Abstract
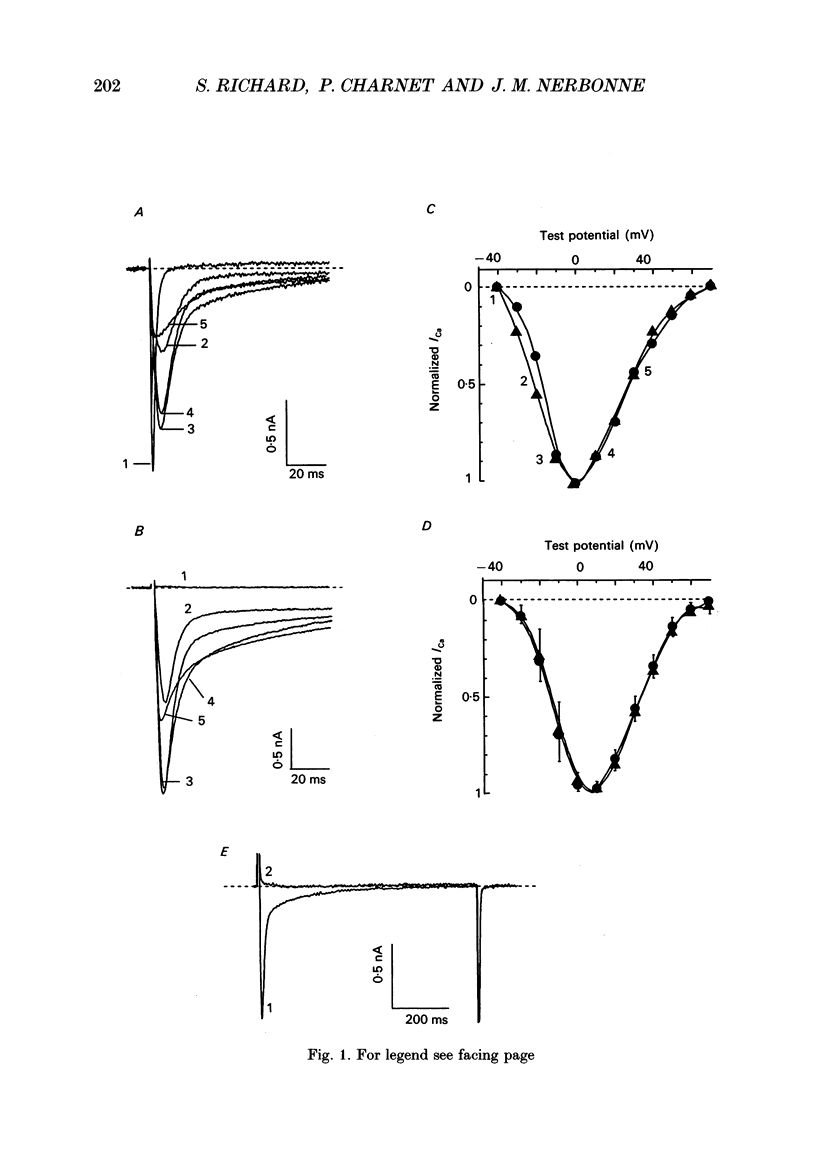
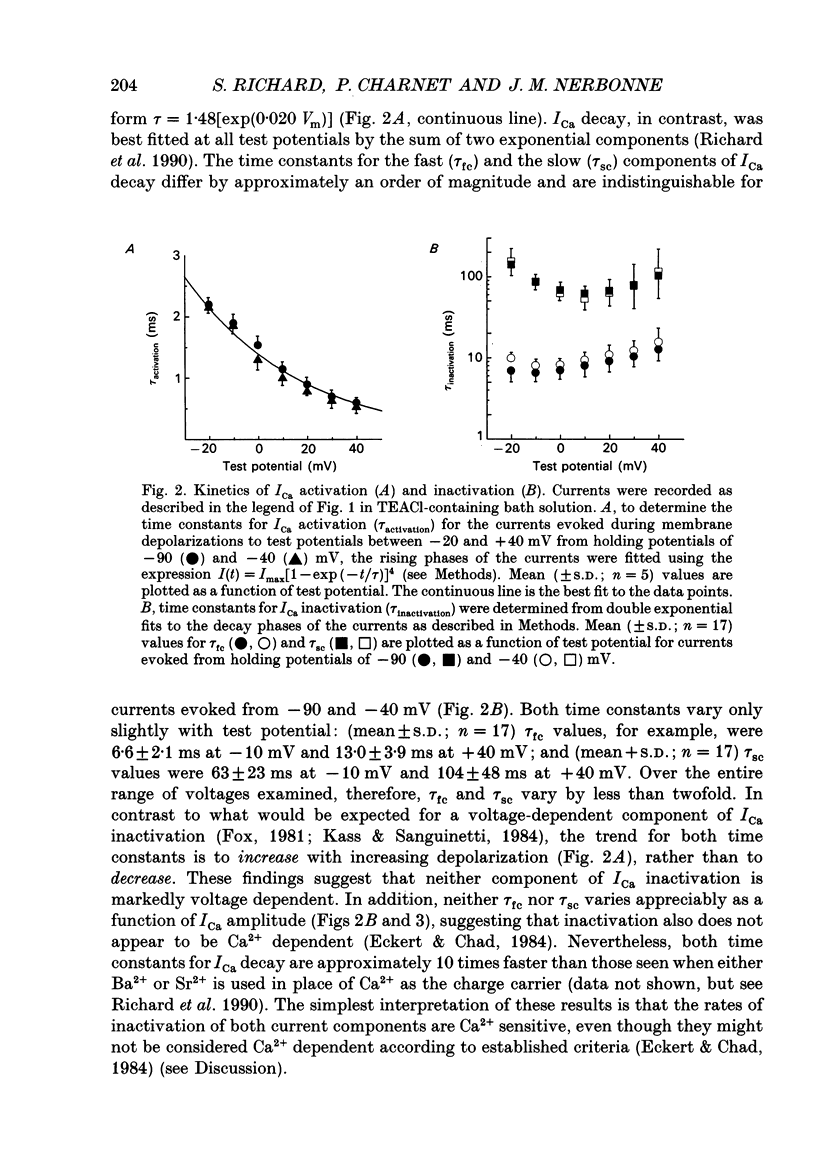
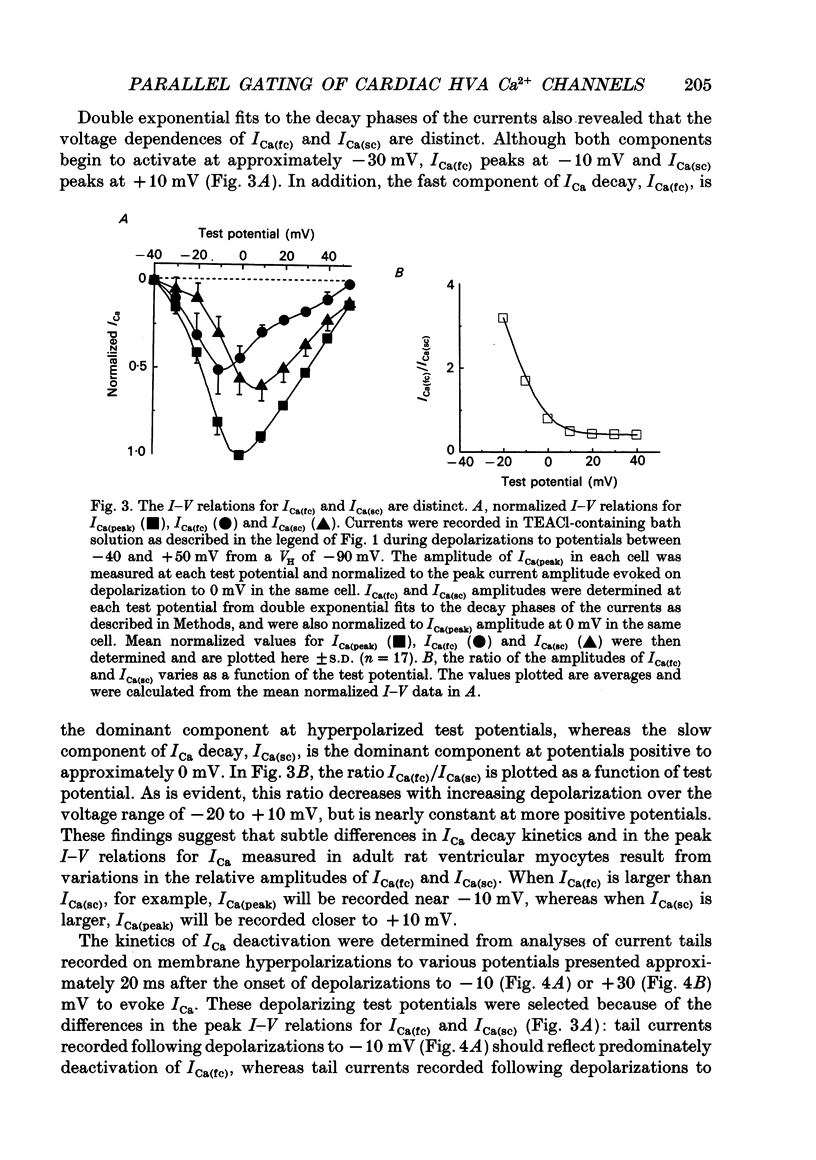
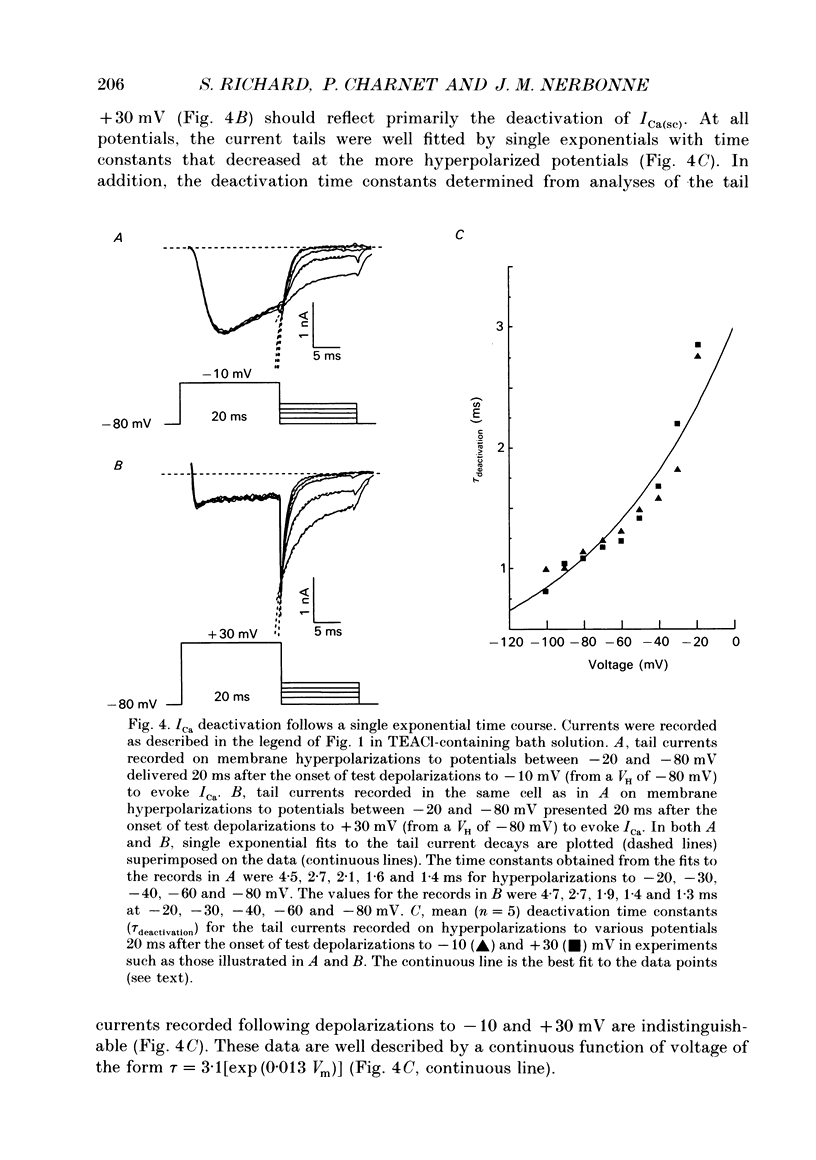
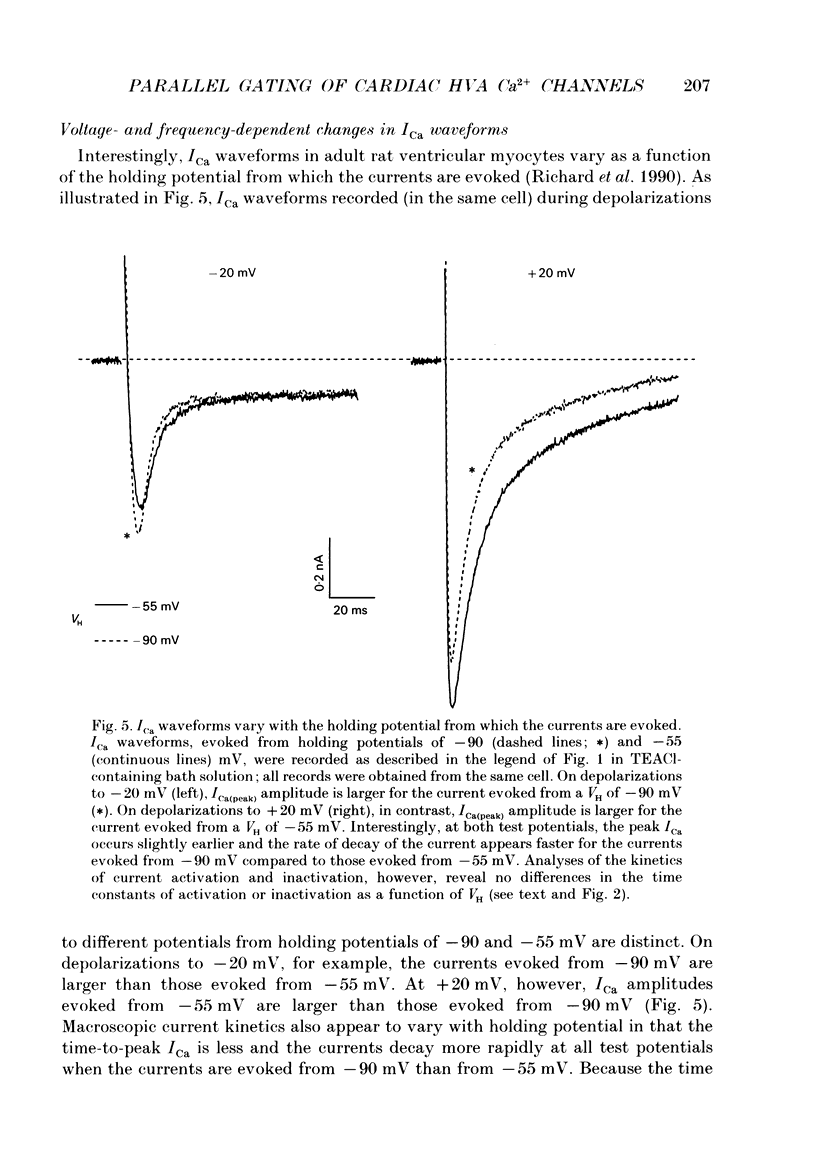
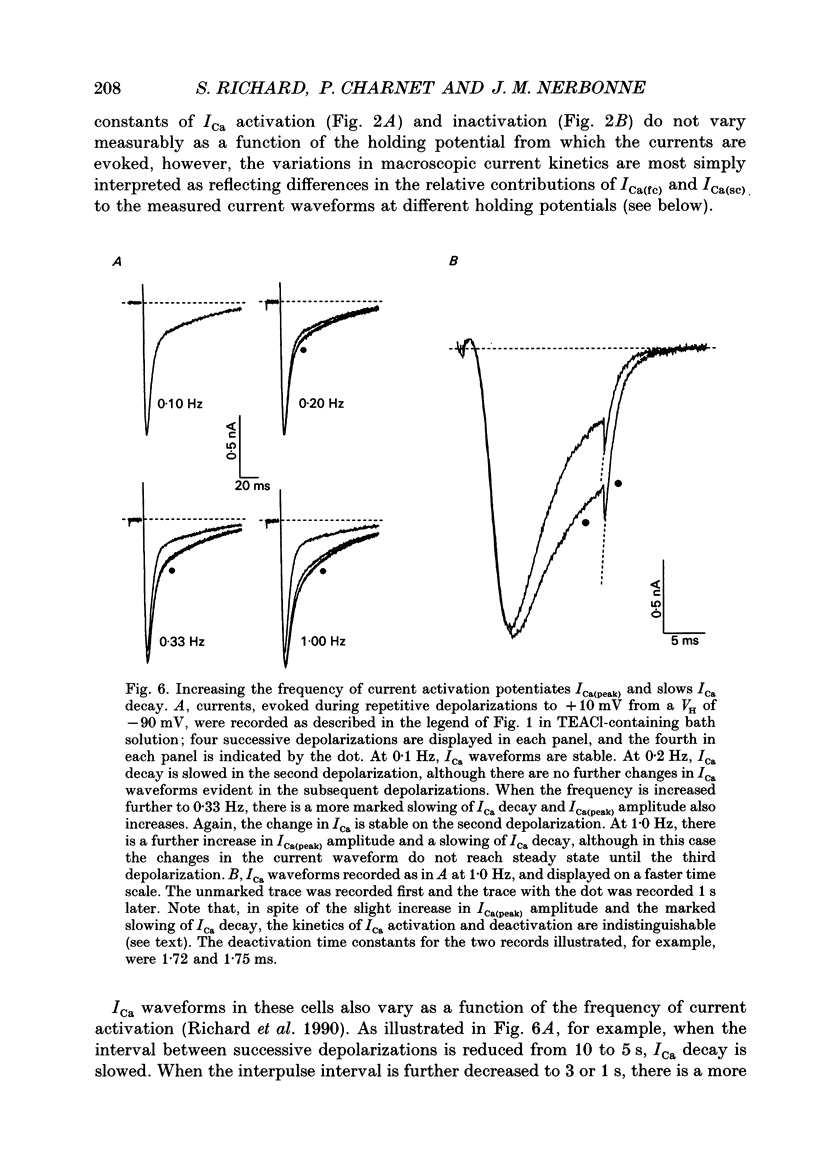
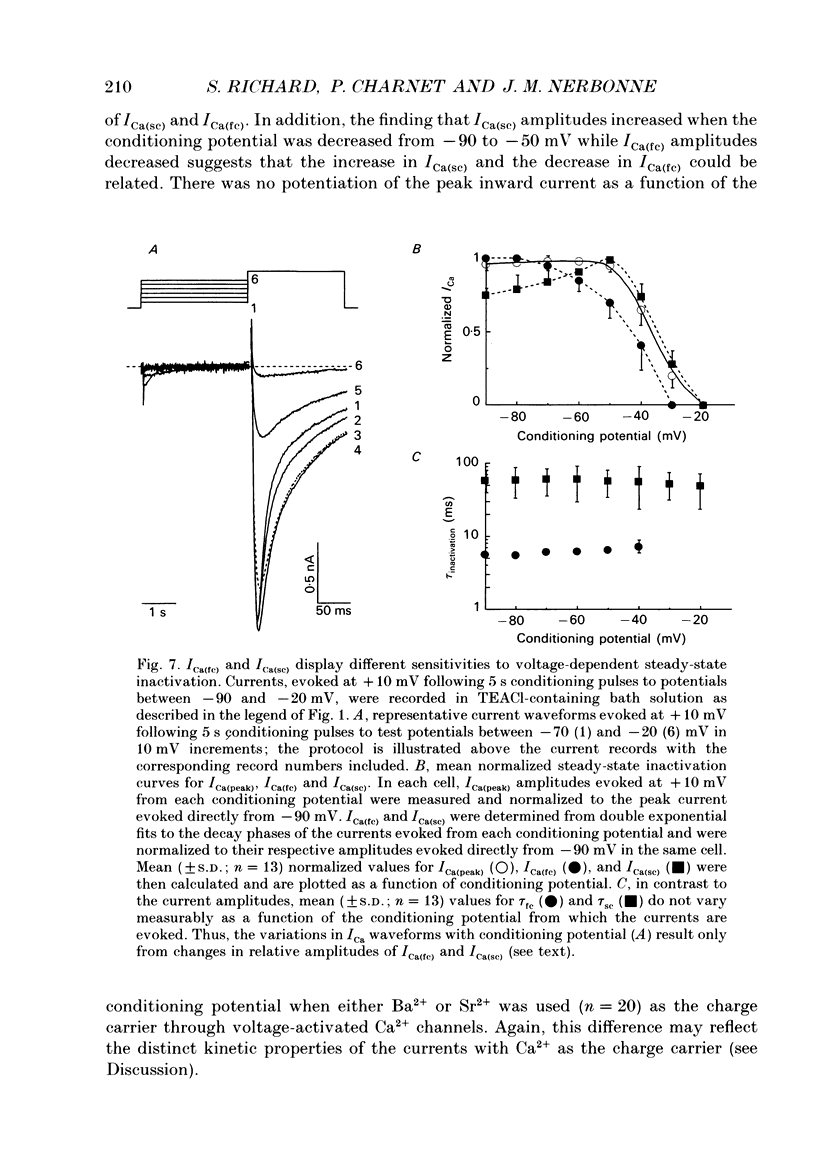
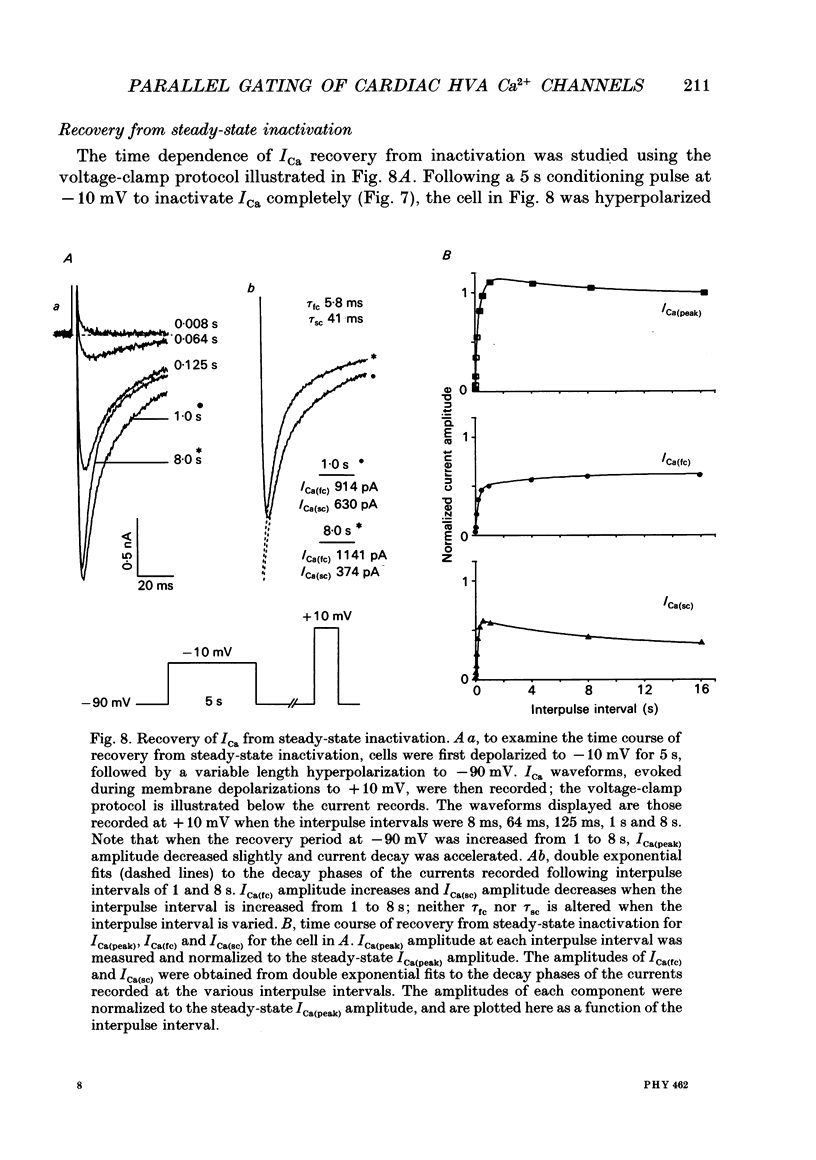
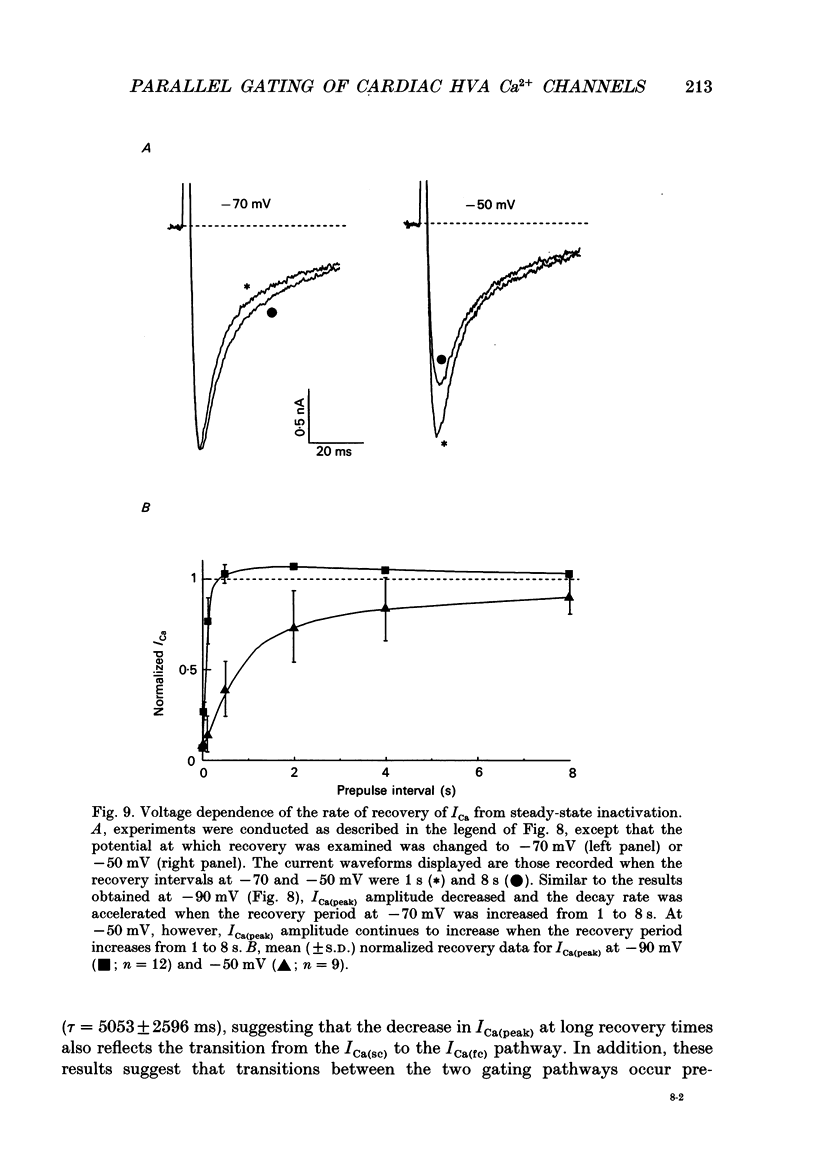
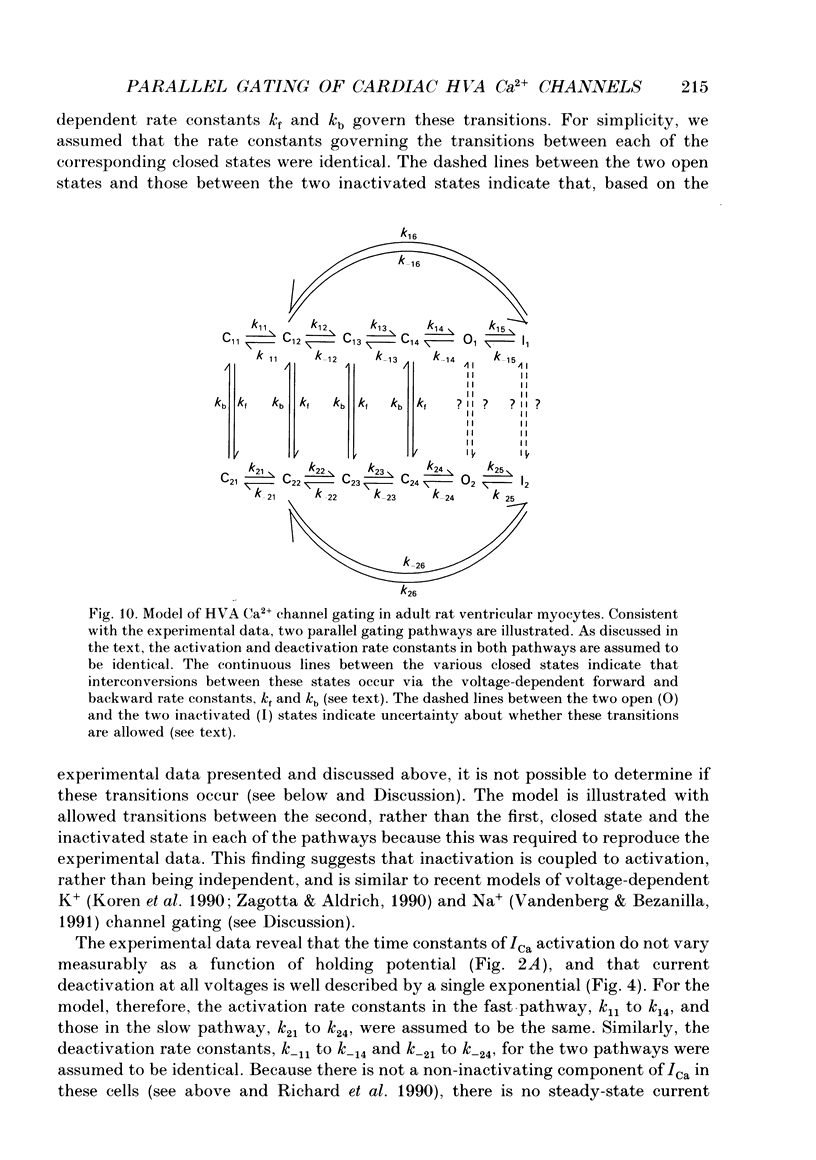
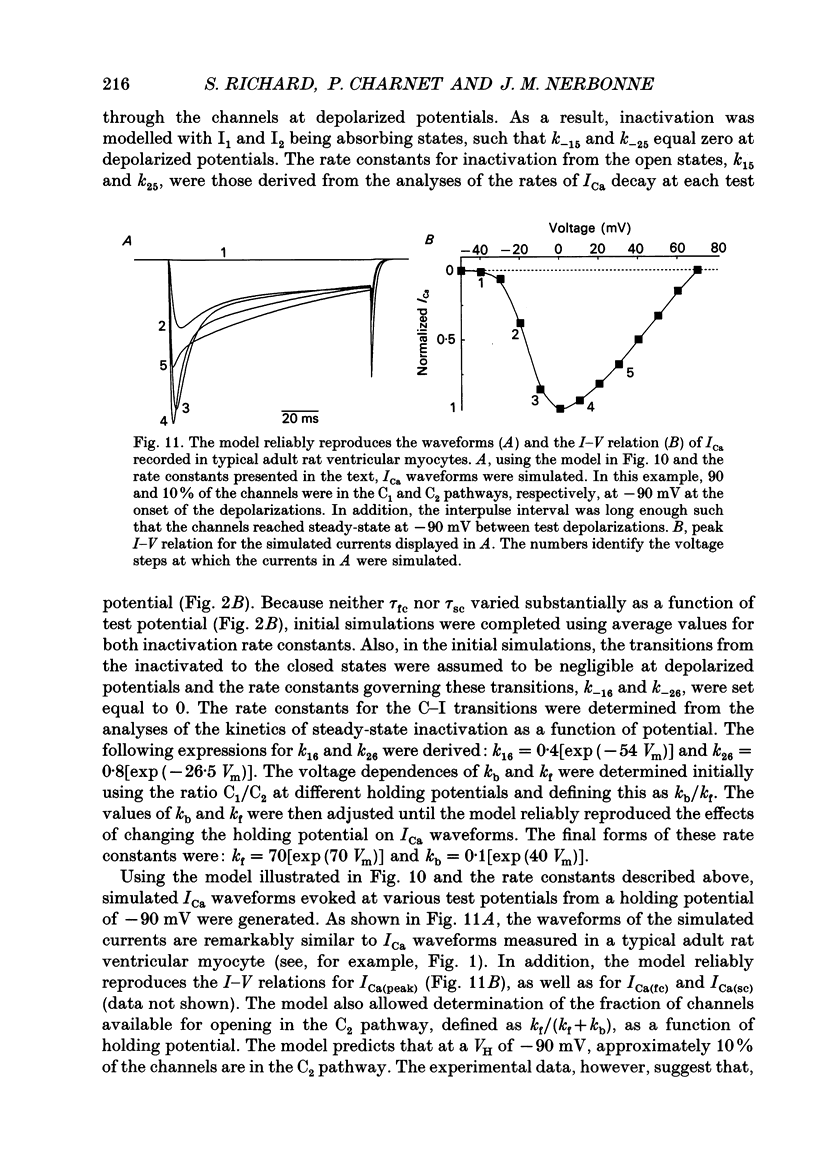
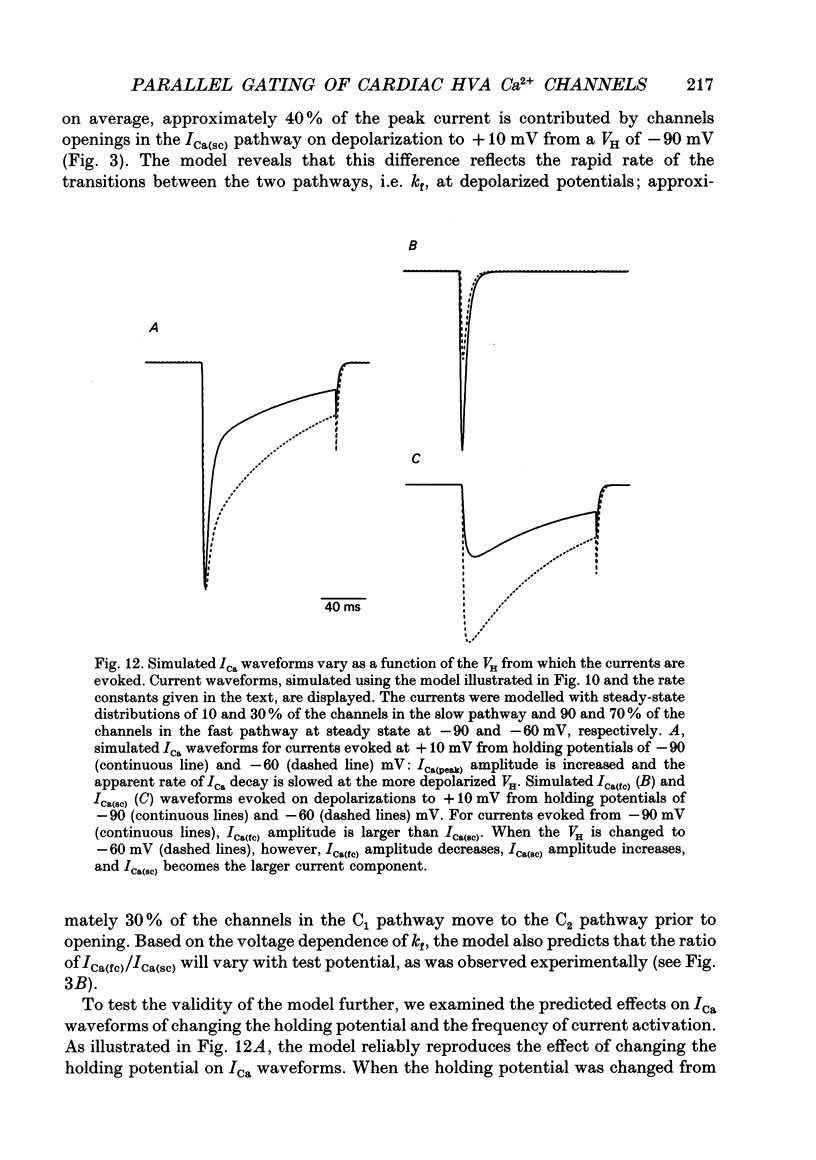
1. High-voltage-activated Ca2+ current (ICa) waveforms in adult rat ventricular myocytes comprise two components, referred to here as ICa(fc) and ICa(sc) to denote the fast and slow components, respectively, of ICa decay. At all test potentials, the two time