Abstract
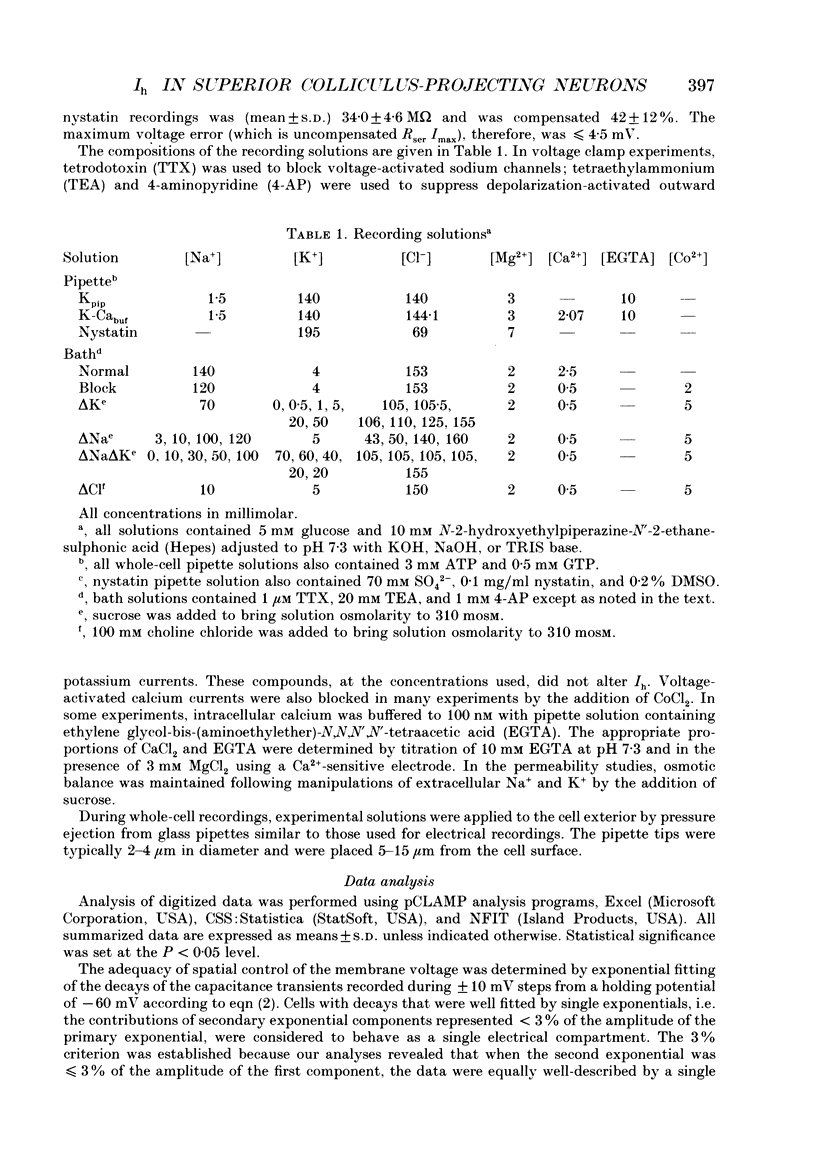
1. In vivo injections of rhodamine beads into the superior colliculus of 4-9 postnatal day rat pups label a population of layer 5 cells in the primary visual cortex that can be identified in tissue sections or dissociated cell cultures. 2. Under voltage clamp, hyperpolarizations of isolated superior colliculus-projecting (SCP) neurons from rest elicit an instantaneous inward current (Iinst) with nearly linear current-voltage properties that is not blocked by extracellular application of 3 mM CsCl. 3. Voltage clamp steps to potentials more negative than -60 mV evoke a slowly activating, non-inactivating inward current that is not blocked by 1 microM TTX, 1 mM 4-aminopyridine (4-AP), 5 mM Co2+, or 25 mM TEA, but is potently blocked by extracellular application of 3 mM CsCl. This current is similar to Ih described in other systems. 