Abstract
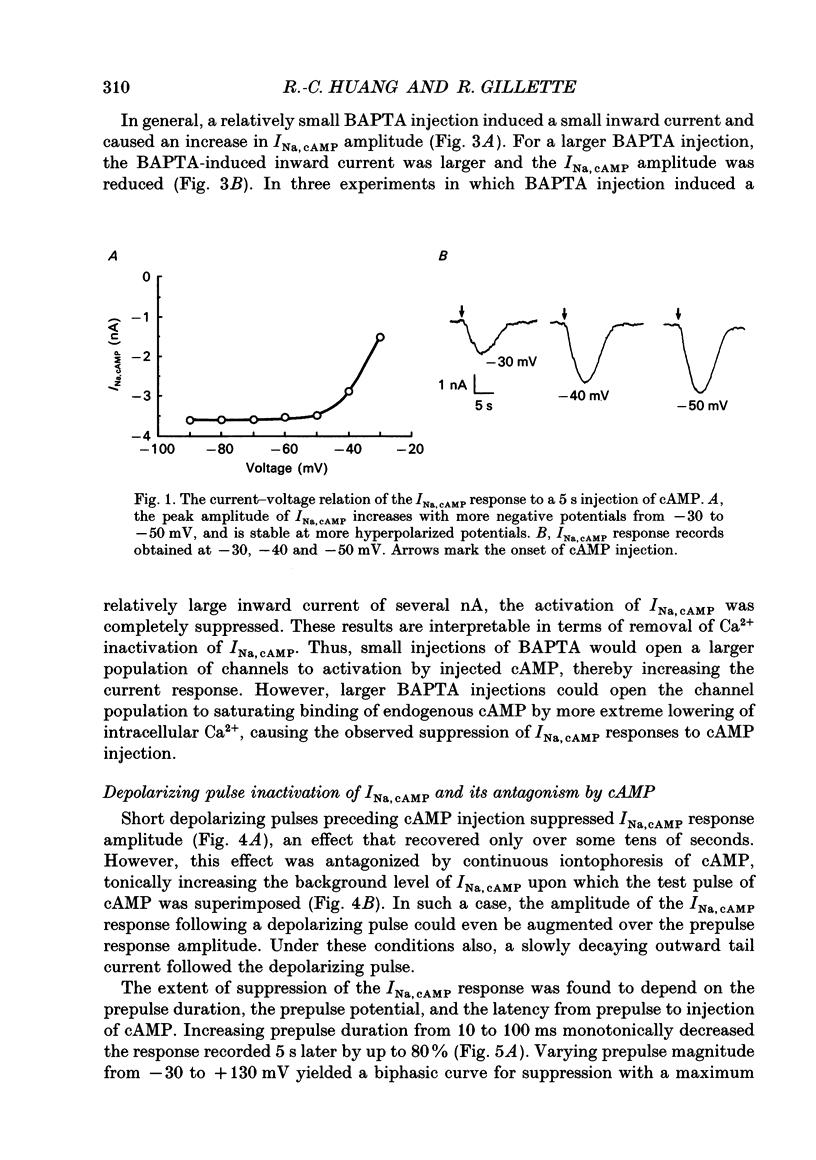
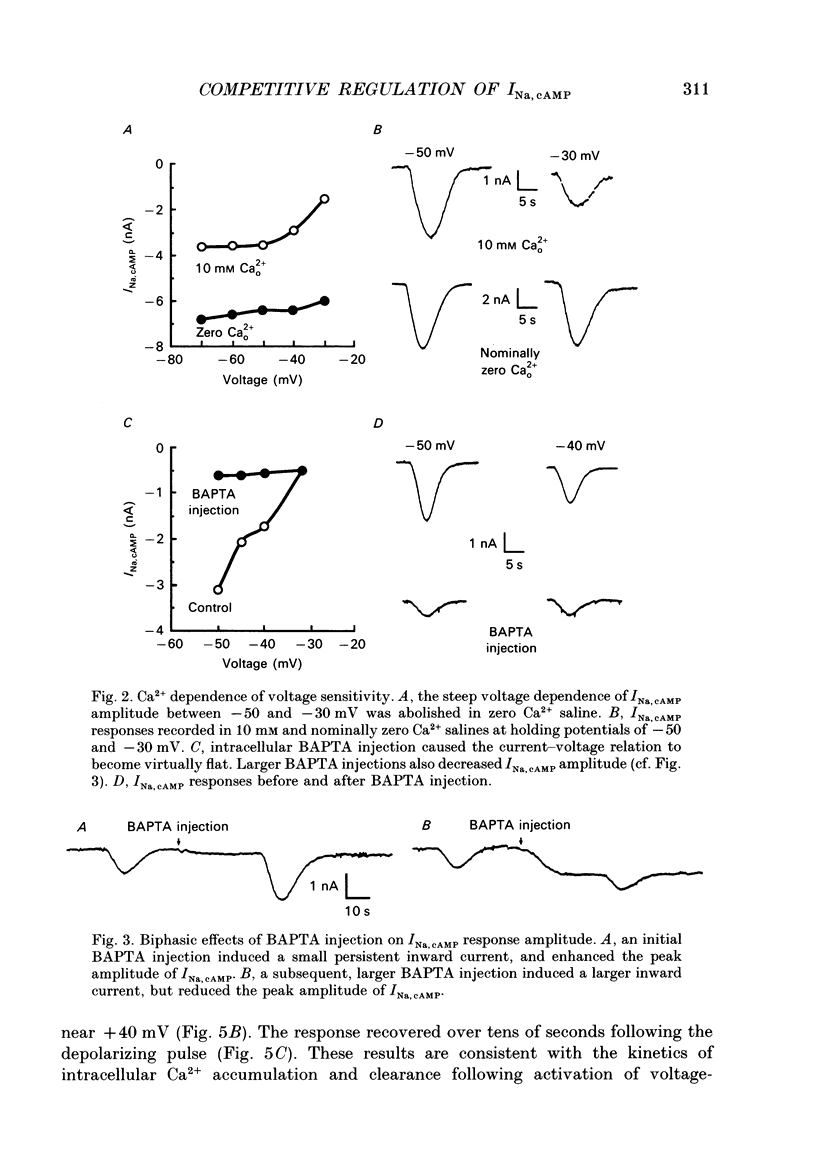
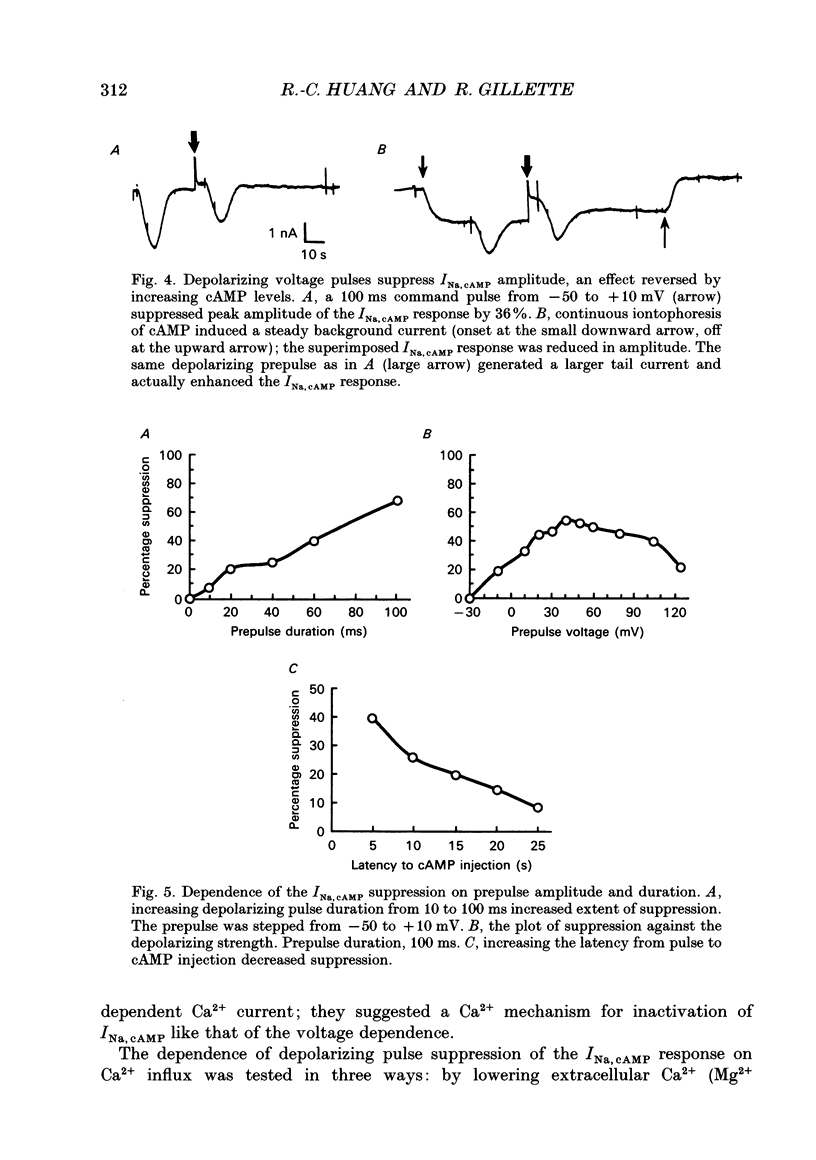
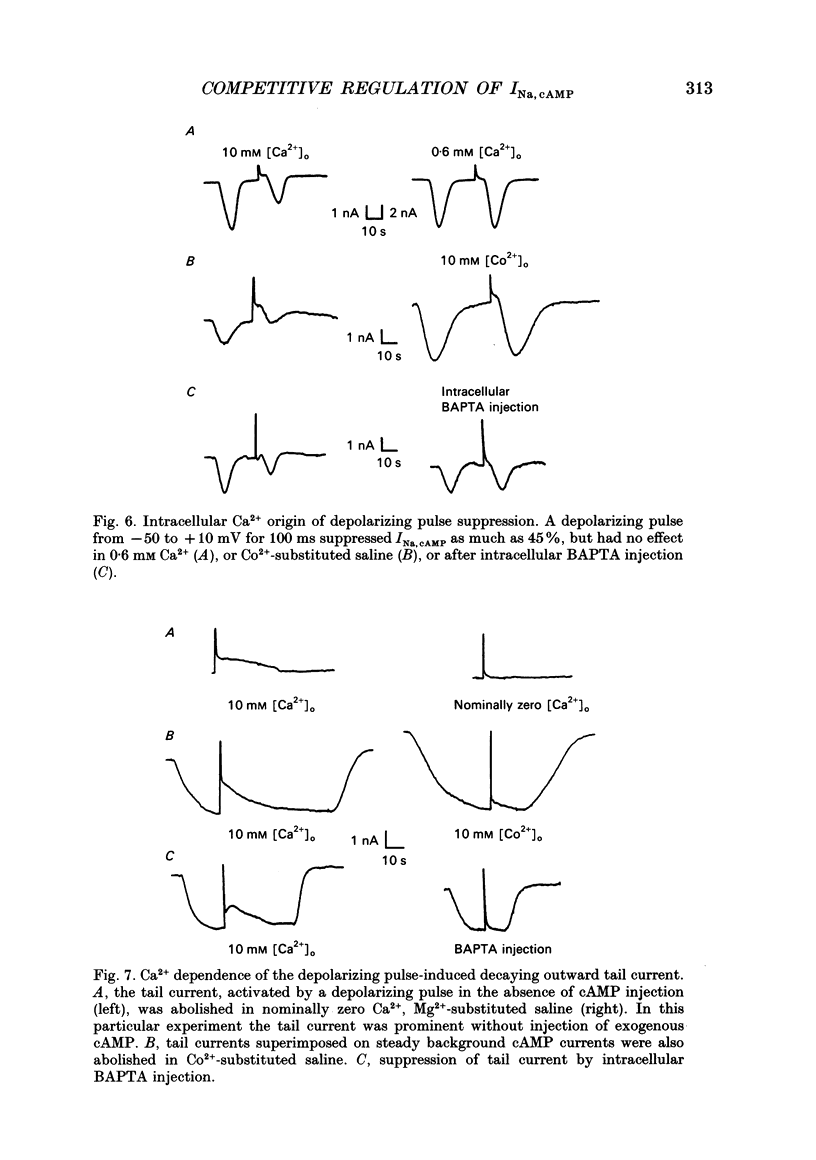
1. The cAMP-gated Na+ current (INa, cAMP) was studied in axotomized neurons of the pedal ganglion of the sea slug Pleurobranchaea. INa, cAMP responses were elicited by iontophoretic injection of cAMP and recorded in voltage clamp. 2. The current-voltage relation for INa, cAMP was flat between -90 and -50 mV, but declined steeply with depolarization from -50 to -30 mV. Depolarizing pulses also suppressed the INa, cAMP response, which recovered slowly over tens of seconds. 3. The inactivating effects of depolarization on the current were abolished both by blockade of Ca2+ current and intracellular injection of Ca2+ chelator. Thus, Ca2+ influx through voltage-dependent Ca2+ channels probably mediates inactivation of INa, cAMP within its normal physiological range of action. 4. Increasing intracellular cAMP levels antagonized the effects of Ca2+ influx on INa, cAMP. The mutual antagonism of the ions suggests that cAMP and Ca2+ act competitively in regulation of the INa, cAMP channel. 5. Measures of fractional inactivation of INa, cAMP provided evidence for the existence of an appreciable basal level of current, and hence cAMP, in the unstimulated neuron. Since INa, cAMP is a direct function of cAMP activity, measures of fractional inactivation permit quantification of cAMP levels in the living neuron. 