constants of ICa decay, tau fc and tau sc, differ by approximately an order of magnitude. Neither tau fc nor tau sc varies appreciably with test potential, however, suggesting that current inactivation is not markedly voltage dependent. 2. Current activation at all test potentials follows a sigmoidal time course and is best described by a power function with n = 4. Deactivation of the currents, examined following variable length depolarizations to various test potentials, however, follows a single exponential time course. In addition, the kinetics of activation and deactivation of ICa(fc) and ICa(sc) are indistinguishable. 3. Although both begin to activate at approximately -30 mV, the voltage dependences of ICa(fc) and ICa(sc) are distinct: ICa(fc) peaks at -10 mV and ICa(sc) peaks at +10 mV. 4. The relative amplitudes of ICa(fc) and ICa(sc) vary with the holding potential from which the currents are evoked and with the frequency of current activation: hyperpolarized holding potentials and low stimulation frequencies reveal preferential activation of ICa(fc), whereas depolarized holding potentials and high stimulation frequencies potentiate ICa(sc). In addition, the observed voltage- and frequency-dependent changes in ICa(fc) and ICa(sc) amplitudes are reciprocal. 5. The apparent voltage dependences of steady-state inactivation of ICa(fc) and ICa(sc) are also distinct. ICa(fc) is reduced to approximately 50% of its maximal amplitude at -45 mV, whereas ICa(sc) is approximately 50% inactivated