Abstract
1. A weak electroneutral sodium channel blocker 6-chloro-3,5-diamino-pyrazine-2-carboxamide was used to perform noise analysis on isolated epithelium from Rana fuscigula to determine the cellular mechanism underlying autoregulation of Na+ channel densities in response to a reduction in the mucosal Na+ concentration. 2. The inherent transport rates of these tissues were generally lower than in other frog skins. The macroscopic sodium current, INa, averaged 10.71 microA/cm2 and was mainly determined by the number of open channels (N(o)) which averaged 21.6 million/cm2. The calculated mean channel open probability (beta') was 0.38, and corresponded very closely to values previously determined by patch clamp. 3. Reducing the mucosal Na+ from 110 to 10 mM caused large increases in the open channel density, which stabilized the Na+ transport rate. N(o) increased from a mean value of 26.6 to 64.3 million/cm2 within 2 min. 4. Autoregulatory changes were induced primarily by increasing beta' by about 60% and to a lesser extent by an increase in NT, the total number of open and closed channels. 5. We also examined the role of the cytoskeleton in the regulation of Na+ channel densities. Colchicine treatment, which disrupted microtubules, had no apparent effect on the ability of the tissues to autoregulate their Na+ channel densities. 6. The integrity of the microfilaments were essential for autoregulatory changes in N(o). After we had disrupted the microfilaments with cytochalasin B, we observed a marked reduction in the ability of the tissues to increase N(o). 