Abstract
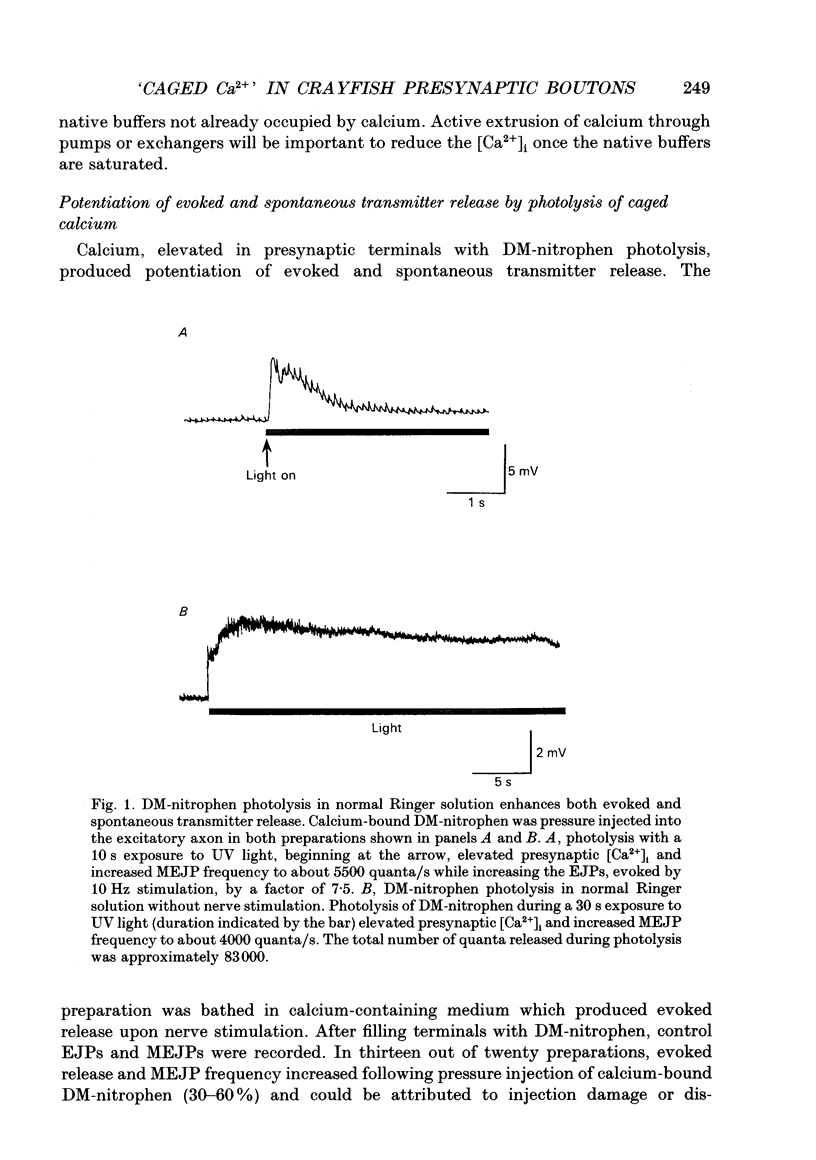
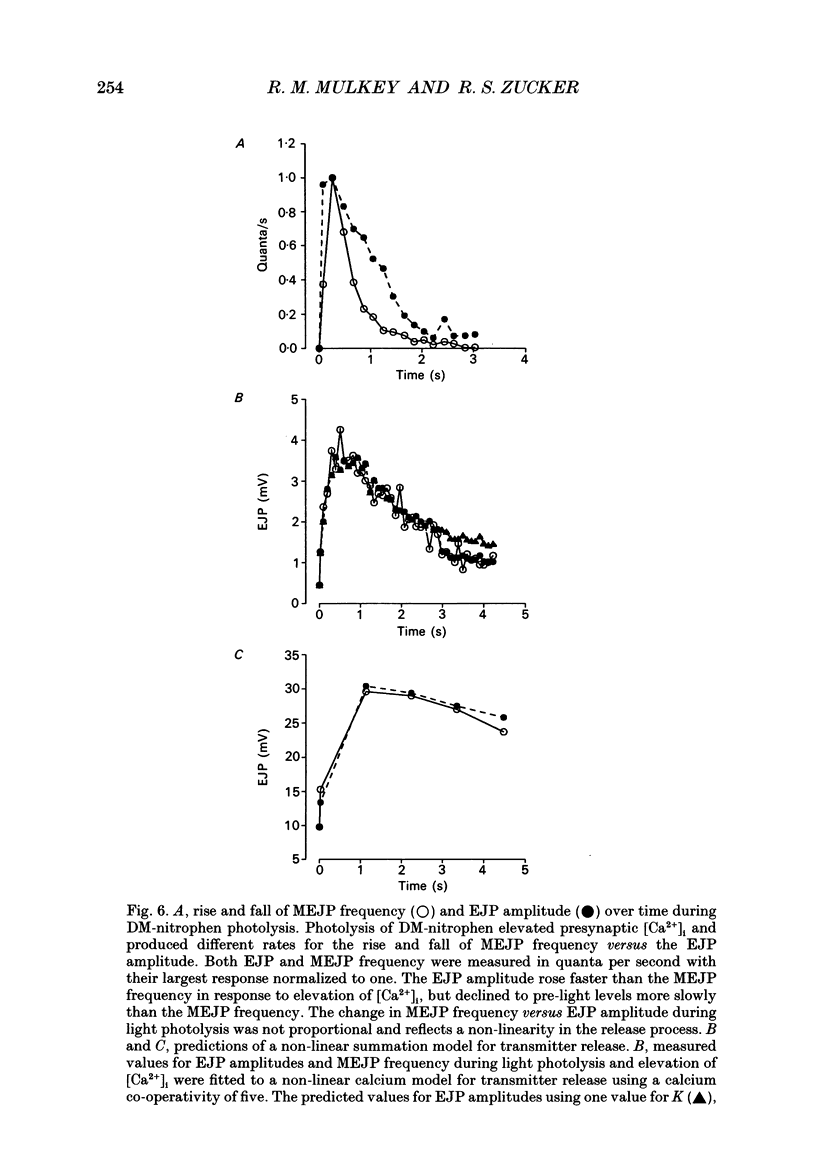
1. Spontaneous and evoked transmitter release at the crayfish neuromuscular junction were potentiated in response to photolytic release of calcium from the 'caged' calcium compound DM-nitrophen, which had previously been injected into presynaptic terminals. 2. The amount of calcium released from DM-nitrophen photolysis depends on the concentration of DM-nitrophen, its photoproducts, Ca2+, Mg2+, H+, ATP and the cell's native buffer. Since none of these are known in the crayfish terminal, the study was conducted in a qualitative fashion. 3. Photolytic release of calcium from DM-nitrophen increased excitatory junctional potentials (EJPs) by a range of 2-31 times over control values and the miniature excitatory junctional potential (MEJP) frequency increased from resting values of 1-10 quanta/s to 3000-11,000 quanta/s. 4. Extracellular calcium was not required for the light-evoked asynchronous release of transmitter. Calcium-bound DM-nitrophen previously pressure injected into crayfish presynaptic terminals increased the MEJP frequency from resting values of 1-8 quanta/s to 800-10,000 quanta/s during photolysis in a calcium-free cobalt Ringer solution. 5. Iontophoresis of calcium-free DM-nitrophen into presynaptic terminals released transmitter upon photolysis, but only in a calcium-containing Ringer solution. This suggests that DM-nitrophen is capable of binding calcium once injected into terminals, but this is dependent on the presence of external calcium. 6. Photolysis of DM-nitrophen at lower light intensities produced a slower rate of transmitter release. 7. Brief light exposures, i.e. those which photolysed 5-20% of the DM-nitrophen, resulted in a rapid decay of postsynaptic responses on extinguishing the light, due to rebinding of photolytically released calcium to unphotolysed DM-nitrophen. Longer light exposures which completely photolysed DM-nitrophen, leaving only the low affinity photoproducts, produced a slow decay of transmitter release after the light pulse, presumably due to the active extrusion of calcium from the presynaptic terminals. 8. During photolysis of DM-nitrophen, the time courses of changes in EJP amplitude and MEJP frequency were different, indicating that the two measures of transmitter release were not linearly related. 9. MEJP frequency and EJP amplitudes during DM-nitrophen photolysis were fitted to a 'non-linear summation model' in which photolytically released calcium sums with calcium entering during an action potential to evoke transmitter release with a calcium co-operativity of five.