at -30 mV. 6. Recovery of ICa(peak) from steady-state inactivation follows a complex time course. Following inactivation at -10 mV, ICa(peak) recovers at -90 mV to its maximal value over a biexponential time course; ICa(peak) then decreases over the next several seconds to a steady-state level. 7. The time course of recovery from steady-state inactivation of ICa(fc) at -90 mV is best described by the sum of two exponentials; the two time constants of recovery differ by approximately a factor of 25. ICa(sc), in contrast, recovers rapidly and over a single exponential time course to its maximal value. When the recovery time at -90 mV is increased, however, ICa(sc) amplitude decreases slowly and over a single exponential time course to a steady-state level.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








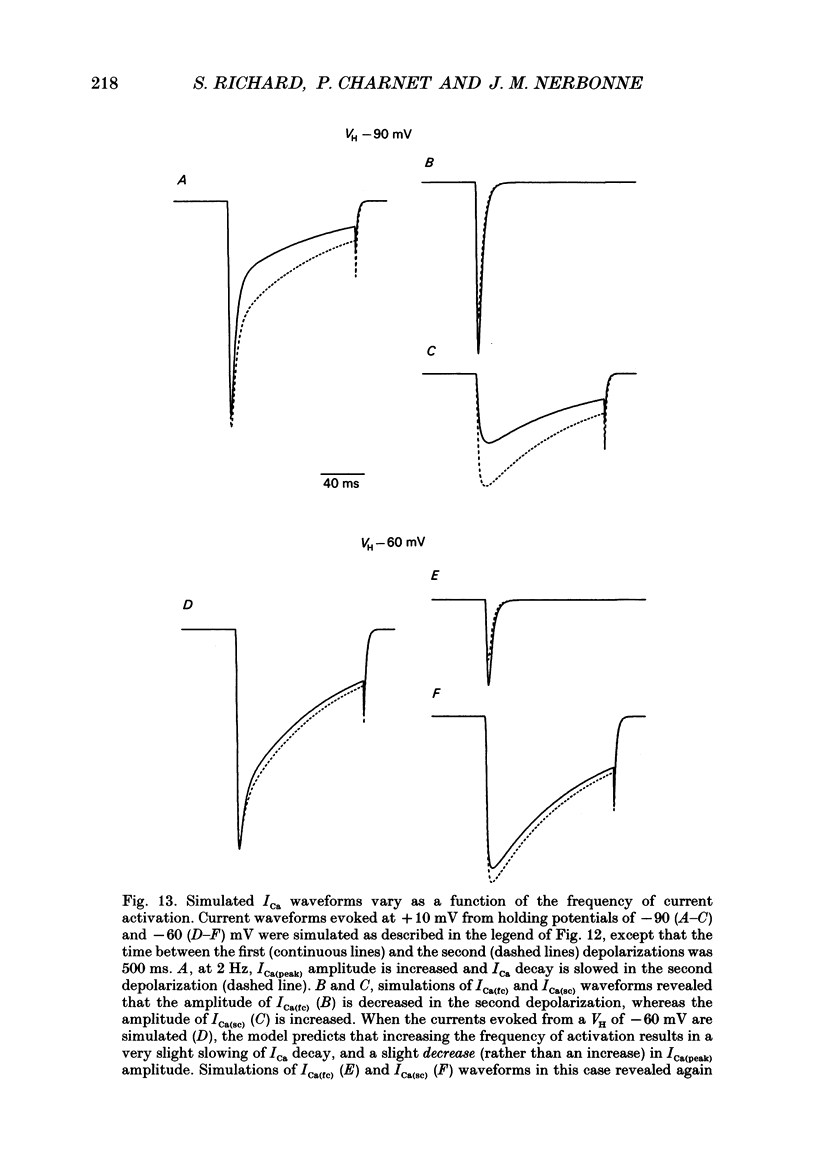

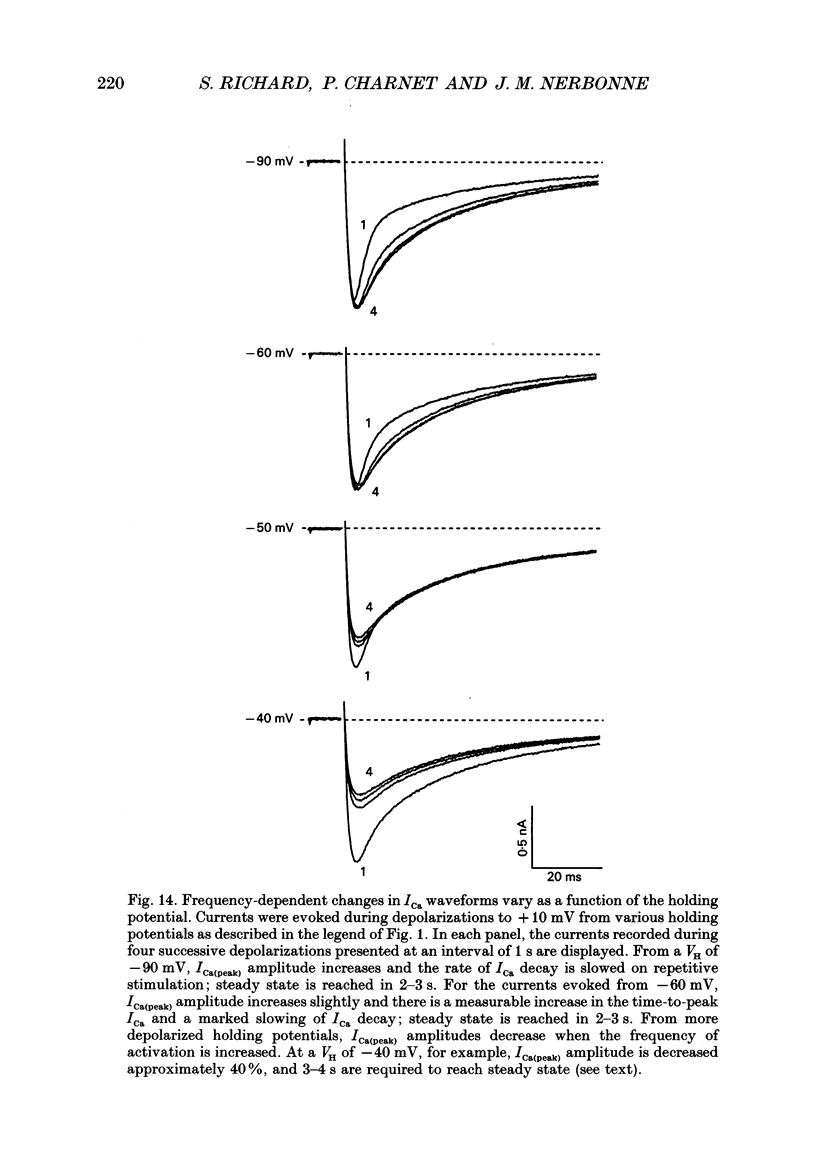








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvallet R., Rougier O. Existence of two calcium currents recorded at normal calcium concentrations in single frog atrial cells. Cell Calcium. 1989 Oct;10(7):499–508. doi: 10.1016/0143-4160(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Use-dependent reduction and facilitation of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:439–460. doi: 10.1113/jphysiol.1988.sp017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci U S A. 1981 Feb;78(2):953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- Le Grand B., Hatem S., Deroubaix