7. The mean N(o) did not increase in response to a drop in mucosal Na+ despite the fact that beta' increased by 69%. We, therefore, assumed that cytochalasin B did not affect Na+ channels already present in the membrane but interfered with recruitment of new channels. Significantly, we did not observe any increase in NT. 8. In kidney and other tight epithelia, microfilaments are responsible for regulating the delivery of newly synthesized membrane proteins. We believe that our results with cytochalasin-treated tissues support the theory that autoregulatory changes in N(o) are also regulated by the recruitment of channels from a cytoplasmic pool.
Full text
PDF



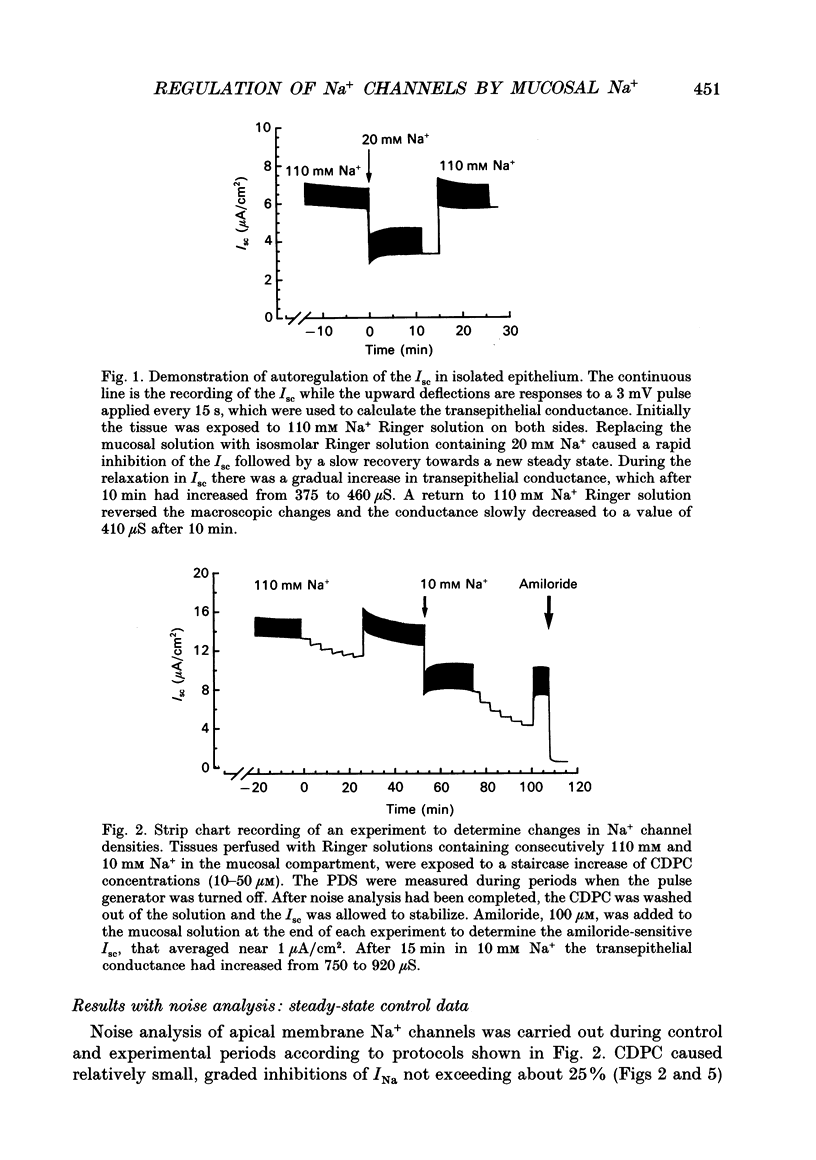

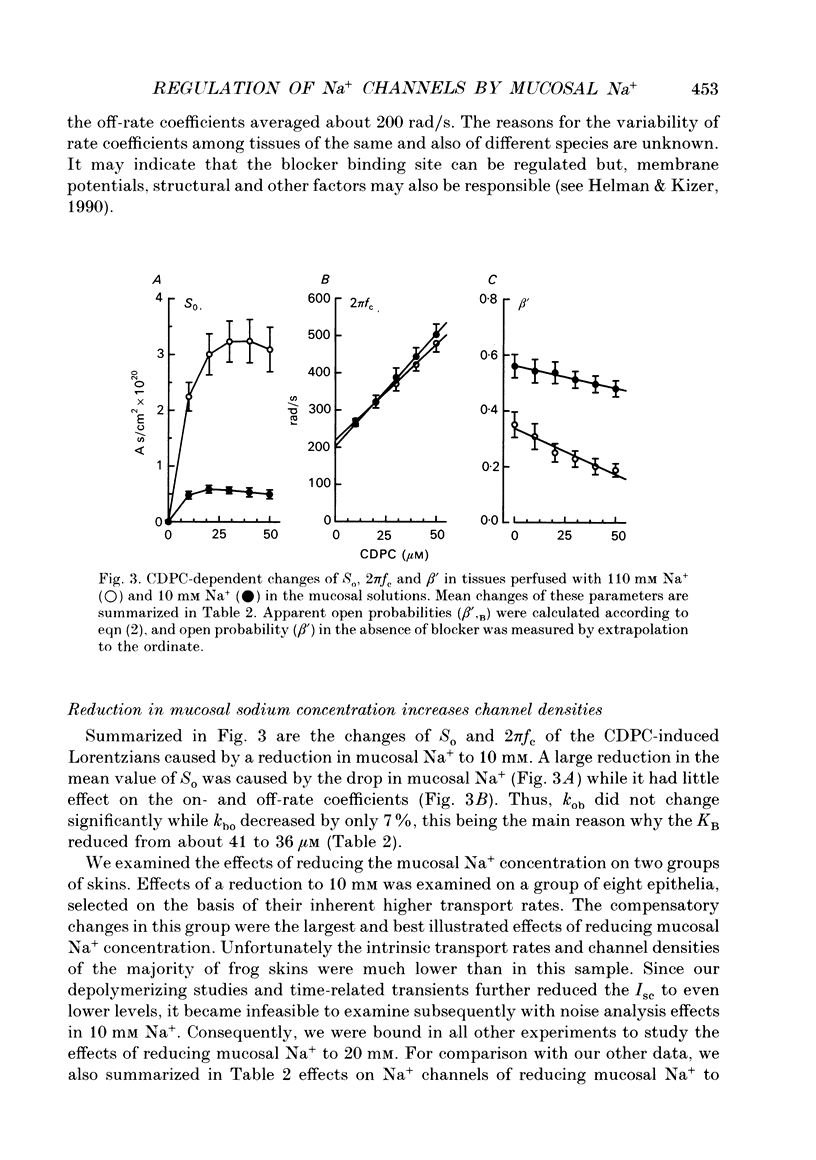
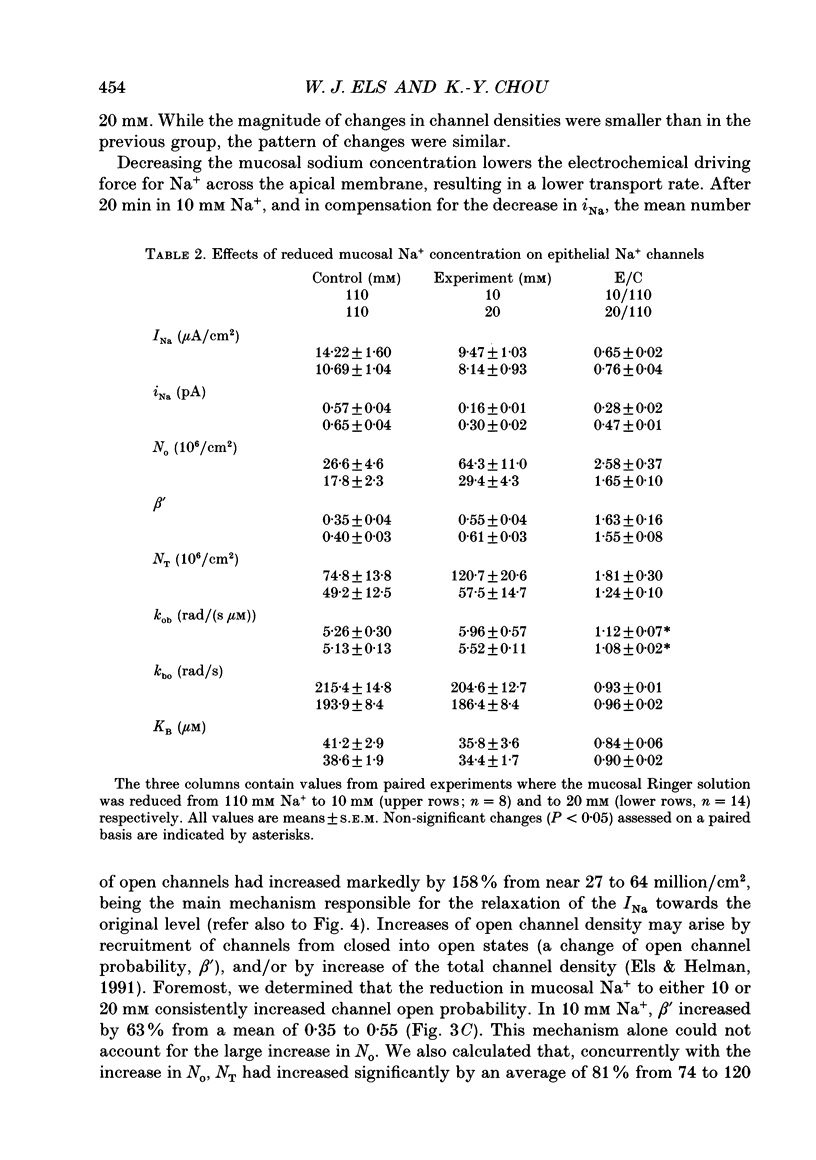
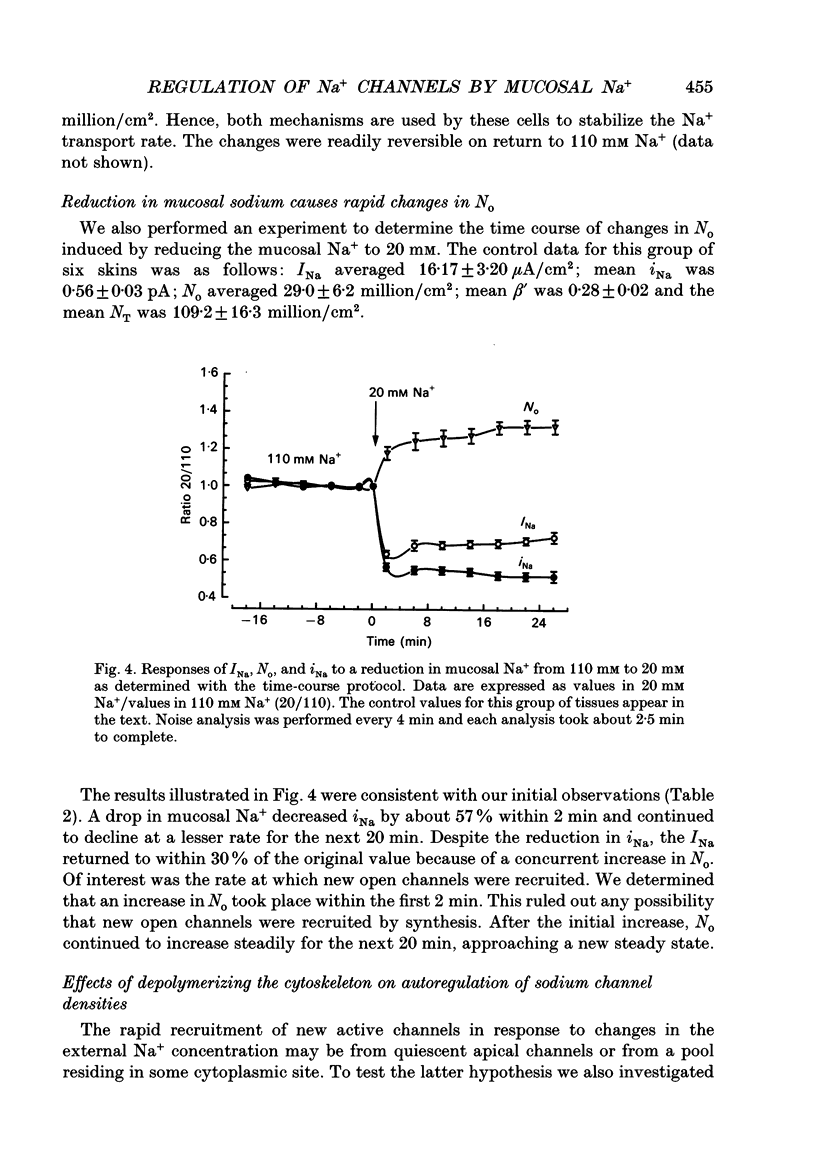








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck F. J., Van Driessche W., Helman S. I. Autoregulation of apical membrane Na+ permeability of tight epithelia. Noise analysis with amiloride and CGS 4270. J Gen Physiol. 1985 Apr;85(4):555–582. doi: 10.1085/jgp.85.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. Membrane recycling and epithelial cell function. Am J Physiol. 1989 Jan;256(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1989.256.1.F1. [DOI] [PubMed] [Google Scholar]
- Els W. J., Helman S. I., Mencio T. Activation of epithelial Na channels by hormonal and autoregulatory mechanisms of action. J Gen Physiol. 1991 Dec;98(6):1197–1220. doi: 10.1085/jgp.98.