Abstract
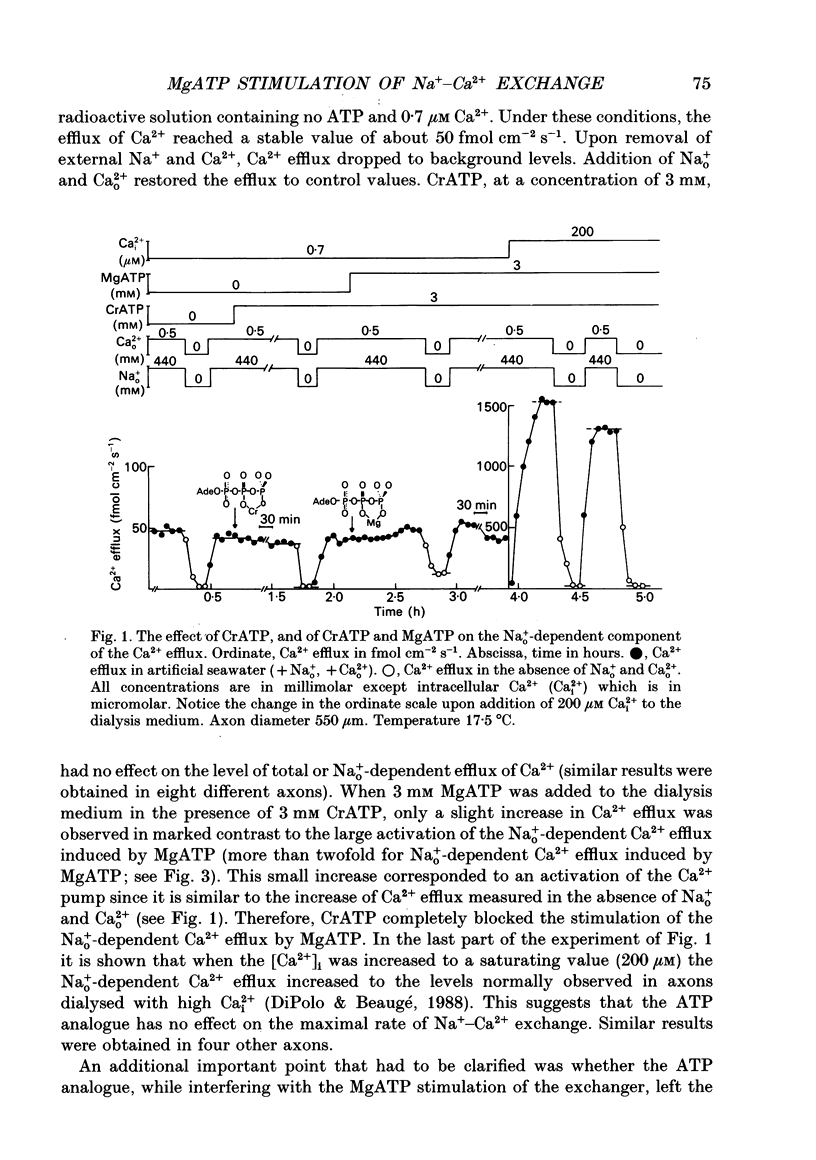
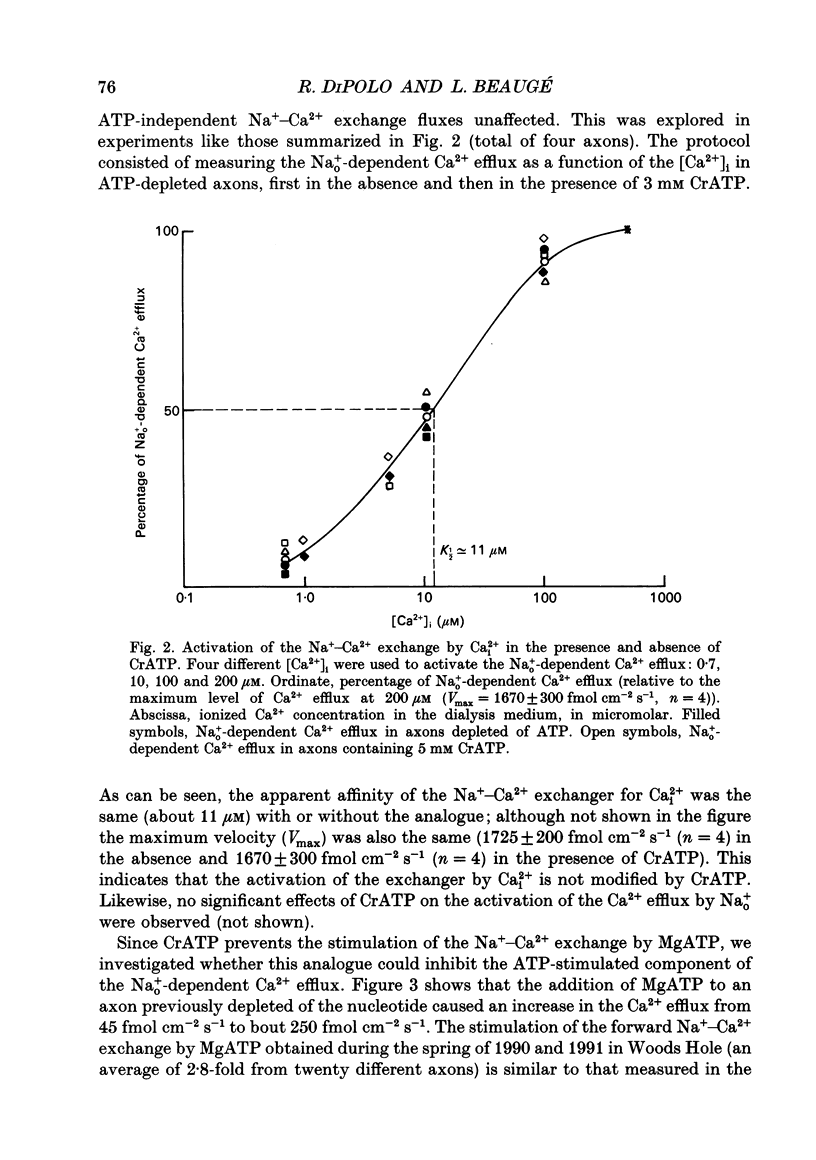
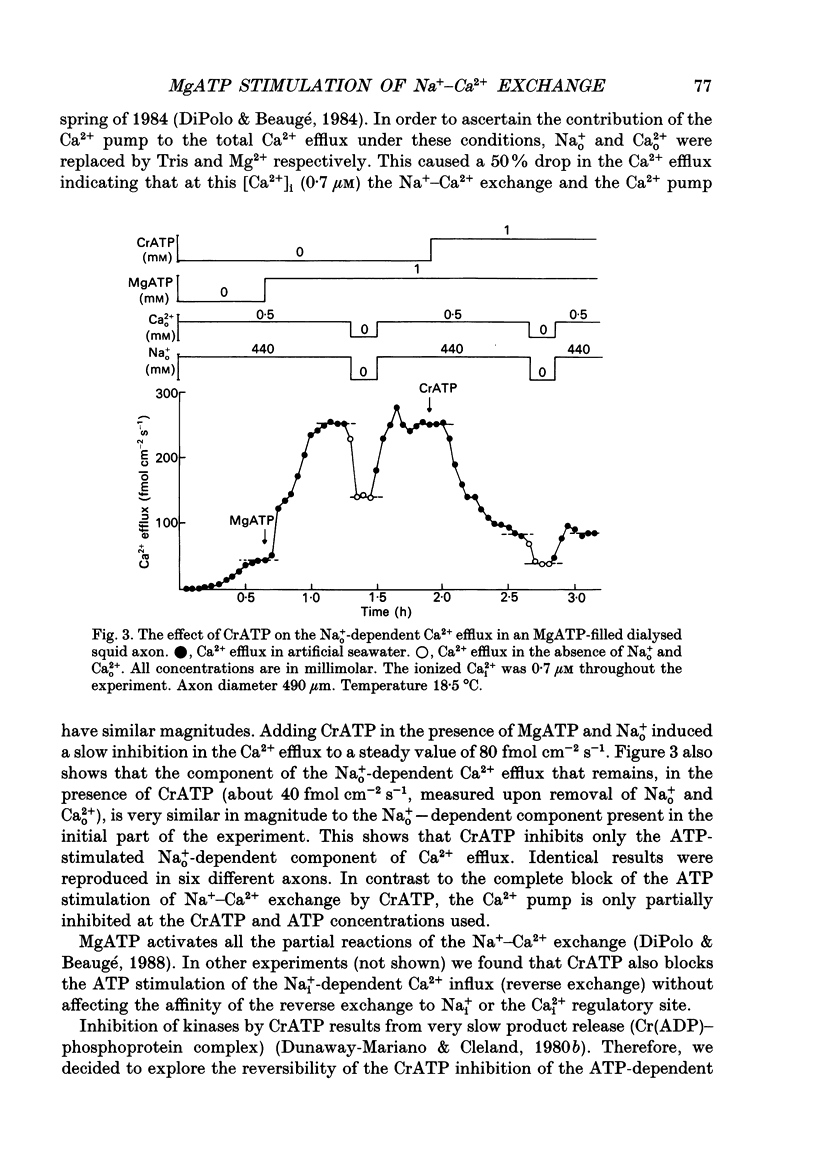
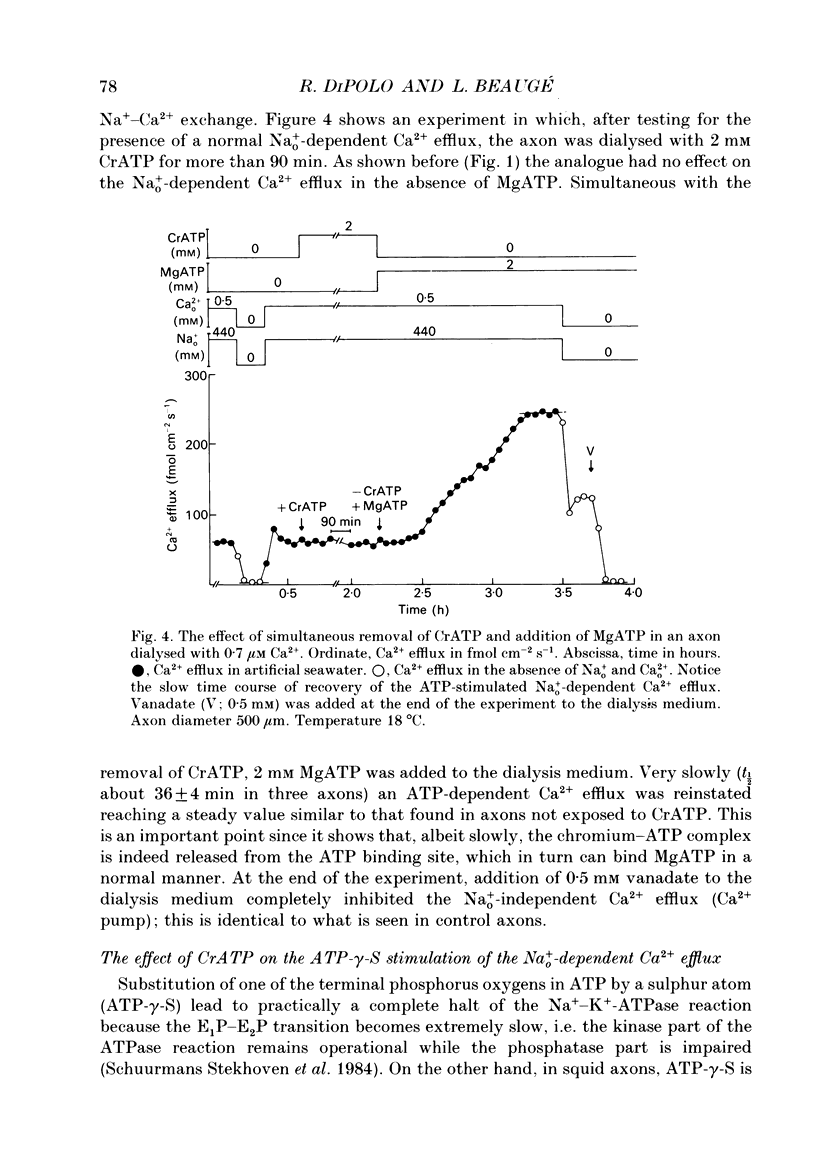
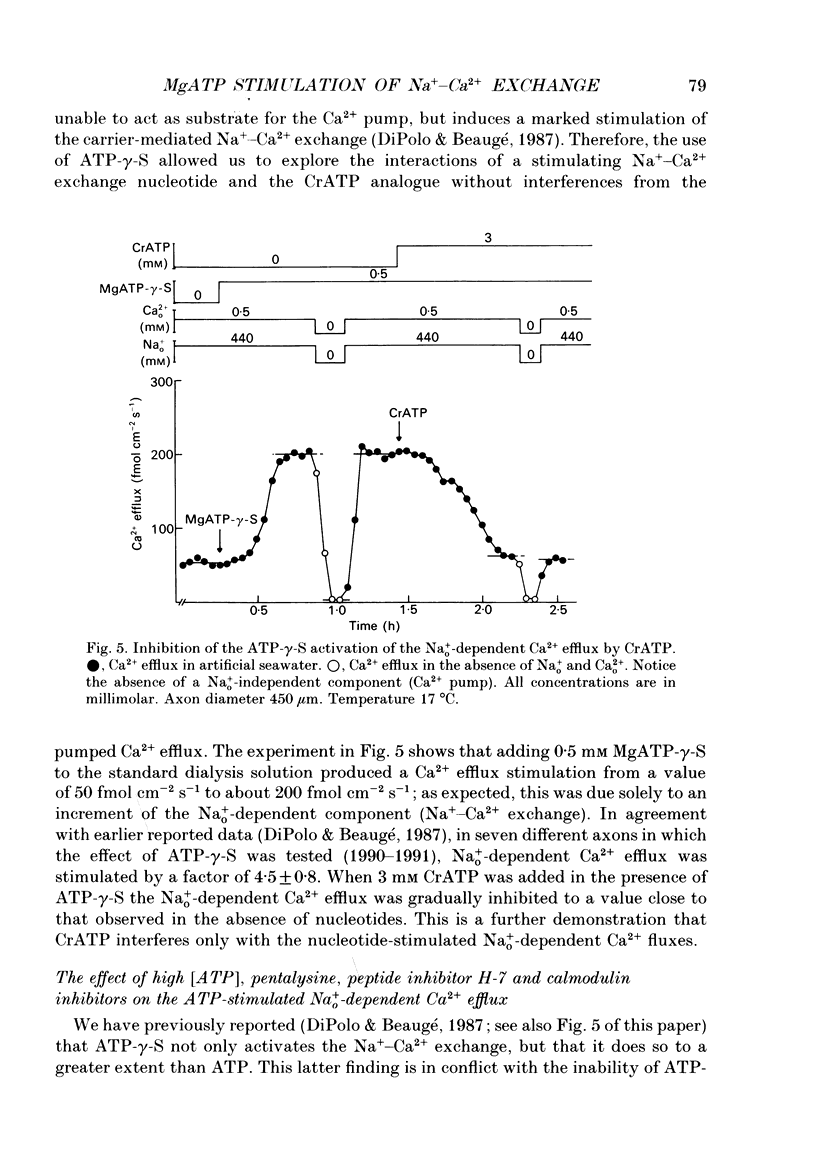
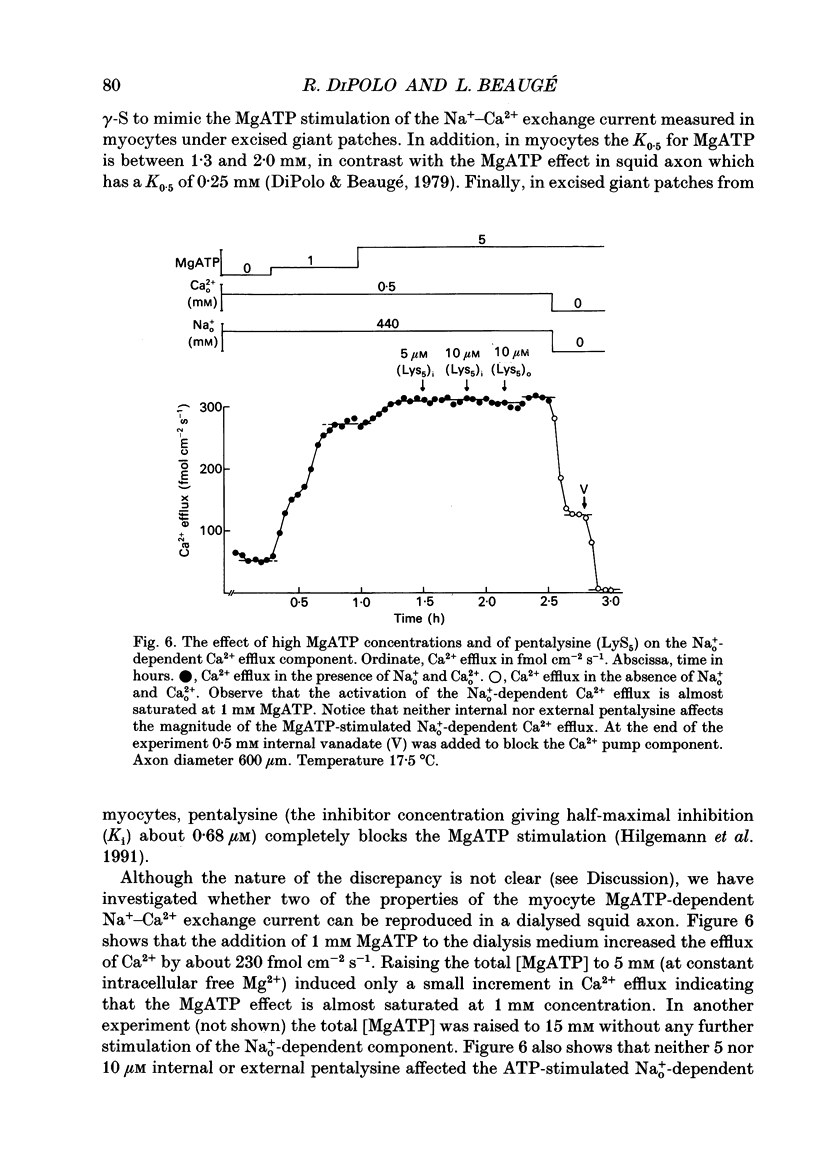
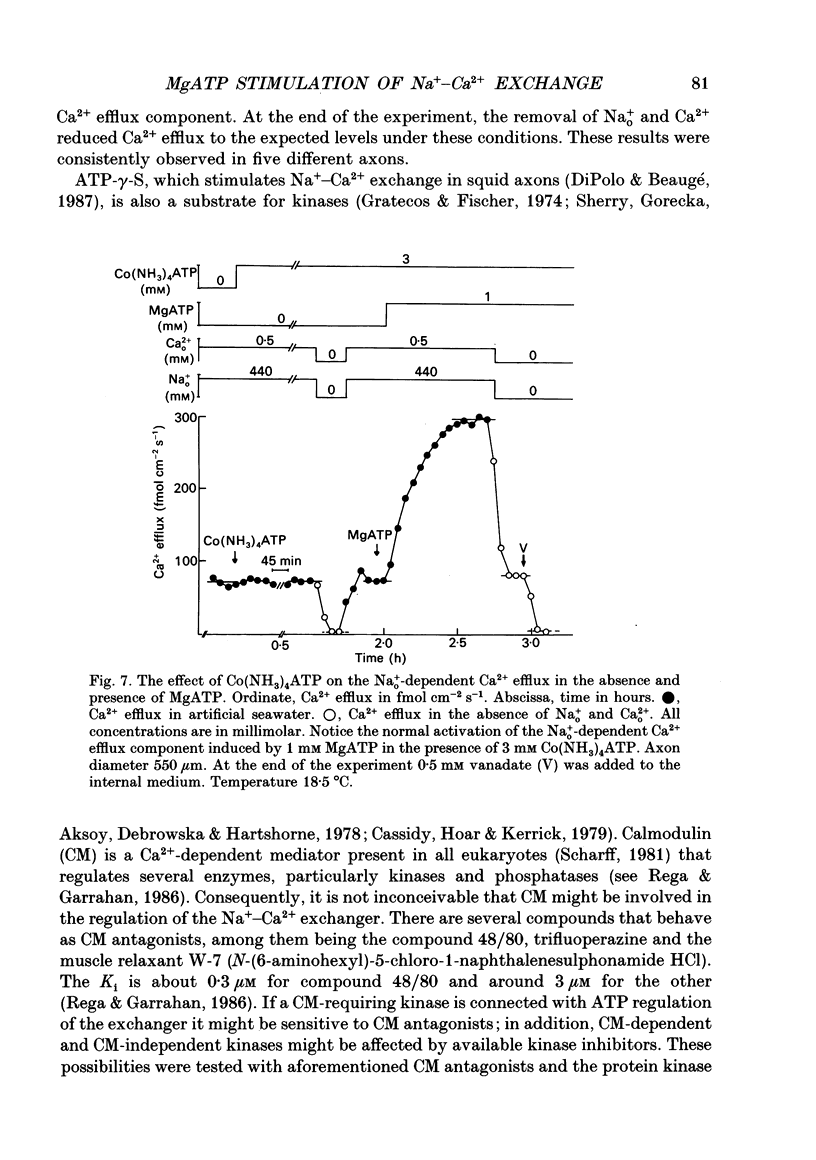
1. Na(+)o-dependent Ca2+ efflux (forward Na(+)-Ca2+ exchange), and in some cases the Na(+)i-dependent Ca2+ influx (reverse Na(+)-Ca2+ exchange) were measured in internally dialysed squid axons under membrane potential control. 2. We tested the effect on the Na(+)-Ca2+ exchange of the MgATP analogue bidentate chromium adenosine-5'-triphosphate (CrATP), substrate of several kinases, and cobalt tetrammine ATP (Co(NH3)4ATP), a poor substrate of most kinases. 3. CrATP completely blocked the MgATP and MgATP-gamma-S (ATP-gamma-S) stimulation of the Na(+)o-dependent Ca2+ efflux (forward exchange) and the Na+i-dependent Ca2+ influx (reverse exchange). The analogue only blocked the nucleotide-dependent fraction of the Na(+)-Ca2+ exchange without modifying any kinetic parameters of the exchange reactions. 