Abstract
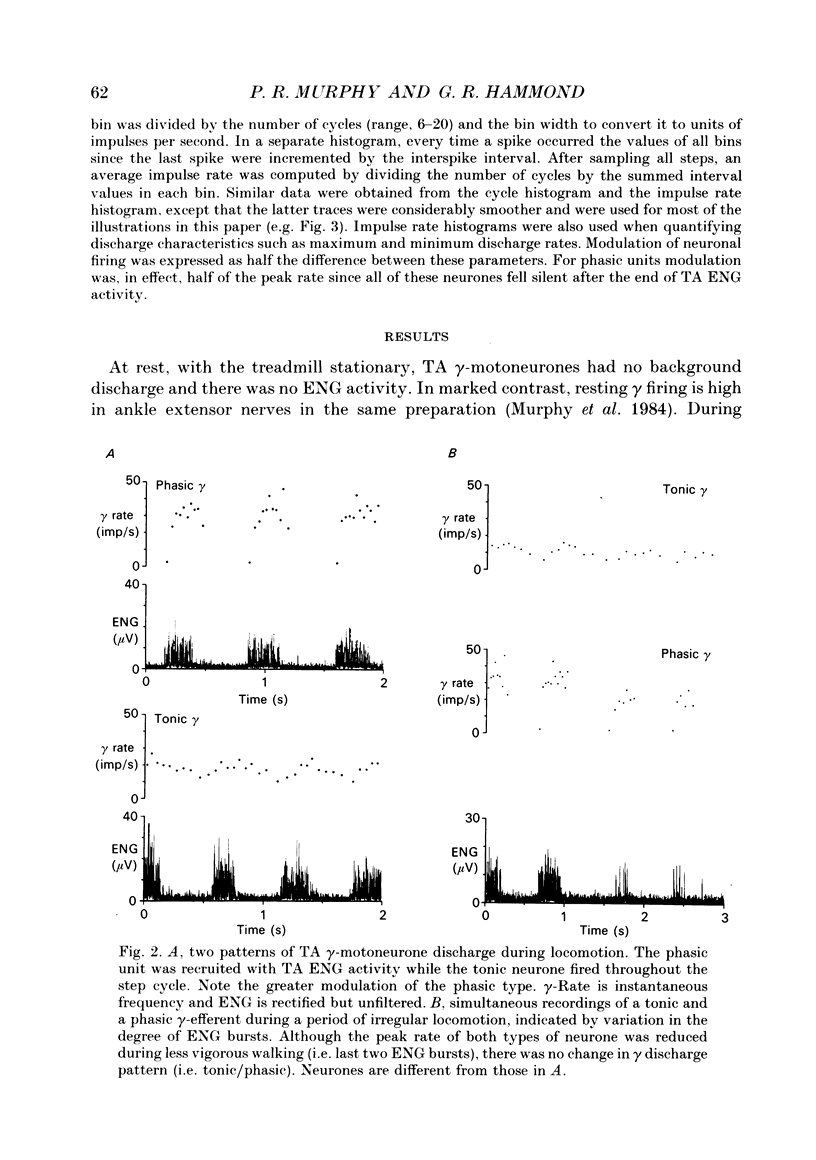
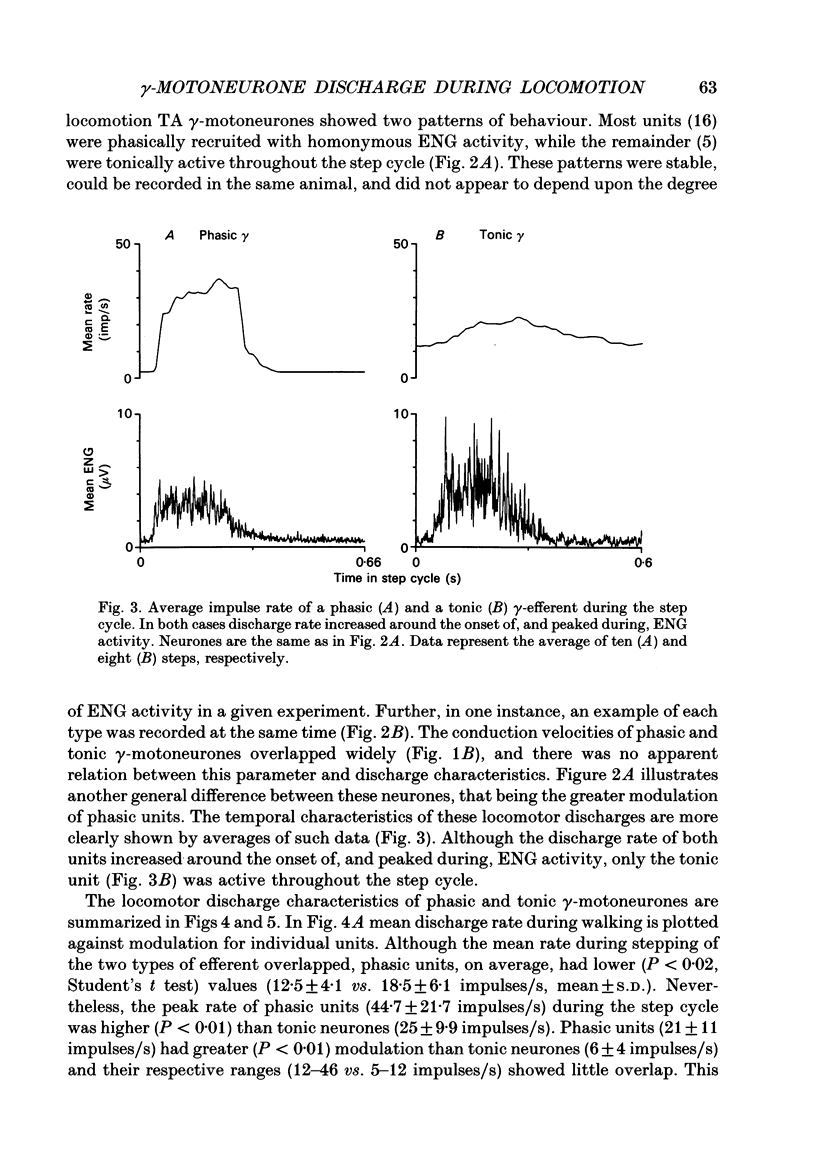
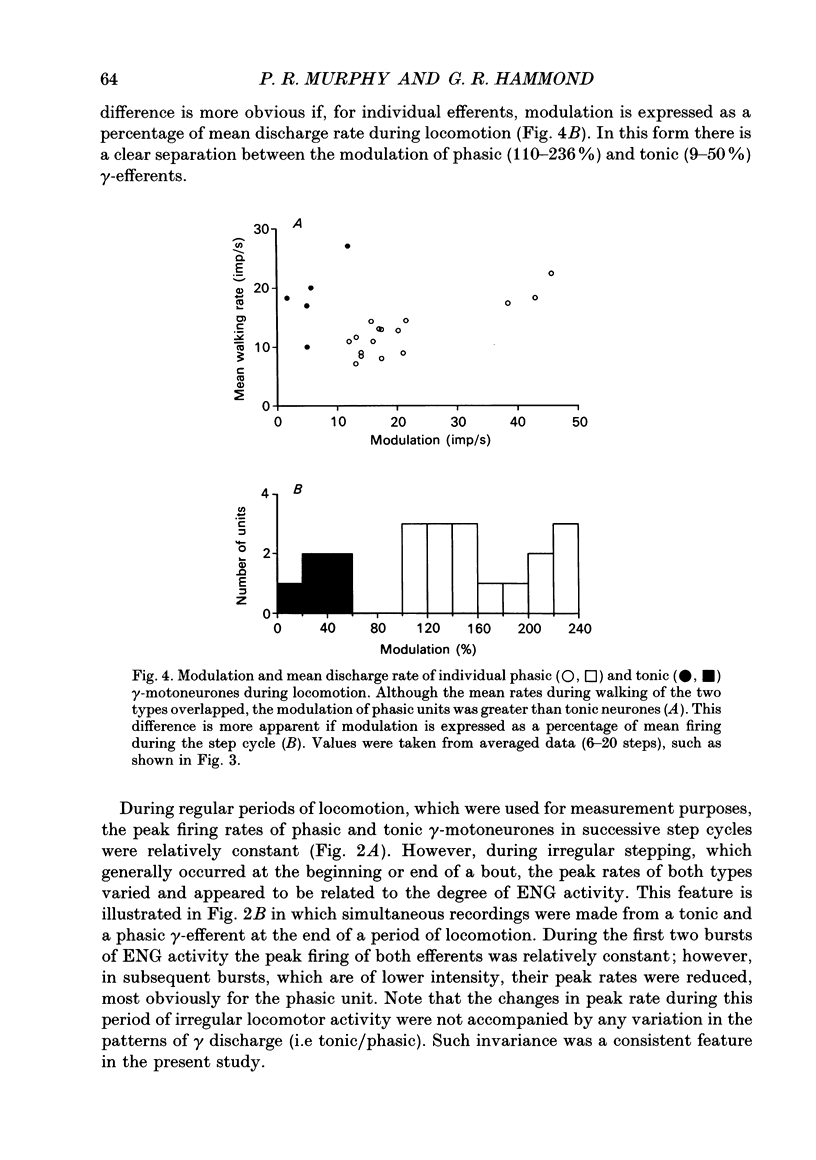
1. The discharge patterns of ankle flexor, tibialis anterior (TA), gamma-motoneurones were recorded during locomotion in the decerebrate cat. 2. At rest gamma-efferents had no background discharge. During locomotion two patterns of gamma activity could be distinguished. Most units (16) were phasically recruited with homonymous electroneurogram (ENG) activity, while the remainder (5) were tonically active throughout the step cycle. 3. The modulation of phasic units was greater (P < 0.01) than tonic neurones. Phasic units had lower (P < 0.02) mean, but higher (P < 0.01) peak, rates during the step cycle. 4. The discharge rate of both types of efferent increased around the onset of ENG activity and peaked during ENG activity, or shortly after its cessation. The conduction velocities of phasic and tonic units overlapped widely. 5. It is proposed, on the basis of muscle spindle afferent recordings during locomotion, that TA phasic and tonic units correspond to static and dynamic gamma-motoneurones, respectively. This correspondence is functionally advantageous for the role of ankle flexor muscles during locomotion. Thus phasic static gamma discharge during flexion would aid muscle contraction via increased Ia afferent activity, while tonic dynamic gamma firing would enhance Ia afferent stretch sensitivity throughout the step cycle. Such enhancement during flexion would oppose unexpected muscle lengthening while, during extension, it would contribute to reciprocal inhibition of ankle extensor muscles. 