4. Ih, the time-dependent inward current in SCP neurons, begins to activate near the resting membrane potential and reaches full activation at -110 mV. The voltage dependence of activation is well fitted by a Boltzmann distribution with the membrane potential at half-maximal activation (V1/2) = -81.0 mV and s (steepness of the curve parameter) = 7.2 mV. Thus, Ih may contribute to setting the resting membrane potential and resting input resistance of SCP neurons. 5. The inward rectification of the whole-cell current vs. voltage relation is accounted for by the voltage dependence of Ih activation. Current through the activated h-conductance shows slight outward rectification that is accounted for by constant field considerations. 6. The h-conductance is substantially permeable only to sodium and potassium and, under normal physiological conditions, is expected to reverse at approximately -22 mV at 20 degrees C. For [K+]o < or = 20 mM and 120 mM > or = [Na+]o > or = 70 mM, Ih obeys independence with a PNa/PK (ratio of Na+ to K+ permeability) of 0.40. 7. Extracellular potassium increases gh. If this effect is modelled as the result of potassium binding to an extracellular conductance-permitting site, potassium has an apparent dissociation constant (Kapp) of 25.7 mM and the ability to maximally increase gh in the order of 10-fold over basal levels. 8. Ih underlies the depolarizing 'sag' and 'overshoot' observed in SCP neurons following the onset and offset, respectively, of hyperpolarizing current injections. In addition, Ih appears to control the duration and the frequency of repetitive firing following the cessation of sustained hyperpolarizing current injections.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




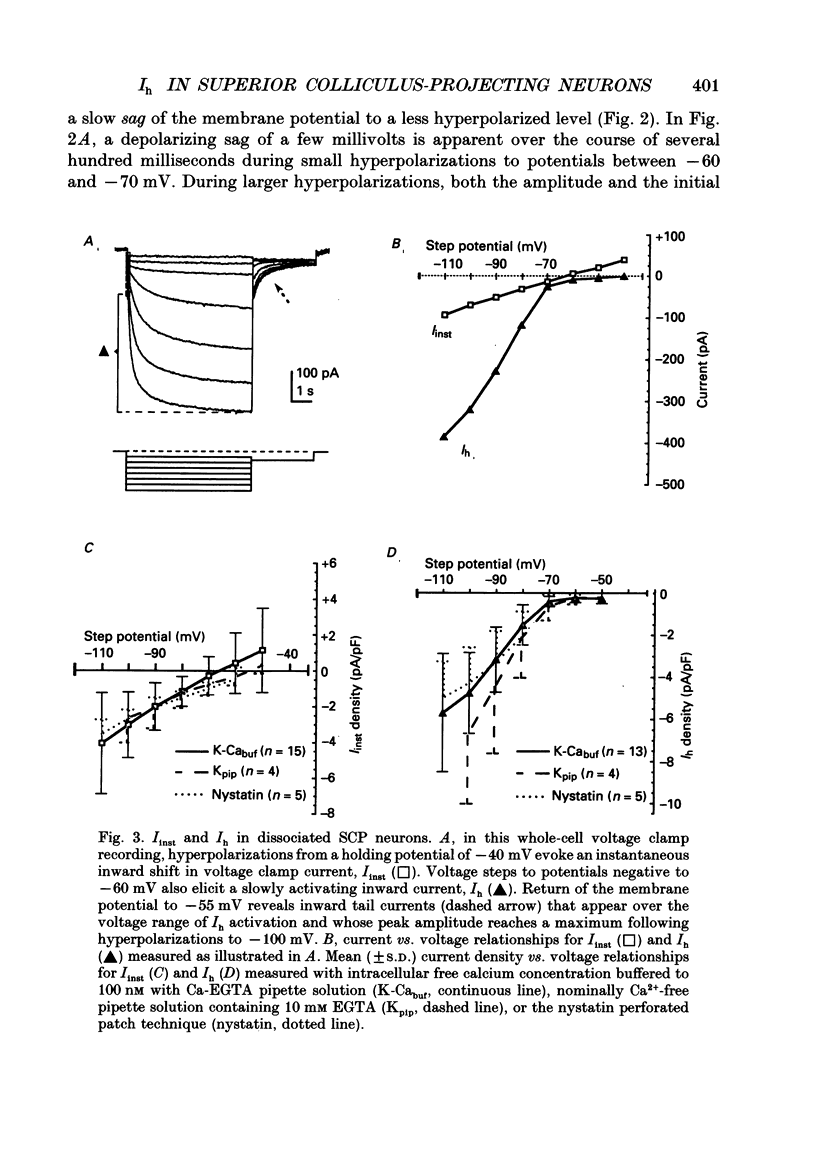

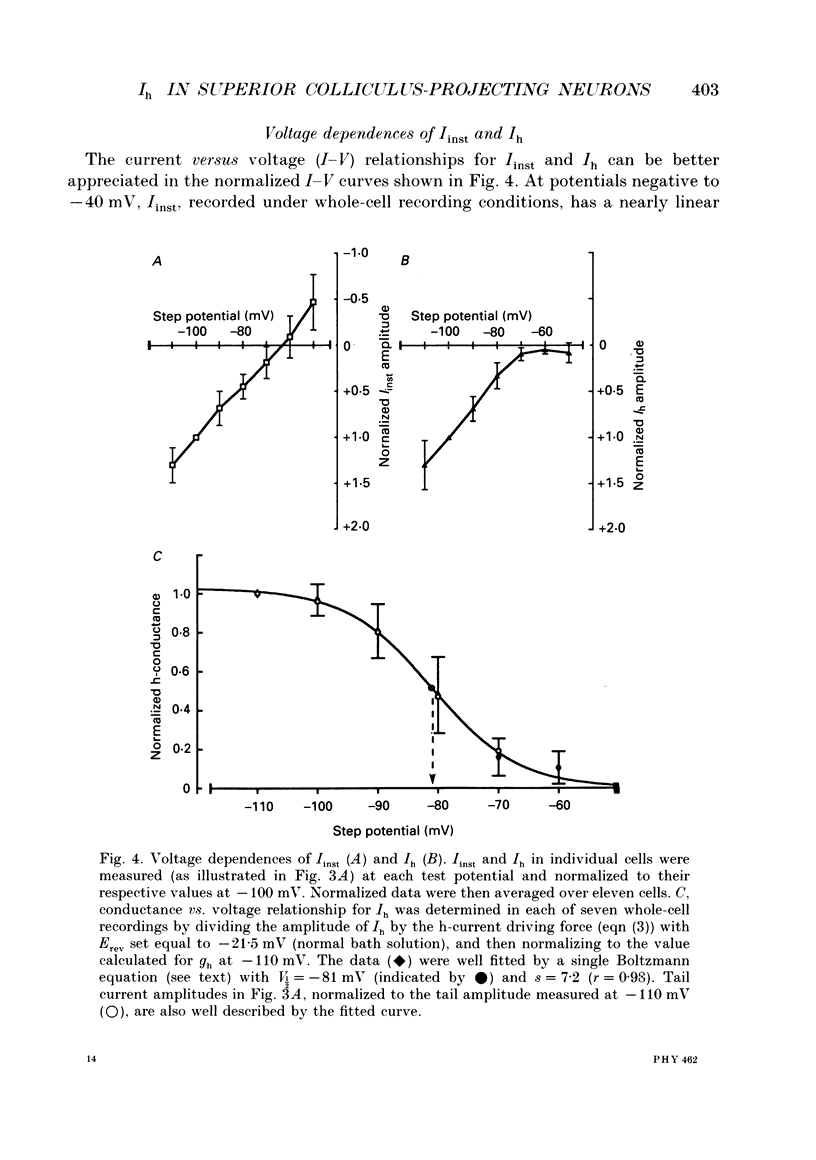