4. The effects of CrATP were fully reversible with a very slow time constant (t 1/2 about 30 min). 5. The MgATP stimulation of the Na(+)-Ca2+ exchange was completely saturated at 1 mM. Higher MgATP concentrations (up to 15 mM) had no additional effects. Pentalysine (internal or external), the protein kinase C inhibitor H-7 (1-(5-isoquinolinylsulphonyl)-2-methylpiperazine) and several calmodulin inhibitors did not inhibit Na(+)-Ca2+ exchange either in the absence or presence of MgATP. 6. Our results do not agree with the idea of an aminophospholipid translocase being responsible for the ATP stimulation of the Na(+)-Ca2+ exchange in squid axons; they suggest that this is due to the action of a kinase system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Glitsch H. G. Voltage-dependent changes in the permeability of nerve membranes to calcium and other divalent cations. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):389–409. doi: 10.1098/rstb.1975.0018. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- DePamphilis M. L., Cleland W. W. Preparation and properties of chromium (3)-nucleotide complexes for use in the study of enzyme mechanisms. Biochemistry. 1973 Sep 11;12(19):3714–3724. doi: 10.1021/bi00743a022. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Ca2+ transport in nerve fibers. Biochim Biophys Acta. 1988 Oct 11;947(3):549–569. doi: 10.1016/0304-4157(88)90007-x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. In squid axons, ATP modulates Na+-Ca2+ exchange by a Ca2+i-dependent phosphorylation. Biochim Biophys Acta. 1987 Mar 12;897(3):347–354. doi: 10.1016/0005-2736(87)90432-9. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Interactions of physiological ligands with the Ca pump and Na/Ca exchange in squid axons. J Gen Physiol. 1984 Dec;84(6):895–914. doi: 10.1085/jgp.84.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Regulation of Na-Ca exchange. An overview. Ann N Y Acad Sci. 1991;639:100–111. doi: 10.1111/j.1749-6632.1991.tb17294.x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. The effects of vanadate on calcium transport in dialyzed squid axons. Sidedness of vanadate-cation interactions. Biochim Biophys Acta. 1981 Jul 20;645(2):229–236. doi: 10.1016/0005-2736(81)90193-0. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Characterization of the ATP-dependent calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1977 Jun;69(6):795–813. doi: 10.1085/jgp.69.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Bezanilla F., Caputo C., Rojas H. Voltage dependence of the Na/Ca exchange in voltage-clamped, dialyzed squid axons. Na-dependent Ca efflux. J Gen Physiol. 1985 Oct;86(4):457–478. doi: 10.1085/jgp.86.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R. Effect of ATP on the calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1974 Oct;64(4):503–517. doi: 10.1085/jgp.64.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway-Mariano D., Cleland W. W. Investigations of substrate specificity and reaction mechanism of several kinases using chromium(III) adenosine 5'-triphosphate and chromium(III) adenosine 5'-diphosphate. Biochemistry. 1980 Apr 1;19(7):1506–1515. doi: 10.1021/bi00548a038. [DOI] [PubMed] [Google Scholar]
- Dunaway-Mariano D., Cleland W. W. Preparation and properties of chromium(III) adenosine 5'-triphosphate, chromium(III) adenosine 5'-diphosphate, and related chromium(III) complexes. Biochemistry. 1980 Apr 1;19(7):1496–1505. doi: 10.1021/bi00548a037. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Goknur A. B., Hunter D. R., Hegge J. O., Berkoff H. A. Inhibition of calcium influx in isolated adult rat heart cells by ATP depletion. Circ Res. 1987 Apr;60(4):586–594. doi: 10.1161/01.res.60.4.586. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Collins A., Cash D. P., Nagel G. A. Cardiac Na(+)-Ca2+ exchange system in giant membrane patches. Ann N Y Acad Sci. 1991;639:126–139. doi: 10.1111/j.1749-6632.1991.tb17296.x. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Cleland W. W. The kinetic mechanism of glycerokinase. J Biol Chem. 1974 Apr 25;249(8):2562–2566. [PubMed] [Google Scholar]