6. The results are discussed in relation to strategies of gamma usage during rhythmic movements. It is postulated that, for such behaviour, muscle contraction is accompanied by coactivity in static and dynamic gamma-motoneurones. A functional rationale is suggested for this strategy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Prochazka A., Proske U., Wand P. Effect of fusimotor stimulation on ia discharge during shortening of cat soleus muscle at different speeds. J Physiol. 1982 Aug;329:509–526. doi: 10.1113/jphysiol.1982.sp014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Joffroy M., Montoya R., Pagès B. Evidence of the co-activation of alpha-motoneurones and static gamma-motoneurones of the sartorius medialis muscle during locomotion in the thalamic cat. Exp Brain Res. 1990;82(1):191–198. doi: 10.1007/BF00230851. [DOI] [PubMed] [Google Scholar]
- Cabelguen J. M. Static and dynamic fusimotor action on the response of spindle primary endings to sinusoidal stretches in the cat. Brain Res. 1979 Jun 15;169(1):45–54. doi: 10.1016/0006-8993(79)90372-x. [DOI] [PubMed] [Google Scholar]
- Cabelguen J. M. Static and dynamic fusimotor controls in various hindlimb muscles during locomotor activity in the decorticate cat. Brain Res. 1981 May 25;213(1):83–97. doi: 10.1016/0006-8993(81)91249-x. [DOI] [PubMed] [Google Scholar]
