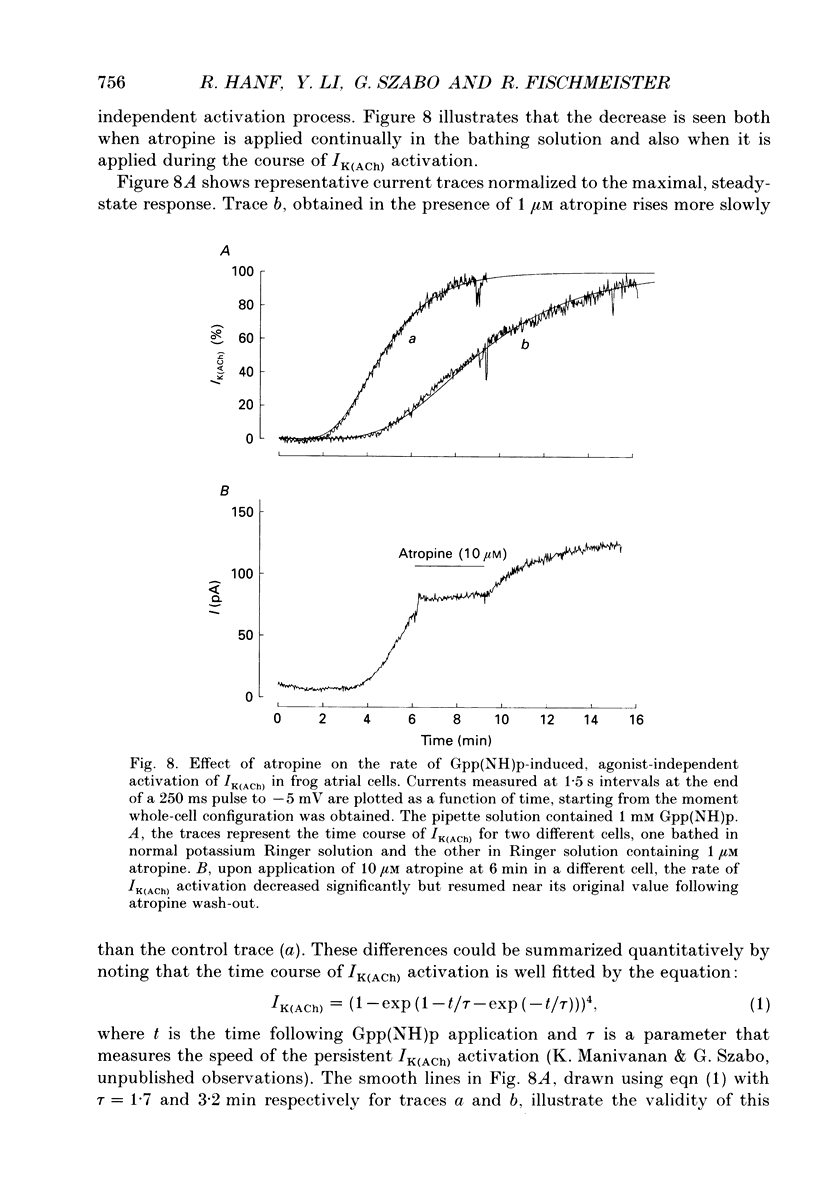







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Guanine nucleotides modulate muscarinic receptor binding in the heart. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1000–1005. doi: 10.1016/s0006-291x(79)80006-6. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. A., Humphrey S. M., Harding S. E. The anti-adrenergic effect of adenosine and its blockade by pertussis toxin: a comparative study in myocytes isolated from guinea-pig, rat and failing human hearts. Br J Pharmacol. 1990 Oct;101(2):484–488. doi: 10.1111/j.1476-5381.1990.tb12734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
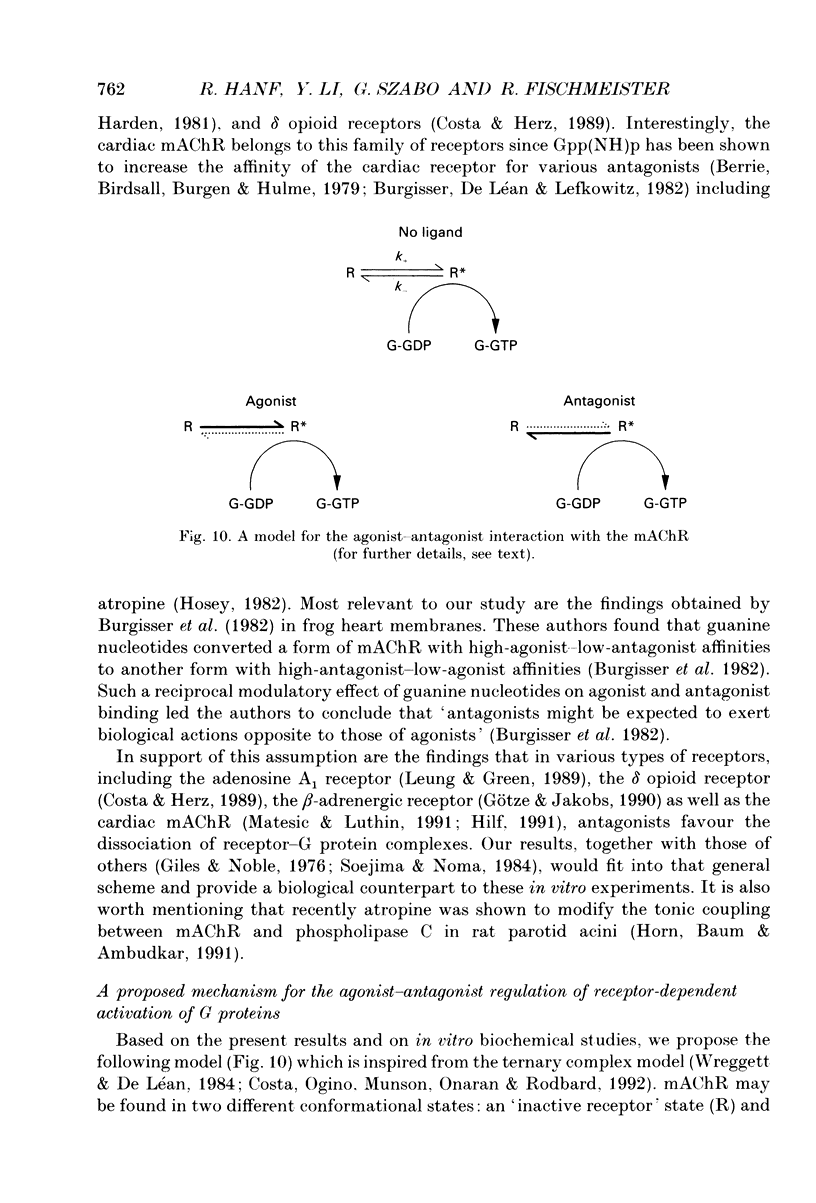
- Burgisser E., De Lean A., Lefkowitz R. J. Reciprocal modulation of agonist and antagonist binding to muscarinic cholinergic receptor by guanine nucleotide. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1732–1736. doi: 10.1073/pnas.79.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T., Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lean A., Kilpatrick B. F., Caron M. G. Guanine nucleotides regulate both dopaminergic agonist and antagonist binding in porcine anterior pituitary. Endocrinology. 1982 Mar;110(3):1064–1066. doi: 10.1210/endo-110-3-1064. [DOI] [PubMed] [Google Scholar]
- Feldman A. M. Experimental issues in assessment of G protein function in cardiac disease. Circulation. 1991 Oct;84(4):1852–1861. doi: 10.1161/01.cir.84.4.1852. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Regulation of calcium current by low-Km cyclic AMP phosphodiesterases in cardiac cells. Mol Pharmacol. 1990 Sep;38(3):426–433. [PubMed] [Google Scholar]
- Fischmeister R., Shrier A. Interactive effects of isoprenaline, forskolin and acetylcholine on Ca2+ current in frog ventricular myocytes. J Physiol. 1989 Oct;417:213–239. doi: 10.1113/jphysiol.1989.sp017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Holmer S. R., Homcy C. J. G proteins in the heart. A redundant and diverse transmembrane signaling network. Circulation. 1991 Nov;84(5):1891–1902. doi: 10.1161/01.cir.84.5.1891. [DOI] [PubMed] [Google Scholar]