Full text
PDF






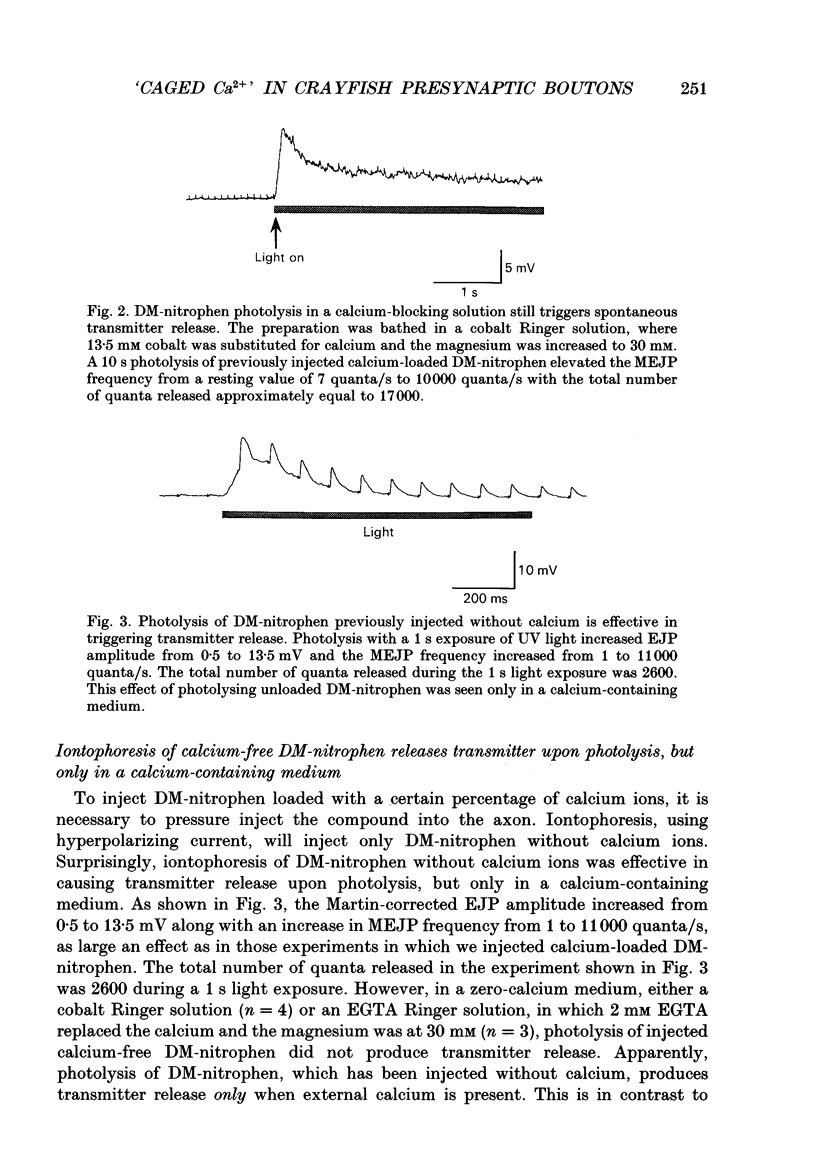






Selected References
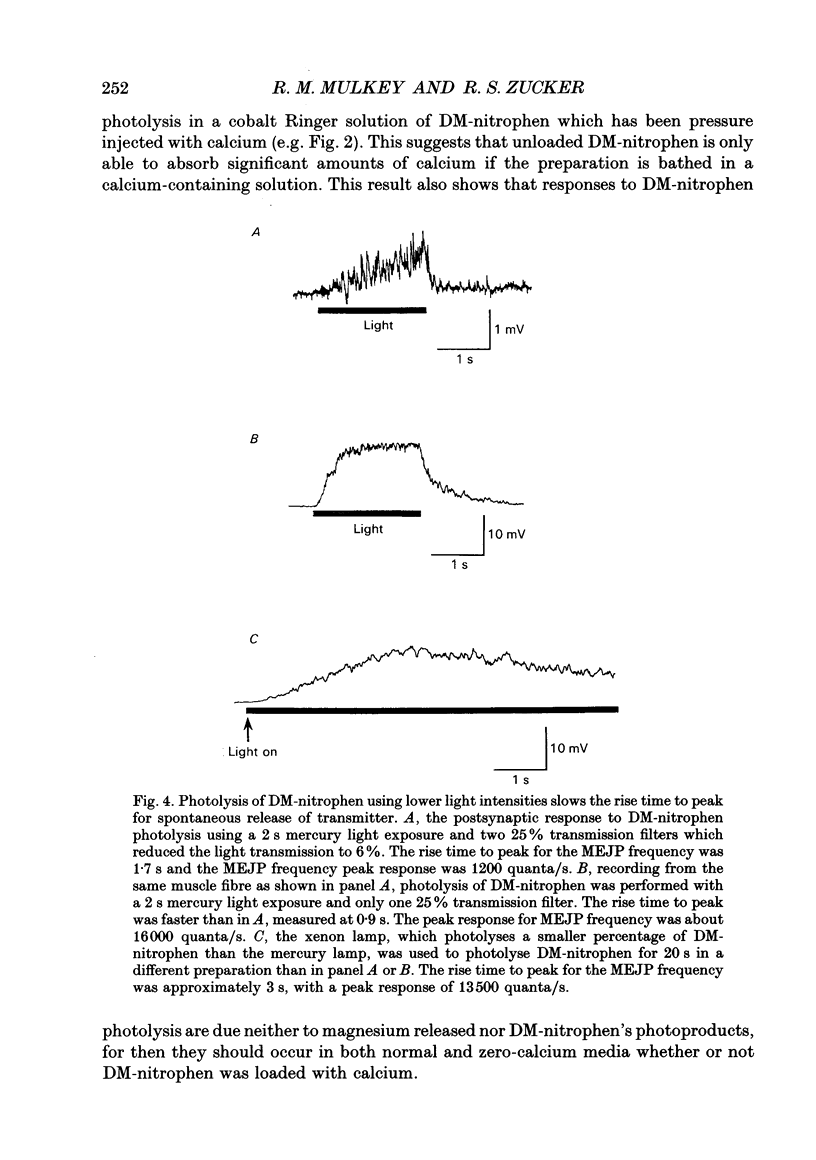
These references are in PubMed. This may not be the complete list of references from this article.
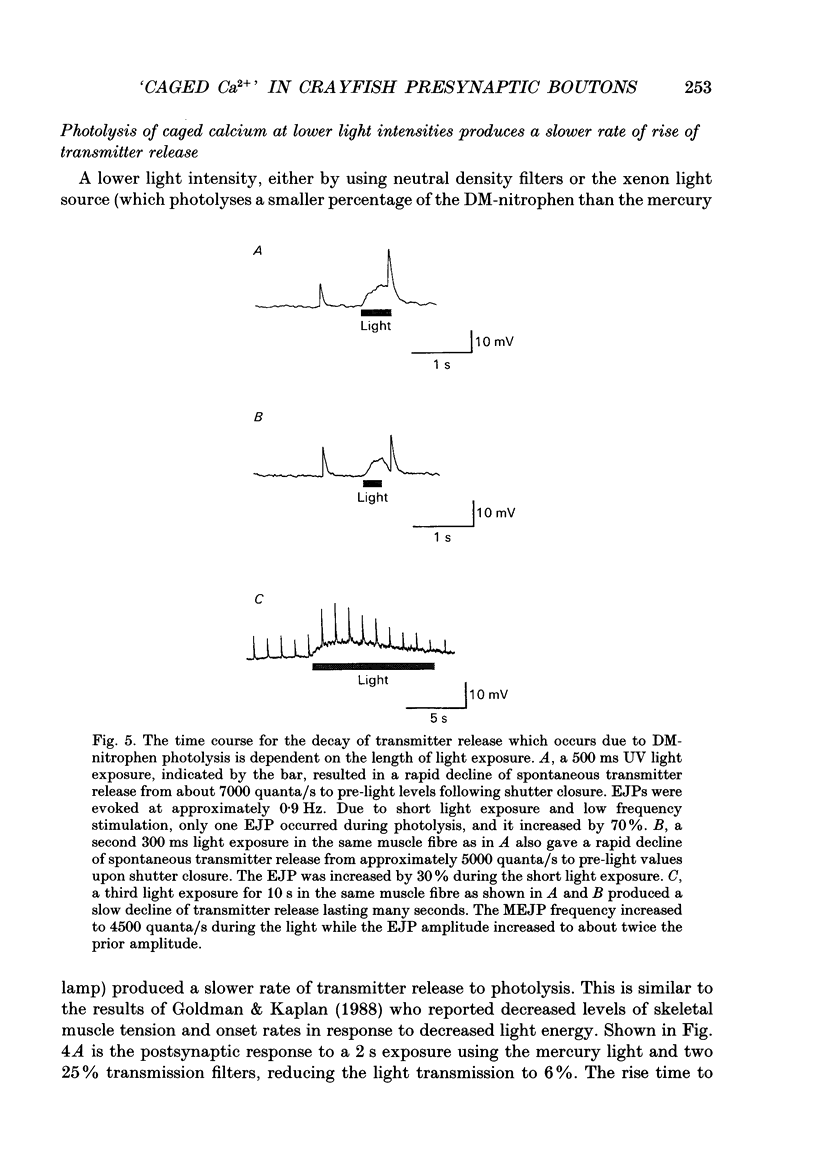
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Gamiño S. M., Rink T. J. Intracellular free magnesium in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1984 Sep;354:303–317. doi: 10.1113/jphysiol.1984.sp015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D., Kennedy D. Quantitative aspects of transmitter release. J Cell Biol. 1970 Dec;47(3):585–592. doi: 10.1083/jcb.47.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S. Calcium released by photolysis of DM-nitrophen stimulates transmitter release at squid giant synapse. J Physiol. 1990 Jul;426:473–498. doi: 10.1113/jphysiol.1990.sp018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagoleva I. M., Liberman E. A., Khashaev Z. Kh. Vliianie razobshchitelei okislitel'nogo fosforilirovaniia na vykhod atsetilkholina iz nervnykh okonchanii. Biofizika. 1970 Jan-Feb;15(1):76–83. [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Membrane noise produced by acetylcholine. Nature. 