E., Couetil J. P., Coraboeuf E. Calcium current depression in isolated human atrial myocytes after cessation of chronic treatment with calcium antagonists. Circ Res. 1991 Aug;69(2):292–300. doi: 10.1161/01.res.69.2.292. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Influence of a change in stimulation rate on action potentials, currents and contractions in rat ventricular cells. J Physiol. 1985 Jul;364:113–130. doi: 10.1113/jphysiol.1985.sp015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., VanDongen A. M., Joho R. H., Brown A. M. Fast and slow gating of sodium channels encoded by a single mRNA. Neuron. 1990 Feb;4(2):243–252. doi: 10.1016/0896-6273(90)90099-2. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Ouadid H., Séguin J., Richard S., Chaptal P. A., Nargeot J. Properties and Modulation of Ca channels in adult human atrial cells. J Mol Cell Cardiol. 1991 Jan;23(1):41–54. doi: 10.1016/0022-2828(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Richard S., Tiaho F., Charnet P., Nargeot J., Nerbonne J. M. Two pathways for Ca2+ channel gating differentially modulated by physiological stimuli. Am J Physiol. 1990 Jun;258(6 Pt 2):H1872–H1881. doi: 10.1152/ajpheart.1990.258.6.H1872. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., Morad M. Regulation of Ca2+ current in frog ventricular myocytes by the holding potential, c-AMP and frequency. Pflugers Arch. 1989 Oct;415(1):1–11. doi: 10.1007/BF00373135. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Bezanilla F. A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J. 1991 Dec;60(6):1511–1533. doi: 10.1016/S0006-3495(91)82186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. P., Best P. M. Increase in T-type calcium current in atrial myocytes from adult rats with growth hormone-secreting tumors. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4655–4659. doi: 10.1073/pnas.87.12.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Y., Potts J. F., Trimmer J. S., Agnew W. S., Sigworth F. J. Multiple gating modes and the effect of modulating factors on the microI sodium channel. Neuron. 1991 Nov;7(5):775–785. doi: 10.1016/0896-6273(91)90280-d. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990 Sep;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]