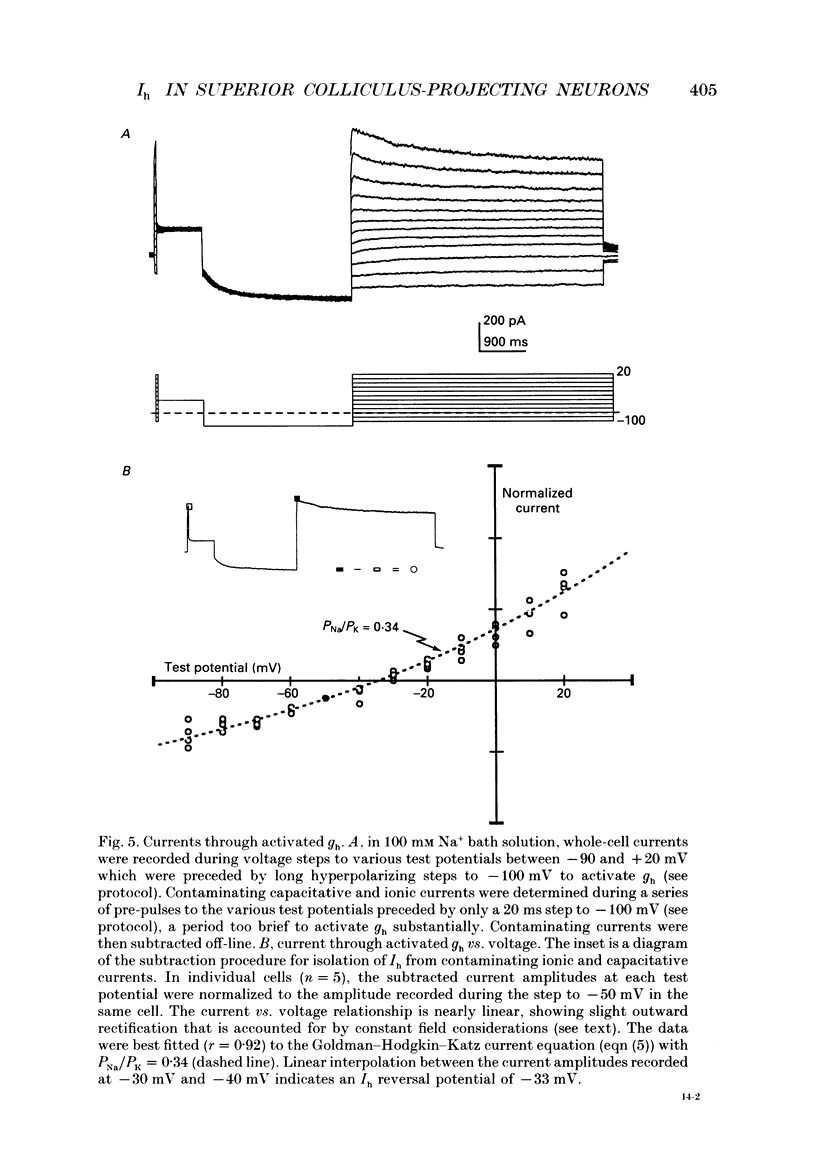

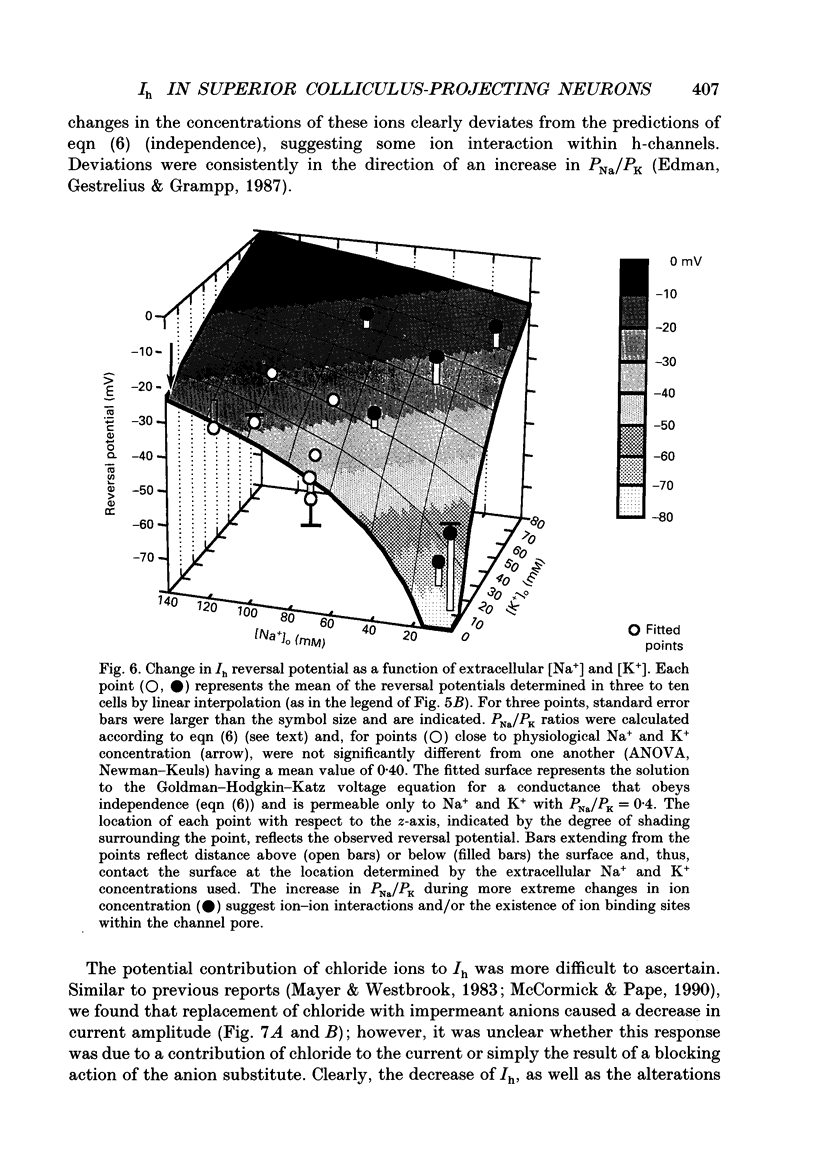
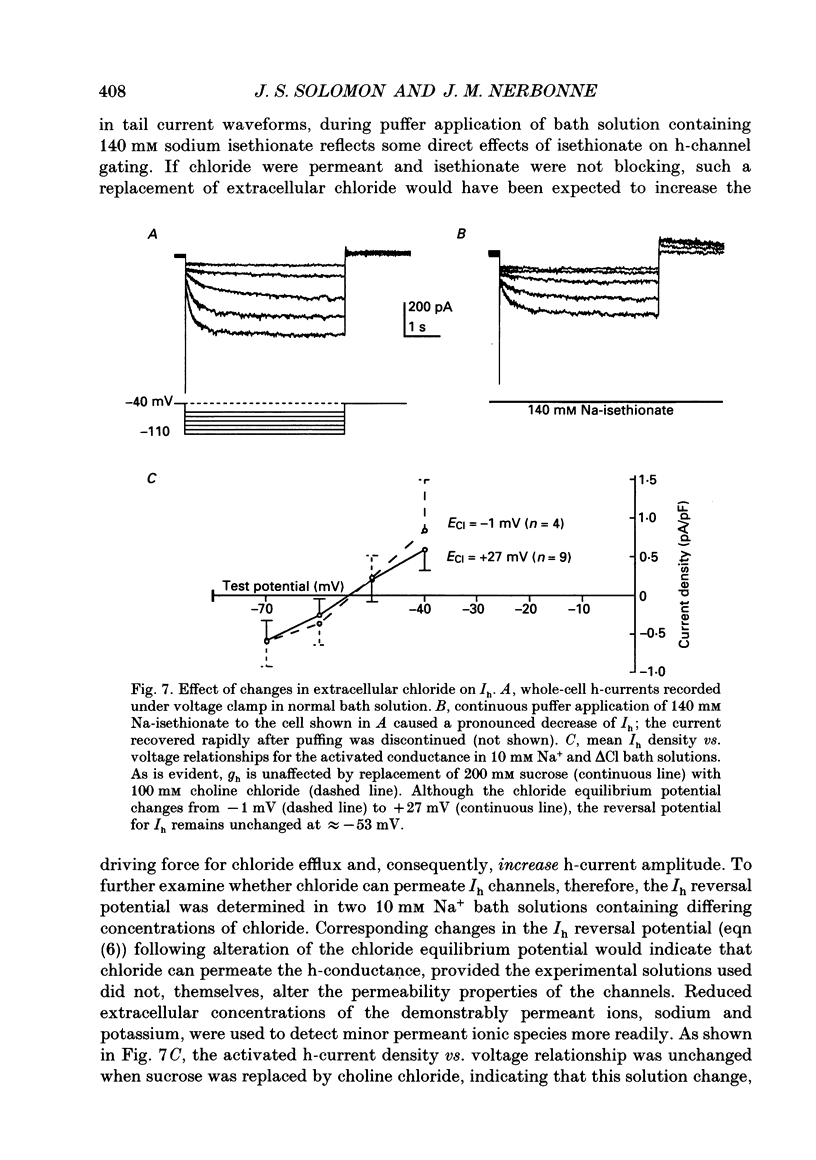

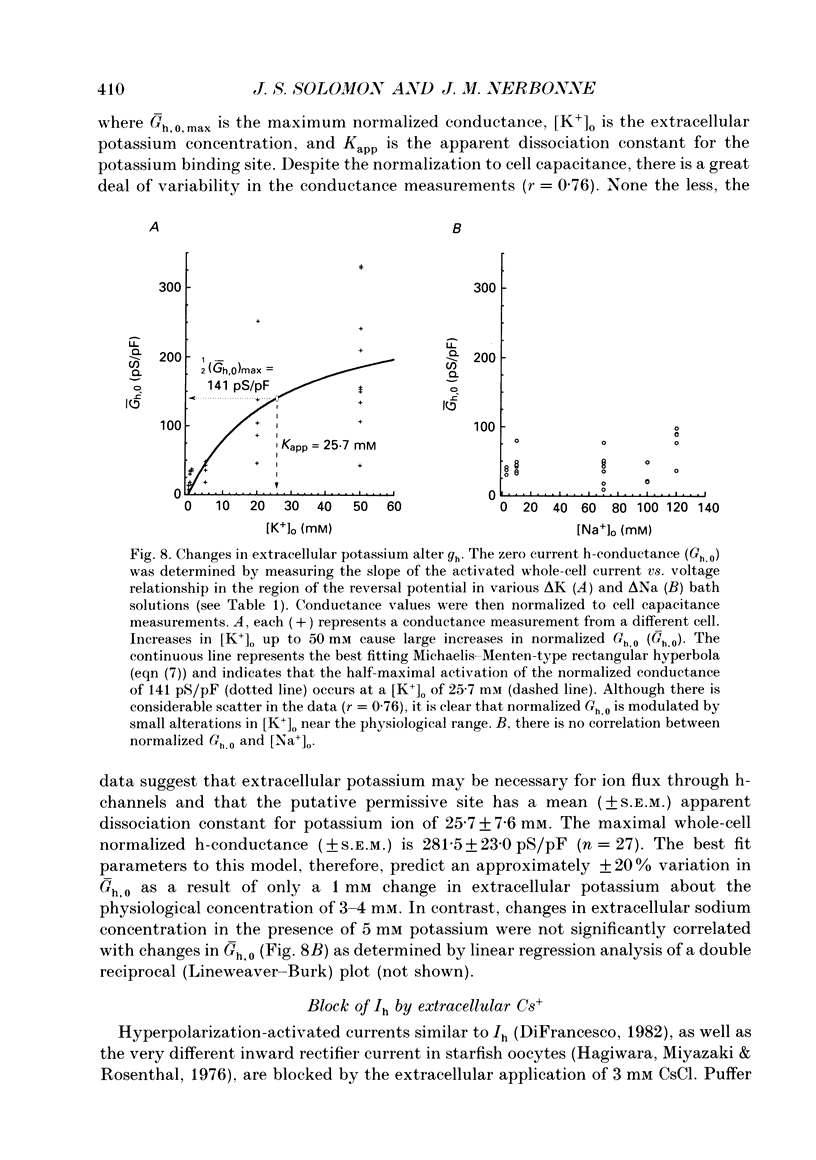
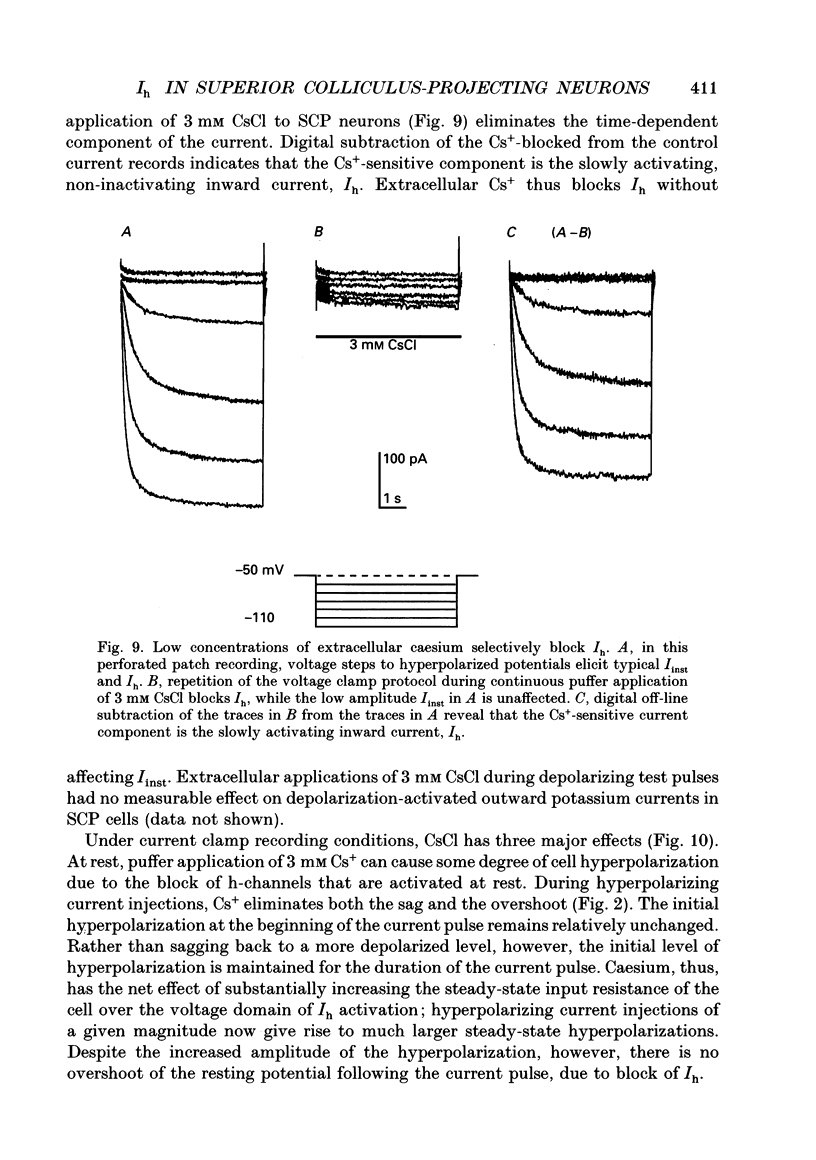
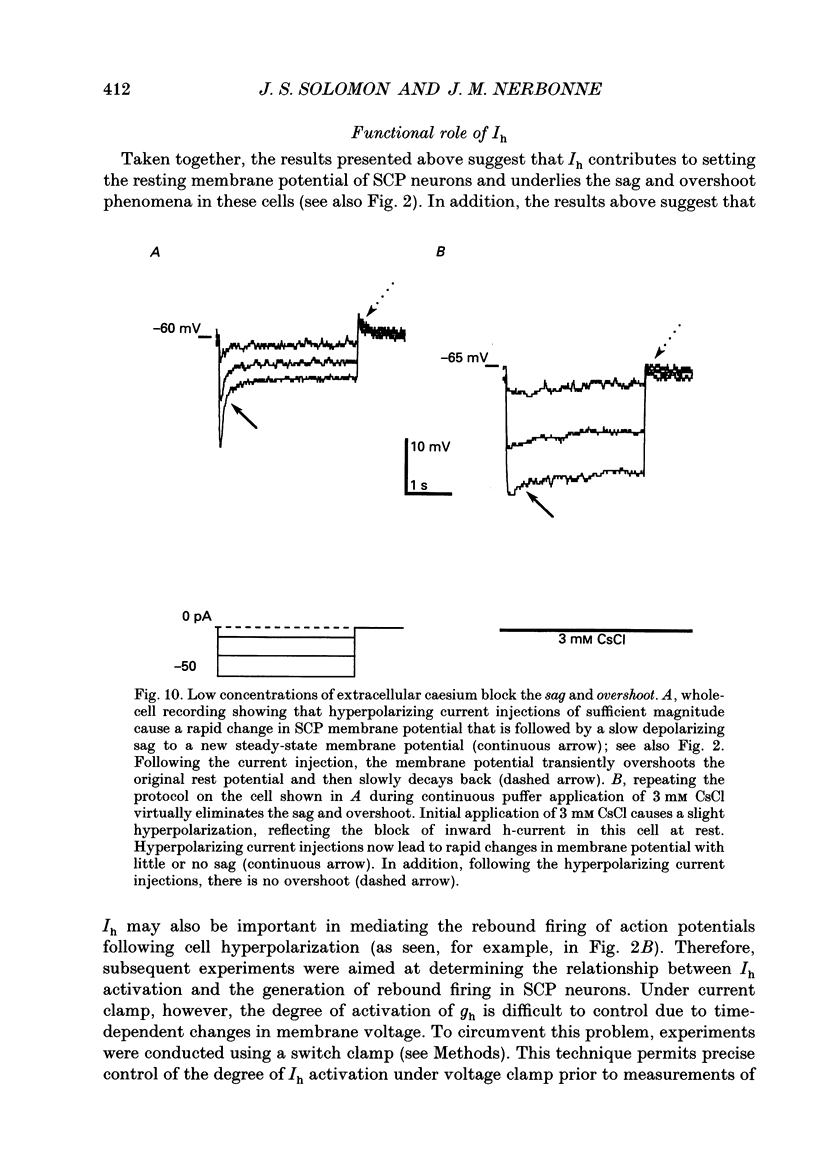
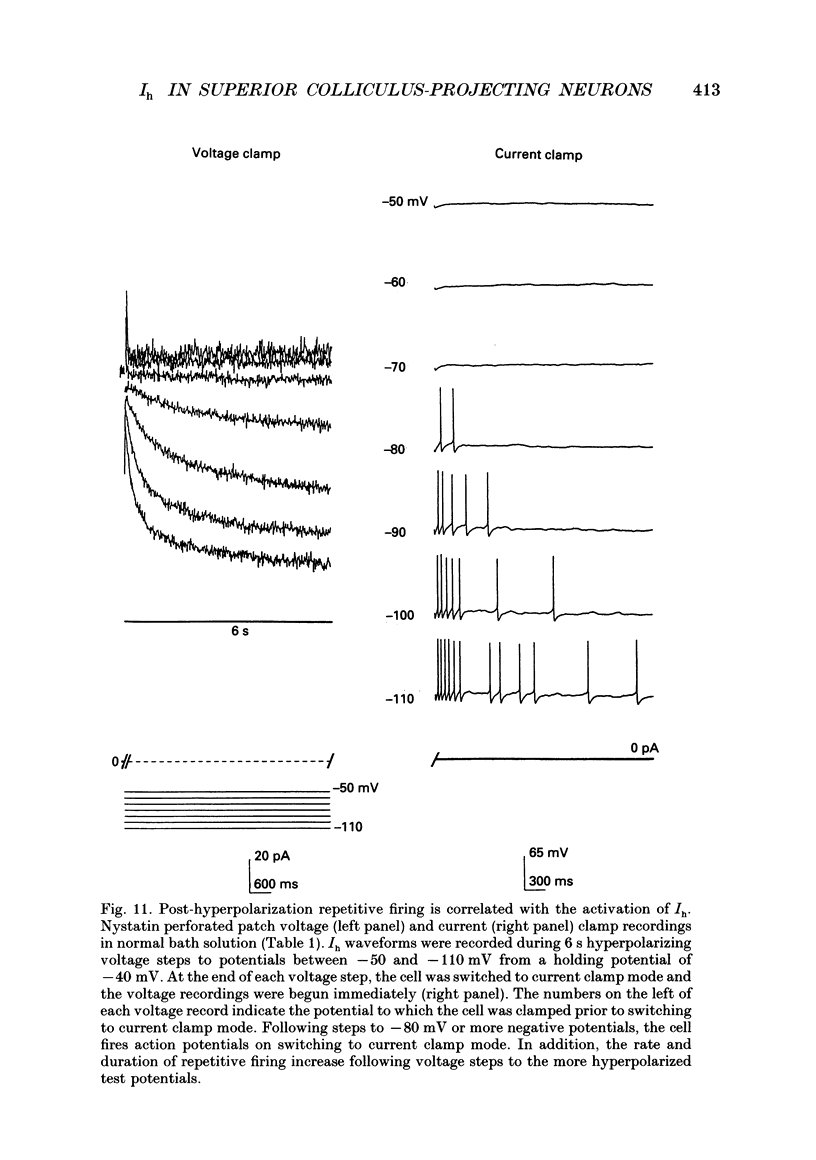
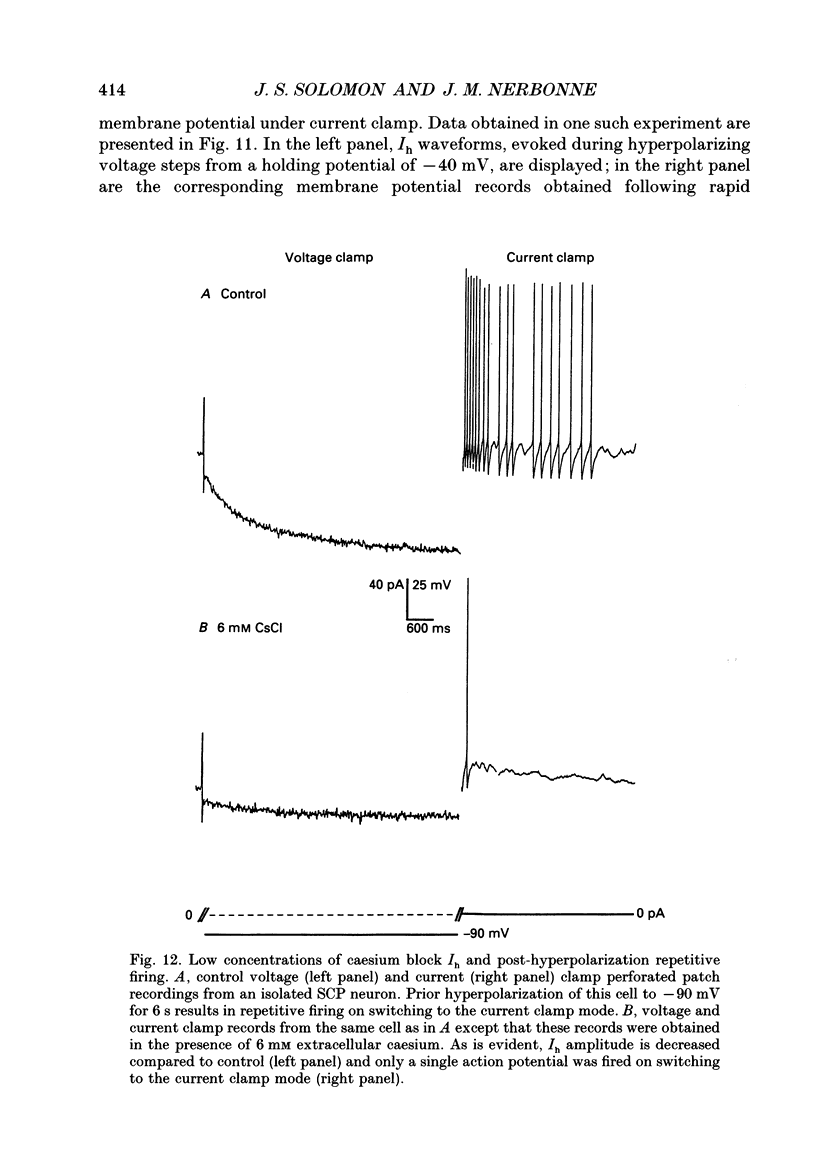






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angstadt J. D., Calabrese R. L. A hyperpolarization-activated inward current in heart interneurons of the medicinal leech. J Neurosci. 1989 Aug;9(8):2846–2857. doi: 10.1523/JNEUROSCI.09-08-02846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
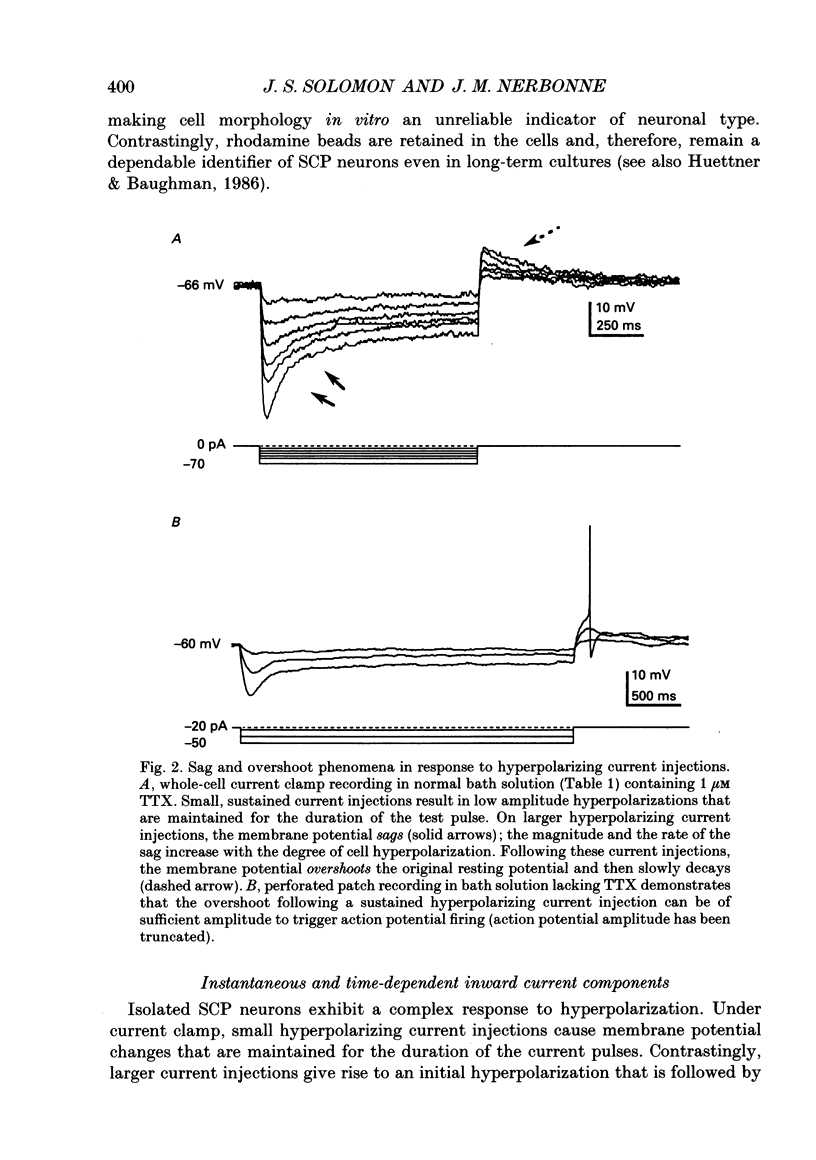
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., DiFrancesco D., Noble S. J. How does adrenaline accelerate the heart? Nature. 1979 Jul 19;280(5719):235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990 Mar;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 1991 Jan;14(1):16–21. doi: 10.1016/0166-2236(91)90178-w. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman A., Gestrelius S., Grampp W. Current activation by membrane hyperpolarization in the slowly adapting lobster stretch receptor neurone. J Physiol. 1987 Mar;384:671–690. doi: 10.1113/jphysiol.1987.sp016476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman A., Grampp W. Ion permeation through hyperpolarization-activated membrane channels (Q-channels) in the lobster stretch receptor neurone. Pflugers Arch. 1989 Jan;413(3):249–255. doi: 10.1007/BF00583537. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Futamachi K. J., Mutani R., Prince D. A. Potassium activity in rabbit cortex. Brain Res. 1974 Jul 19;75(1):5–25. doi: 10.1016/0006-8993(74)90767-7. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Martin K. A., Whitteridge D. Evidence for the connections between a clutch cell and a corticotectal neuron in area 17 of the cat visual cortex. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):385–391. doi: 10.1098/rspb.1988.0028. [DOI] [PubMed] [Google Scholar]
- Giffin K., Solomon J. S., Burkhalter A., Nerbonne J. M. Differential expression of voltage-gated calcium channels in identified visual cortical neurons. Neuron. 1991 Mar;6(3):321–332. doi: 10.1016/0896-6273(91)90242-r. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman L. E., Schofield B. R., Lin C. S. Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol. 1988 Jun 1;272(1):149–160. doi: 10.1002/cne.902720111. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol. 1987 Sep;390:319–333. doi: 10.1113/jphysiol.1987.sp016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Sypert G. W., Ward A. A., Jr Extracellular potassium concentration changes during propagated seizures in neocortex. Exp Neurol. 