- Khoyi M. A., Bjur R. A., Westfall D. P. Norepinephrine increases Na-Ca exchange in rabbit abdominal aorta. Am J Physiol. 1991 Oct;261(4 Pt 1):C685–C690. doi: 10.1152/ajpcell.1991.261.4.C685. [DOI] [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll D. A., Longoni S., Philipson K. D. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science. 1990 Oct 26;250(4980):562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Pauls H., Serpersu E. H., Kirch U., Schoner W. Chromium(III)ATP inactivating (Na+ + K+)-ATPase supports Na+-Na+ and Rb+-Rb+ exchanges in everted red blood cells but not Na+,K+ transport. Eur J Biochem. 1986 Jun 16;157(3):585–595. doi: 10.1111/j.1432-1033.1986.tb09706.x. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Reaction sequence of the K plus-dependent phosphatase. Biochim Biophys Acta. 1970 Sep 16;212(3):509–511. doi: 10.1016/0005-2744(70)90259-7. [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G., Fahlbusch K., Schoner W. Demonstration of cooperating alpha subunits in working (Na+ + K+)-ATPase by the use of the MgATP complex analogue cobalt tetrammine ATP. Eur J Biochem. 1987 Oct 1;168(1):123–131. doi: 10.1111/j.1432-1033.1987.tb13396.x. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F. M., Swarts H. G., Fu Y. F., Kuijpers G. A., De Pont J. J., Bonting S. L. Thiophosphorylation of (Na + K+)-ATPase yields an ADP-sensitive phosphointermediate. Biochim Biophys Acta. 1984 Jul 25;774(2):277–287. doi: 10.1016/0005-2736(84)90302-x. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Bunk S., Schoner W. How do MgATP analogues differentially modify high-affinity and low-affinity ATP binding sites of Na+/K(+)-ATPase? Eur J Biochem. 1990 Jul 31;191(2):397–404. doi: 10.1111/j.1432-1033.1990.tb19135.x. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Simons T. J. The interaction of ATP-analogues possessing a blocked gamma-phosphate group with the sodium pump in human red cells. J Physiol. 1975 Jan;244(3):731–739. doi: 10.1113/jphysiol.1975.sp010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Duval D., Frelin C., Lazdunski M. The Na+/Ca2+ antiporter in aortic smooth muscle cells. Characterization and demonstration of an activation by phorbol esters. J Biol Chem. 1988 Jun 15;263(17):8078–8083. [PubMed] [Google Scholar]