6. Calcium inactivation of INa, cAMP completes a negative feedback loop that can contribute to endogenous burst activity. Over the burst cycle, depolarization and action potential activity driven by INa, cAMP would lead to Ca2+ influx, consequent inactivation of the inward current, and hyperpolarization. This mechanism of endogenous bursting resembles other in which the burst cycle has been found to be regulated by kinetics of Ca2+ influx and removal. However, INa, cAMP may vary in its Ca2+ sensitivity in different neurons and these variations may affect the functional expression of endogenous oscillatory activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B., Levitan I. B. Voltage and ion dependences of the slow currents which mediate bursting in Aplysia neurone R15. J Physiol. 1985 Mar;360:69–93. doi: 10.1113/jphysiol.1985.sp015604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff J. B., Hofmeier G., Lux H. D., Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res. 1983 Oct 16;276(2):289–296. doi: 10.1016/0006-8993(83)90736-9. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol. 1984 Sep;354:139–162. doi: 10.1113/jphysiol.1984.sp015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Gillette R., Gillette M. U., Davis W. J. Action-potential broadening and endogenously sustained bursting are substrates of command ability in a feeding neuron of Pleurobranchaea. J Neurophysiol. 1980 Mar;43(3):669–685. doi: 10.1152/jn.1980.43.3.669. [DOI] [PubMed] [Google Scholar]