1973 Jan;38(1):20–26. doi: 10.1016/0014-4886(73)90004-6. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Burkhalter A., Dreyer W. J. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984 Aug 9;310(5977):498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Larkman A., Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990 May;10(5):1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990 May;10(5):1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Obrenovitch T. P., Hartell N., Barratt S., Bachelard H. S., Symon L. Simultaneous recording of tissue PCO2, interstitial pH and potassium activity in the rat cerebral cortex during anoxia and the subsequent recovery period. Neurol Res. 1989 Sep;11(3):153–159. doi: 10.1080/01616412.1989.11739882. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Schielke G. P., Moises H. C., Betz A. L. Blood to brain sodium transport and interstitial fluid potassium concentration during early focal ischemia in the rat. J Cereb Blood Flow Metab. 1991 May;11(3):466–471. doi: 10.1038/jcbfm.1991.89. [DOI] [PubMed] [Google Scholar]
- Schofield B. R., Hallman L. E., Lin C. S. Morphology of corticotectal cells in the primary visual cortex of hooded rats. J Comp Neurol. 1987 Jul 1;261(1):85–97. doi: 10.1002/cne.902610107. [DOI] [PubMed] [Google Scholar]
- Silva L. R., Amitai Y., Connors B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991 Jan 25;251(4992):432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Thong I. G., Dreher B. The development of the corticotectal pathway in the albino rat. Brain Res. 1986 Mar;390(2):227–238. doi: 10.1016/s0006-8993(86)80231-1. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Kritz N., Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972 Apr 14;39(1):255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]