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Els W. J., Helman S. I. Regulation of epithelial sodium channel densities by vasopressin signalling. Cell Signal. 1989;1(6):533–539. doi: 10.1016/0898-6568(89)90061-2. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Erlij D., Helman S. I. Intracellular voltage of isolated epithelia of frog skin: apical and basolateral cell punctures. J Gen Physiol. 1980 Oct;76(4):447–453. doi: 10.1085/jgp.76.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W., Larsen E. H., Lindemann B. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol. 1977 May;267(1):137–166. doi: 10.1113/jphysiol.1977.sp011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Garty H., Edelman I. S. Amiloride-sensitive trypsinization of apical sodium channels. Analysis of hormonal regulation of sodium transport in toad bladder. J Gen Physiol. 1983 Jun;81(6):785–803. doi: 10.1085/jgp.81.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Baxendale L. M. Blocker-related changes of channel density. Analysis of a three-state model for apical Na channels of frog skin. J Gen Physiol. 1990 Apr;95(4):647–678. doi: 10.1085/jgp.95.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Cox T. C., Van Driessche W. Hormonal control of apical membrane Na transport in epithelia. Studies with fluctuation analysis. J Gen Physiol. 1983 Aug;82(2):201–220. doi: 10.1085/jgp.82.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman P. S., Clark R. A., Hoilien C. A., Linas S. L., Molitoris B. A. Role of microfilaments in maintenance of proximal tubule structural and functional integrity. Am J Physiol. 1990 Aug;259(2 Pt 2):F279–F285. doi: 10.1152/ajprenal.1990.259.2.F279. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Ifshin M. S., Loo D. D., Diamond J. M. Studies of sodium channels in rabbit urinary bladder by noise analysis. J Membr Biol. 1984;80(2):135–151. doi: 10.1007/BF01868770. [DOI] [PubMed] [Google Scholar]
- Li J. H., Palmer L. G., Edelman I. S., Lindemann B. The role of sodium-channel density in the natriferic response of the toad urinary bladder to an antidiuretic hormone. J Membr Biol. 1982;64(1-2):77–89. doi: 10.1007/BF01870770. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol. 1989 Jun;256(6 Pt 2):F1094–F1103. doi: 10.1152/ajprenal.1989.256.6.F1094. [DOI] [PubMed] [Google Scholar]
- Linshaw M. A. Volume control of isolated rabbit proximal tubules. Semin Nephrol. 1989 Mar;9(1):83–90. [PubMed] [Google Scholar]
- Olans L., Sariban-Sohraby S., Benos D. J. Saturation behavior of single, amiloride-sensitive Na+ channels in planar lipid bilayers. Biophys J. 1984 Dec;46(6):831–835. doi: 10.1016/S0006-3495(84)84082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J Gen Physiol. 1988 Jul;92(1):121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol. 1987 Aug;253(2 Pt 2):F333–F339. doi: 10.1152/ajprenal.1987.253.2.F333. [DOI] [PubMed] [Google Scholar]
- Parczyk K., Haase W., Kondor-Koch C. Microtubules are involved in the secretion of proteins at the apical cell surface of the polarized epithelial cell, Madin-Darby canine kidney. J Biol Chem. 1989 Oct 5;264(28):16837–16846. [PubMed] [Google Scholar]