- Gillette R., Green D. J. Calcium dependence of voltage sensitivity in adenosine 3',5'-cyclic phosphate-stimulated sodium current in Pleurobranchaea. J Physiol. 1987 Dec;393:233–245. doi: 10.1113/jphysiol.1987.sp016821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Patch- and voltage-clamp analysis of cyclic AMP-stimulated inward current underlying neurone bursting. Nature. 1983 Dec 22;306(5945):784–785. doi: 10.1038/306784a0. [DOI] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Regulation of cAMP-stimulated ion current by intracellular pH, Ca2+, and calmodulin blockers. J Neurophysiol. 1988 Jan;59(1):248–258. doi: 10.1152/jn.1988.59.1.248. [DOI] [PubMed] [Google Scholar]
- Hara N., Sawada M., Maeno T. Influences of pressure-injected cyclic AMP on the membrane current and characteristics of an identified neuron of Aplysia kurodai. Jpn J Physiol. 1985;35(6):985–1012. doi: 10.2170/jjphysiol.35.985. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Yamane T. Compartmentalization of cyclic AMP elevation in neurons of Aplysia californica. Cell Mol Neurobiol. 1987 Mar;7(1):19–33. doi: 10.1007/BF00734987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. C., Gillette R. Kinetic analysis of cAMP-activated Na+ current in the molluscan neuron. A diffusion-reaction model. J Gen Physiol. 1991 Oct;98(4):835–848. doi: 10.1085/jgp.98.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., McAdoo D. J. The cyclic GMP-induced inward current in neuron R14 of Aplysia californica: similarity to a FMRFamide-induced inward current. J Neurobiol. 