- Horn V. J., Baum B. J., Ambudkar I. S. Muscarinic receptor antagonist effects in parotid acini and M1-CHO cells: evidence of G protein involvement. Biochem Biophys Res Commun. 1991 Jun 14;177(2):784–789. doi: 10.1016/0006-291x(91)91857-9. [DOI] [PubMed] [Google Scholar]
- Hosey M. M. Regulation of antagonist binding to cardiac muscarinic receptors. Biochem Biophys Res Commun. 1982 Jul 16;107(1):314–321. doi: 10.1016/0006-291x(82)91706-5. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Ito H., Sugimoto T., Kobayashi I., Takahashi K., Katada T., Ui M., Kurachi Y. On the mechanism of basal and agonist-induced activation of the G protein-gated muscarinic K+ channel in atrial myocytes of guinea pig heart. J Gen Physiol. 1991 Sep;98(3):517–533. doi: 10.1085/jgp.98.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Kaibara M., Nakajima T., Irisawa H., Giles W. Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol. 1991 Feb;433:589–613. doi: 10.1113/jphysiol.1991.sp018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E., Green R. D. Density gradient profiles of A1 adenosine receptors labeled by agonist and antagonist radioligands before and after detergent solubilization. Mol Pharmacol. 1989 Sep;36(3):412–419. [PubMed] [Google Scholar]
- Matesic D. F., Luthin G. R. Atropine dissociates complexes of muscarinic acetylcholine receptor and guanine nucleotide-binding protein in heart membranes. FEBS Lett. 1991 Jun 24;284(2):184–186. doi: 10.1016/0014-5793(91)80680-2. [DOI] [PubMed] [Google Scholar]
- Milligan G., Green A. Agonist control of G-protein levels. Trends Pharmacol Sci. 1991 Jun;12(6):207–209. doi: 10.1016/0165-6147(91)90551-3. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot J., Garnier D., Rougier O. Analysis of the negative inotropic effect of acetylcholine on frog atrial fibres. J Physiol (Paris) 1981 Mar;77(8):829–843. [PubMed] [Google Scholar]
- Okabe K., Yatani A., Brown A. M. The nature and origin of spontaneous noise in G protein-gated ion channels. J Gen Physiol. 1991 Jun;97(6):1279–1293. doi: 10.1085/jgp.97.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in the beta-adrenergic modulation of calcium currents in rabbit ventricular cells. Circ Res. 1992 Jan;70(1):104–115. doi: 10.1161/01.res.70.1.104. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Li Y., Szabo G. Receptor-mediated deactivation of Gk in cardiac myocytes. Pflugers Arch. 1991 Jan;417(5):543–545. doi: 10.1007/BF00370953. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Lagrutta A., White R. E., Hartzell H. C. Regulation of Ca2+ current in frog ventricular cardiomyocytes by 5'-guanylylimidodiphosphate and acetylcholine. J Physiol. 1991 Jan;432:593–620. doi: 10.1113/jphysiol.1991.sp018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. Reciprocal modulation of agonist and antagonist binding to A1 adenosine receptors by guanine nucleotides is mediated via a pertussis toxin-sensitive G protein. J Pharmacol Exp Ther. 1988 Sep;246(3):1194–1200. [PubMed] [Google Scholar]
- Reithmann C., Gierschik P., Sidiropoulos D., Werdan K., Jakobs K. H. Mechanism of noradrenaline-induced heterologous desensitization of adenylate cyclase stimulation in rat heart muscle cells: increase in the level of inhibitory G-protein alpha-subunits. Eur J Pharmacol. 1989 Aug 15;172(3):211–221. doi: 10.1016/0922-4106(89)90051-5. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Szabo G., Otero A. S. G protein mediated regulation of K+ channels in heart. Annu Rev Physiol. 1990;52:293–305. doi: 10.1146/annurev.ph.52.030190.001453. [DOI] [PubMed] [Google Scholar]
- Tietje K. M., Goldman P. S., Nathanson N. M. Cloning and functional analysis of a gene encoding a novel muscarinic acetylcholine receptor expressed in chick heart and brain. J Biol Chem. 1990 Feb 15;265(5):2828–2834. [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K. Guanine nucleotides modulate the affinity of antagonists at beta-adrenergic receptors. J Cyclic Nucleotide Res. 1981;7(5):303–312. [PubMed] [Google Scholar]
- Wong Y. H., Federman A., Pace A. M., Zachary I., Evans T., Pouysségur J., Bourne H. R. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991 May 2;351(6321):63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., De Léan A. The ternary complex model. Its properties and application to ligand interactions with the D2-dopamine receptor of the anterior pituitary gland. Mol Pharmacol. 1984 Sep;26(2):214–227. [PubMed] [Google Scholar]