- Capaday C., Cody F. W., Stein R. B. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. J Neurophysiol. 1990 Aug;64(2):607–616. doi: 10.1152/jn.1990.64.2.607. [DOI] [PubMed] [Google Scholar]
- Corda M., von Euler C., Lennerstrand G. Reflex and cerebellar influences on alpha and on 'rhythmic' and 'tonic' gamma activity in the intercostal muscle. J Physiol. 1966 Jun;184(4):898–923. doi: 10.1113/jphysiol.1966.sp007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKLUND G., VON EULER, RUTKOWSKI S. SPONTANEOUS AND REFLEX ACTIVITY OF INTERCOSTAL GAMMA MOTONEURONES. J Physiol. 1964 May;171:139–163. doi: 10.1113/jphysiol.1964.sp007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
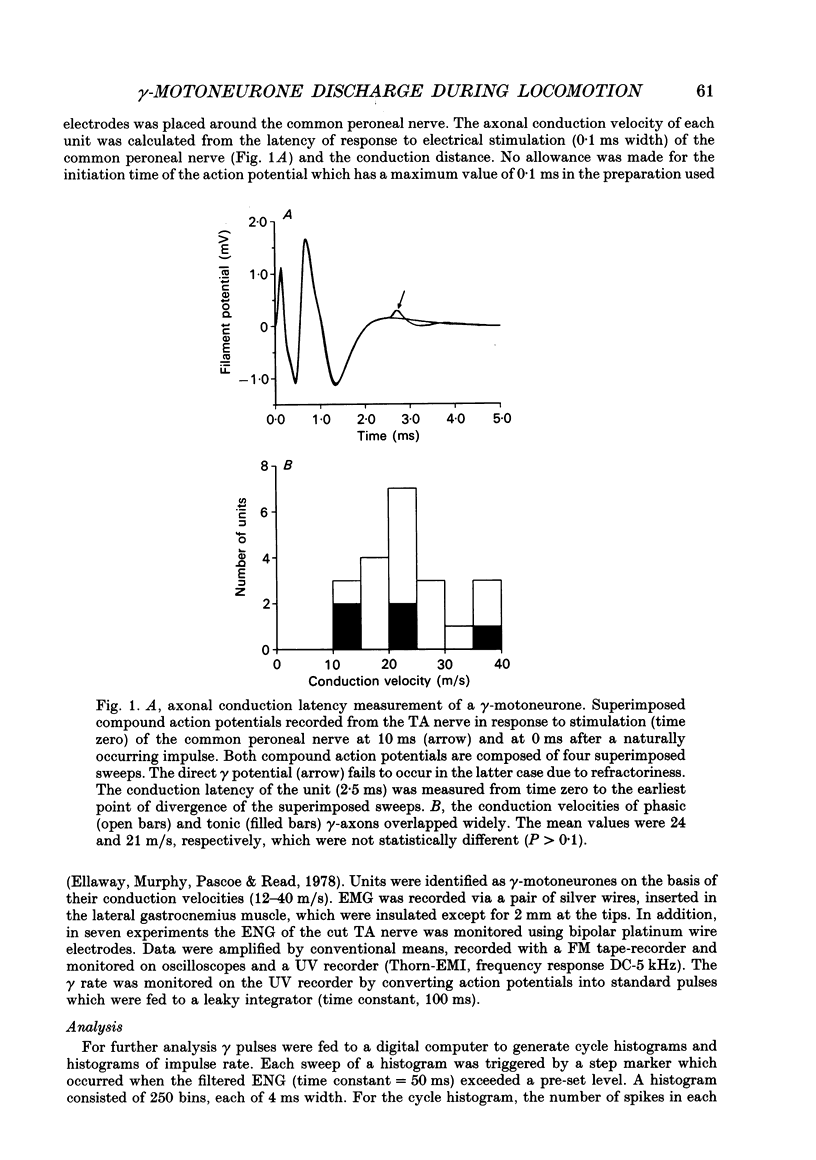
- Ellaway P. H., Murphy P. R., Pascoe J. E., Read G. L. Use of a delay line to measure conduction velocity of an axon [proceedings]. J Physiol. 1978 Jul;280:5P–6P. [PubMed] [Google Scholar]
- Greer J. J., Stein R. B. Fusimotor control of muscle spindle sensitivity during respiration in the cat. J Physiol. 1990 Mar;422:245–264. doi: 10.1113/jphysiol.1990.sp017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975 Apr;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Iliya A. R., Dum R. P. Somatotopic relations between the motor nucleus and its innervated muscle fibers in the cat tibialis anterior. Exp Neurol. 1984 Nov;86(2):272–292. doi: 10.1016/0014-4886(84)90186-9. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987;65(2):294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Stein R. B., Taylor J. Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol. 1984 Aug;52(2):228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Perret C., Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activity in the cat. Exp Brain Res. 1973 Sep 29;18(2):178–188. doi: 10.1007/BF00234722. [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Chan A. K., Goslow G. E., Jr The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol. 1978 Mar;155(3):253–269. doi: 10.1002/jmor.1051550302. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefchyk S. J., Stein R. B., Jordan L. M. Synaptic transmission from muscle afferents during fictive locomotion in the mesencephalic cat. J Neurophysiol. 1984 May;51(5):986–997. doi: 10.1152/jn.1984.51.5.986. [DOI] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- Taylor J., Stein R. B., Murphy P. R. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985 Feb;53(2):341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]
- Wand P., Prochazka A., Sontag K. H. Neuromuscular responses to gait perturbations in freely moving cats. Exp Brain Res. 1980;38(1):109–114. doi: 10.1007/BF00237937. [DOI] [PubMed] [Google Scholar]
- von Euler C., Peretti G. Dynamic and static contributions to the rhythmic y activation of primary and secondary spindle endings in external intercostal muscle. J Physiol. 1966 Dec;187(3):501–516. doi: 10.1113/jphysiol.1966.sp008106. [DOI] [PMC free article] [PubMed] [Google Scholar]