1989 Jan;20(1):10–24. doi: 10.1002/neu.480200103. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Cyclic AMP-induced slow inward current in depolarized neurons of Aplysia californica. J Neurosci. 1990 Oct;10(10):3194–3207. doi: 10.1523/JNEUROSCI.10-10-03194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Cyclic AMP-induced slow inward current: its synaptic manifestation in Aplysia neurons. J Neurosci. 1990 Oct;10(10):3208–3218. doi: 10.1523/JNEUROSCI.10-10-03208.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G., Shcherbatko A. D. The effect of intracellular cAMP injections on stationary membrane conductance and voltage- and time-dependent ionic currents in identified snail neurons. Brain Res. 1983 Jun 6;268(2):321–338. doi: 10.1016/0006-8993(83)90499-7. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-induced inactivation of calcium current causes the inter-burst hyperpolarization of Aplysia bursting neurones. J Physiol. 1985 May;362:131–160. doi: 10.1113/jphysiol.1985.sp015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrohan C. R., Gillette R. Cyclic AMP-stimulated sodium current in identified feeding neurons of Lymnaea stagnalis. Brain Res. 1988 Jan 12;438(1-2):115–123. doi: 10.1016/0006-8993(88)91330-3. [DOI] [PubMed] [Google Scholar]
- Swandulla D. Cationic membrane conductances induced by intracellularly elevated cAMP and Ca2+: measurements with ion-selective microelectrodes. Can J Physiol Pharmacol. 1987 May;65(5):898–903. doi: 10.1139/y87-145. [DOI] [PubMed] [Google Scholar]