1970 Jun 6;226(5249):962–963. doi: 10.1038/226962a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Calcium ionophore X-537A increases spontaneous and phasic quantal release of acetylcholine at frog neuromuscular junction. Nature. 1974 Aug 23;250(5468):658–660. doi: 10.1038/250658a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Action potentials must admit calcium to evoke transmitter release. Nature. 1991 Mar 14;350(6314):153–155. doi: 10.1038/350153a0. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Posttetanic potentiation at the crayfish neuromuscular junction is dependent on both intracellular calcium and sodium ion accumulation. J Neurosci. 1992 Nov;12(11):4327–4336. doi: 10.1523/JNEUROSCI.12-11-04327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Hovav G., Parnas I. Effect of Ca2+ diffusion on the time course of neurotransmitter release. Biophys J. 1989 May;55(5):859–874. doi: 10.1016/S0006-3495(89)82885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S., Bittner G. D., Brodwick M. S. Calcium-activated potassium conductance in presynaptic terminals at the crayfish neuromuscular junction. J Gen Physiol. 1991 Dec;98(6):1161–1179. doi: 10.1085/jgp.98.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Liesegang G. W., Berger R. L., Czerlinski G., Podolsky R. J. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal Biochem. 1984 Nov 15;143(1):188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J. The action of ionophores at the frog neuromuscular junction. Life Sci. 1975 Nov 1;17(9):1401–1406. doi: 10.1016/0024-3205(75)90159-9. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S. Reversal potentials of L-glutamate and the excitatory transmitter at the neuromuscular junction of the crayfish. Biochim Biophys Acta. 1971 Aug 13;241(2):700–703. doi: 10.1016/0005-2736(71)90071-x. [DOI] [PubMed] [Google Scholar]
- Wallin B. G. Intracellular ion concentrations in single crayfish axons. Acta Physiol Scand. 1967 Jul-Aug;70(3):419–430. doi: 10.1111/j.1748-1716.1967.tb03640.x. [DOI] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Delaney K. R., Mulkey R., Tank D. W. Presynaptic calcium in transmitter release and posttetanic potentiation. Ann N Y Acad Sci. 1991;635:191–207. doi: 10.1111/j.1749-6632.1991.tb36492.x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Lara-Estrella L. O. Post-tetanic decay of evoked and spontaneous transmitter release and a residual-calcium model of synaptic facilitation at crayfish neuromuscular junctions. J Gen Physiol. 1983 Mar;81(3):355–372. doi: 10.1085/jgp.81.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]