- Pearl M., Taylor A. Role of the cytoskeleton in the control of transcellular water flow by vasopressin in amphibian urinary bladder. Biol Cell. 1985;55(3):163–172. doi: 10.1111/j.1768-322x.1985.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Benos D. J. The amiloride-sensitive sodium channel. Am J Physiol. 1986 Feb;250(2 Pt 1):C175–C190. doi: 10.1152/ajpcell.1986.250.2.C175. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Benos D. J. Epithelial Na+ channels. Annu Rev Physiol. 1991;53:509–530. doi: 10.1146/annurev.ph.53.030191.002453. [DOI] [PubMed] [Google Scholar]
- Valenti G., Hugon J. S., Bourguet J. To what extent is microtubular network involved in antidiuretic response? Am J Physiol. 1988 Dec;255(6 Pt 2):F1098–F1106. doi: 10.1152/ajprenal.1988.255.6.F1098. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Erlij D. Noise analysis of inward and outward Na+ currents across the apical border of ouabain-treated frog skin. Pflugers Arch. 1983 Aug;398(3):179–188. doi: 10.1007/BF00657149. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Lindemann B. Concentration dependence of currents through single sodium-selective pores in frog skin. Nature. 1979 Nov 29;282(5738):519–520. doi: 10.1038/282519a0. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Zweifach A. Recent advances in the characterization of epithelial ionic channels. Biochim Biophys Acta. 1987 Apr 27;906(1):1–31. doi: 10.1016/0304-4157(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Zeiske W., Van Driessche W. The sensitivity of apical Na+ permeability in frog skin to hypertonic stress. Pflugers Arch. 1984 Feb;400(2):130–139. doi: 10.1007/BF00585030. [DOI] [PubMed] [Google Scholar]


